1. INTRODUCTION
Klebsiella pneumoniae is a Gram-negative bacterium that may induce a variety of illnesses in healthcare settings, such as pneumonia, bloodstream infections, wound or surgical site infections and meningitis [1]. It was subsequently known as a saprophyte microbe capable of inhabiting the human gastrointestinal tract, skin and nasopharynx, as well as causing infections of the urinary and biliary tracts, osteomyelitis and bacteraemia [2]. Antibiotic resistance has been identified as a global concern in modern medicine after more than 70 years of misuse of antibiotics to treat numerous infections. K. pneumoniae is a multidrug-resistant (MDR) bacterium and the ESBL producing strains-producing strains. ESBL-producing strains are also a prime contributor to hospital infections, which are marked by high morbidity and mortality due to limited therapeutic approaches [3]. Furthermore, ESBL infection is hard to treat not only due to its tolerance to extended spectrum cephalosporins but rather because many ESBL genes are found on various plasmids that also incorporate genes encoding resistance to a variety of other antibiotics, including amino glycosides, chloramphenicol, sulfonamides and tetracyclines [4].
Biofilm formation of K. pneumoniae on the catheter’s interior and external surfaces has been identified as the primary cause of catheter-associated urinary tract infections (CAUTIs) [5]. Many preceding reports have demonstrated that bacterial biofilms are more resistant to environmental challenges, including heat, than their planktonic suspension counterparts and in addition, biofilm has a pivotal role in safeguarding cells from antibiotics, permitting them to endure in harsh environments [6]. Bacterial adhesion to surfaces is primarily influenced by the properties of the materials that make up the contact surface, ambient challenges and media composition [5]. So far, crystal violet (CV) staining has been the most frequently used approach for assessing biofilms. Nevertheless, there is a scarcity of information on characterization and quantification of K. pneumoniae biofilms.
The development of biofilms is one of the bacteria most efficient means of surviving in the presence of antibiotics, particularly for K. pneumoniae, one of the most prevalent bacterial sources of biofilm-related contamination of medical equipment [7]. It has been found that planktonic bacterial resistance to antibiotics is substantially lower than tolerance specific to biofilms [8]. As a result, biofilm-related illnesses are more challenging to treat and more likely to persist [9]. The quantitative relationship between the development of biofilms and antibiotic resistance is not yet known.
The purpose of this study is to establish the antibiotic resistance profiles of K. pneumoniae as well as to screen for ESBL-producing isolates, assess biofilm development on two distinct catheter surfaces (silicone elastomer bonded and PVC) by employing the CV staining approach, analyze the influence of sugar supplements in biofilm formation and to establish the antibiotic resistance pattern of K. pneumoniae isolated from biofilm.
2. MATERIALS AND METHODS
2.1. Collection of Strains
All the strains used in this study were kindly provided by K.A.P. Viswanatham Government College Hospital in Tiruchirappalli, Tamil Nadu, India.
2.2. Reconfirmation of Bacterial Isolates
First, the accrued isolates were maintained overnight at 37° C on tryptic soya agar (TSA) (HiMedia) and then transferred to MacConkey agar (HiMedia) for morphological assessment, subsequently preceded by a biochemical confirmation study [10].
2.3. Assessment of Antibiotic Resistance Pattern
Disk diffusion method was applied to screen the antibiotic resistance characteristics of the identified K. pneumoniae bacterial isolates. The overnight bacterial suspensions were diluted into a 0.85% NaCl and the turbidity was comparable to the McFarland standards. A suspension suitable for 0.5 McFarland turbidity (1.5-2 × 108 colony forming units; cfu/mL) was prepared in sterile saline water [4]. Sterile cotton swabs were used to swab the bacterial culture over the Mueller-Hinton Agar (MHA) (HiMedia) and the antibiotic disks were placed and incubated at 37° C for 24 h. The zone of inhibition was determined after incubation. The antibiotic disks employed in this experiment were of ampicillin (AMP) (10 μg), aztreonam (AT) (30 μg), cefepime (CPM) (30 μg), cefotaxime (CTX) (30 μg), cefuroxime (CXM) (30 μg), cephalothine (CT) (10 μg), co-trimoxazole (COT) (25 μg), doripenem (DOR) (10 μg), ertapenem (ETP) (10 μg), gentamycin (GEN) (10 μg), imipenem (IMP) (10 μg) and meropenem (MRP) (10 μg) (HiMedia).
2.4. Phenotypic Confirmatory Disk Diffusion Test (PCDDT)
For the initial screening of ESBL production, antibiotic disks of ceftazidime (CAZ) (30 μg), AT (30 μg), CTX (30 μg) and ceftriaxone (CRO) (30 μg) were utilized. Then, 0.5 McFarland standard inoculums were made from colonies on agar plates. MHA plates were infected with a germ-free cotton swab using the lawn culture procedure. The aforesaid disks were placed on the MHA plate using sterile pincers and the plate was incubated at 37° C for 18-24 h [11].
2.5. Double Disk Synergy Test (DDST)
A disk approximation test was used to determine the isolates that produce ESBL. Using sterile cotton swab, the 18 h old bacterial suspension equivalent to McFarland standard was inoculated on a sterilized MHA plates. CAZ (30 μg), CPM (30 μg) and CTX (30 μg) were positioned around an amoxicillin-clavulanic acid (AMC) (20-10 μg) disk at a distance of 16-20 mm. Finally, the plates were incubated for 24 h at 37° C. When a zone of inhibition was found in cephalosporin disks and a clear-cut rise toward AMC (30 μg) disk, the bacterium synthesizing ESBL was recognized [12].
2.6. Modified Double Disk Synergy Test (MDDST)
The MDDST approach was used to determine ESBL in clinical isolates that produce AmpC. A disk of AMC (30 μg) was positioned in the center and then, disks of AT (30 μg), CPM (30 μg), CTX (30 μg) and CAZ (30 μg) were positioned around it at 16-20 mm distance from the AMC (30 μg) disk (center to center) and a disk of piperacillin-tazobactam (TZP) (100 μg/10 μg) was placed at 22 and 25 mm over the agar plate swabbed with 18 h old culture equivalent to McFarland standard. When the zone of inhibition surrounding CPM (30 μg) or any of the extended spectrum cephalosporin disks showed a clear-cut rise toward the AMC disk or TZP disk, the organisms were determined to be producing ESBL [12].
2.7. Modified Three-Dimensional Test (MTDT)
2.7.1. Direct modified three-dimensional test (DMTDT)
The test strains comparable to 0.5 McFarland standards were swabbed on sterile MHA plates as conducted in DDST and the disks of AT (30 μg), CTX (30 μg) or CAZ (30 μg) were positioned in the center of the plate. Inoculum (30 μL) of the test strain in Tryptone Soya Broth (TSB), comparable to 5.0 McFarland standards were seeded into a well with a diameter of 6 mm. An ESBL production was distinguished by a heart-shaped distortion of the zone of inhibition, with test organism growth emerging behind and approaching the well [13].
2.7.2. Indirect modified three-dimensional test (IMTDT)
The E. coli ATCC 25922 strain comparable to 0.5 McFarland standard instead of the test strain was swabbed on the sterilized MHA plates. Inoculums (30 μL) of the test strain in TSB comparable to 5.0 McFarland standards were seeded into a well with a diameter of 6 mm. ESBL production was evidenced by a heart-shaped distortion of the zone of inhibition around the β-lactam disk [12].
2.8. Testing of Biofilm Production on Silicone Elastomer Bonded and PVC Catheter Surface by K. pneumoniae using TSB and TSBG Media
The CV staining test was conducted to evaluate bacterial adhesion on catheters made of two distinct materials (Silicone elastomer bonded and PVC). The catheters (Teleflex Medical Pvt. Ltd. and Royal Surgicare Pvt. Ltd., India) were cut into 13 mm long vertically and placed in a tubes containing 10 mL of sterile TSB and TSBG, then inoculated with 100 μL (initial concentration - 0.3 at OD595 nm) of overnight bacterial suspension and incubated at 37° C for 24 h, respectively. At the end of incubation, the glass tubes were decanted and washed with sterile water to remove the detached cells. Then, the catheters were shifted to sterile glass tubes to avoid considering cells adhered to the preceding glass tubes after incubation. Later, the adhered cells were stained with 1 mL of 0.5% CV (HiMedia). Excess stains were washed with sterile water and the stain in the adhered cells was decolorized with 1 mL of 99% ethanol, respectively. This solution was used for assessing the biofilm formation on the surface materials tested. Finally, the OD was taken at 595 nm using an UV Spectrophotometer (Cary-60 UV-Vis, Agilent Technologies, U.S.A) [6].
2.9. Assessment of Antibiotic Resistance Pattern after Biofilm Formation on Both the Tested Catheter Surfaces (Silicone Elastomer Bonded and PVC)
The disk diffusion method was employed to obtain the antibiotic resistance profile of K. pneumoniae after biofilm formation on the tested surfaces. After the biofilm formation, using the sterile cotton swab, biofilm cells were collected from both the surfaces tested and diluted in sterile 0.85% saline to obtain the turbidity equivalent to McFarland standards. The antibiotic disks utilized in the antibiotic susceptibility test before biofilm formation were used in this experiment.
3. RESULTS
3.1. Assessment of K. pneumoniae Antibiotic Resistance Profile
The percentage of antibiotic resistance of all the strains tested is shown in Table 1. All 47 isolates tested positive for resistance to at least one of the antibiotics tested. In the ESBL antibiotic test, the test isolates showed strong resistance to AMP (92%), CT (79%), CAZ (77%), CPM (64%), CTX (60%), COT (51%), AT (44%) and GEN (27%).
Table 1: Antibiotic resistance profile of Klebsiella pneumoniae.
| Antibiotics | Resistant (%) | Intermediate (%) | Susceptible (%) |
|---|---|---|---|
| AMP | 92 | 2 | 6 |
| CT | 79 | 2 | 19 |
| CXM | 77 | 0 | 23 |
| CPM | 64 | 6 | 30 |
| CTX | 60 | 0 | 40 |
| COT | 51 | 2 | 47 |
| AT | 44 | 9 | 47 |
| IMP | 40 | 22 | 38 |
| GEN | 27 | 9 | 64 |
| MRP | 15 | 6 | 79 |
| ETP | 13 | 2 | 85 |
| DOR | 0 | 0 | 100 |
AMP: Ampicillin, AT: Aztreonam, CPM: Cefepime, CTX: Cefotaxime, CXM: Cefuroxime, CT: Cephalothine, COT: Co–trimoxazole, DOR: Doripenem, ETP: Ertapenem, GEN: Gentamycin, IMP: Imipenem, MRP: Meropenem.
The different types of antibiotic resistant profile for K. pneumoniae strains were studied. There were a total of 29 distinct types of resistance patterns found among the strains tested. Among them, 74% (n = 35/47) of the tested isolated confirmed the existence of MDR strains. In addition, 36% (n = 17/47) of isolates tested positive for carbapenem antibiotic resistance and 97.8% (n=46/47) tested positive for ESBL antibiotic resistance. In a carbapenem antibiotic resistance test, high resistance was disk overed against IMP (40%), MRP (15%) and ETP (13%). DOR sensitivity was determined to be 100% in all strains. KPC strains made up 36% of the total.
3.2. Comparison of Various ESBL Detection Approaches
The incidence of ESBL-producing K. pneumoniae was determined using the following assays: PCDDT, DDST, MDDST, DMTDT and IMTDT as per CLSI recommendations. The results obtained with the different methods used to detect ESBL are given in Table 2. The results demonstrated that the MDDST method was the most sensitive, while the DMTDT method was the least sensitive, when compared to the other techniques employed to detect ESBL-producing strains.
Table 2: Comparison of various methods of extended–spectrum–β–lactamase detection.
| ESBL detection approaches | Positive (%) |
|---|---|
| PCDDT | 44 (93.6) |
| DDST | 39 (82.9) |
| MDDST | 46 (97.8) |
| DMTDT | 17 (36.1) |
| IMTDT | 41 (87.2) |
ESBL: Extended–spectrum–β–lactamase, PCDDT: Phenotypic confirmatory disk diffusion test, DDST: Double disk synergy test, MDDST: Modified double disk synergy test, DMTDT: Direct modified three-dimensional test, IMTDT: Indirect modified three-dimensional test.
3.3. Testing of Biofilm Production on Silicone Elastomer Bonded Catheter Surface by K. pneumoniae using TSB and TSBG Media
The biofilm formation of K. pneumoniae on silicone elastomer bonded catheter surface using TSB and TSBG is illustrated in Figure 1. In TSB and TSBG, all 47 isolates formed consistent biofilms. In TSBG, there was a considerable growth reduction in strains 14 and 28 compared to TSB. In the case of TSBG, strains 26, 35, 36 and 43-46 showed a substantial rise in growth trend, with a higher proportion of TSBG than TSB with an increase of more than 20%. Similarly, strains 1, 6, 8, 18, 21, 22, 24, 27, 29-34, 38-42 and 47 showed a considerable rise in TSBG compared with an increase of 20%. Strains 2-5, 7, 9-7, 19, 20 and 23, 25 grew at a slower rate in TSBG, with an increase of 10% compared to TSB. No significant changes were observed against strain 37 among TSB and TSBG. In TSBG, strain 38 had the highest biofilm development while strain 4 had the lowest.
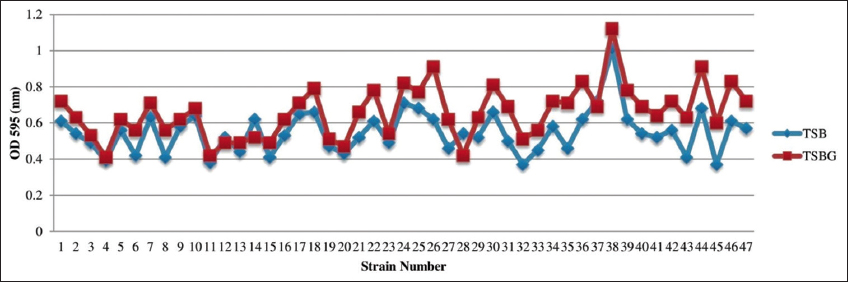 | Figure 1: Biofilm formation of K. pneumoniae on silicone elastomer bonded catheter surface using TSB and TSBG. [Click here to view] |
3.4. Testing of Biofilm Production on PVC Catheter Surface by K. pneumoniae using TSB and TSBG Media
The biofilm development of K. pneumoniae on the PVC catheter surface using TSB and TSBG is depicted in the Figure 2. In the case of TSBG, strains 2, 4, 15, 18, 21-24, 27-29, 33, 37, 39 and 45-47 demonstrated a marginal increase in growth trend, with a proportion of >60% than TSB. Similarly, strains 1, 3, 7, 10-12, 16, 17, 20, 24-26, 30 and 40-43 exhibited a significant increase in TSBG when compared to TSB, increasing by >40%. Strains 5, 13, 19, 22, 31, 32, 35 and 44 expanded at a slower rate in TSBG, with an increase of >20% compared to TSB, while strains 6, 34, 36 and 38 grew at the slowest rate, by <20%. In TSBG, strain 46 developed the highest bacterial growth, whereas strain 6 developed the least.
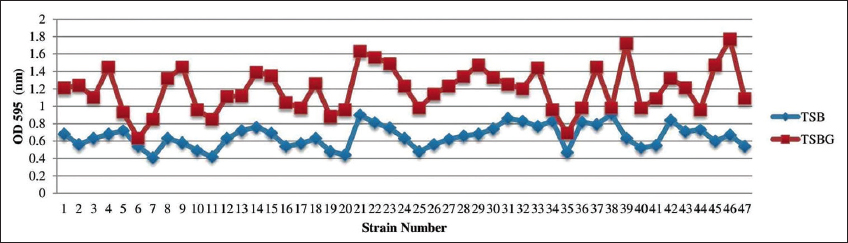 | Figure 2: Biofilm formation of K. pneumoniae on PVC catheter surface using TSB and TSBG. [Click here to view] |
The comparison of K. pneumoniae growth over two distinct catheter materials (silicone elastomer bonded and PVC) tested using TSB and TSBG is depicted in Figure 3. When compared to silicone elastomer bonded catheter, PVC-based catheter demonstrated higher biofilm development in both TSB and TSBG. In addition, the higher growth was observed in TSBG rather than TSB on both the surfaces tested. The evaluated strains exhibited 11% more adhesion on a PVC surface than on a silicone elastomer bonded catheter surface in TSB. Similarly, in TSBG, the tested strains expressed 54% more on a PVC surface than on a silicone elastomer bonded catheter surface. The development of biofilm was found highest in TSBG over PVC-based catheter and lowest in TSB over silicone elastomer bonded catheter.
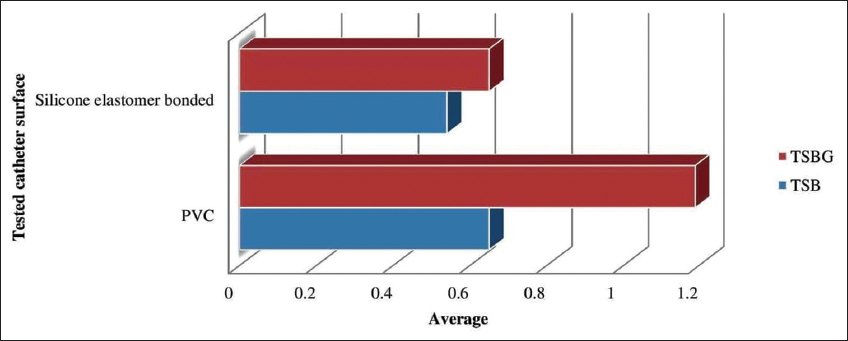 | Figure 3: Comparison of biofilm formation by K. pneumoniae on two distinct catheter surfaces. [Click here to view] |
3.5. Assessment of K. pneumoniae Antibiotic Resistance Profile after Biofilm Formation on Silicone Elastomer Bonded Catheter Surface
The antibiotic resistance profile of K. pneumoniae after biofilm development on silicone elastomer bonded catheter surface is depicted in the Figure 4. When compared to the antibiotic resistance profile of K. pneumoniae before biofilm formation, the resistance of tested isolates increased marginally. The tested strains had 15% ETP resistance (which is increased by 2%), 19% MRP resistance (which increased by 4%), 31% GEN resistance (which increased by 4%) and 46% IMP resistance (which increased by 6%), 53% COT resistance (which is increased by 2%), 45% AT resistance (which increased by 1%), 62% CTX resistance (which increased by 2%), 67% CPM resistance (which increased by 3%), 79% CXM resistance (which increased by 2%), 81% CT resistance (which increased by 2%) and 94% AMP resistance (which increased by 2%) after biofilm formation.
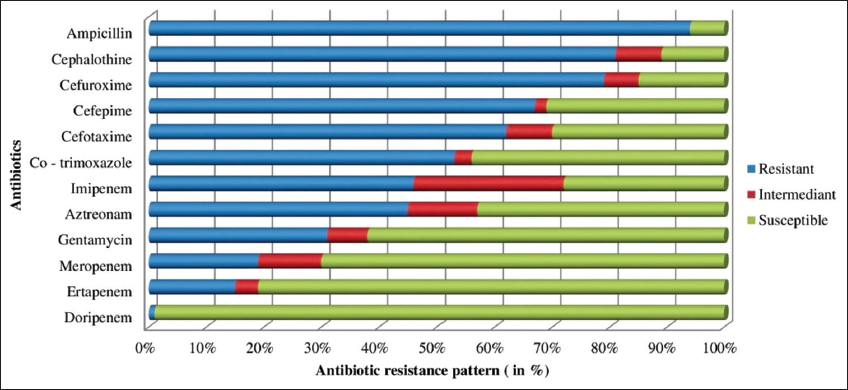 | Figure 4: Antibiotic resistant profile of K. pneumoniae after biofilm formation on silicone elastomer bonded surface. [Click here to view] |
3.6. Assessment of K. pneumoniae Antibiotic Resistance Profile after Biofilm Formation on PVC Catheter Surface
The antibiotic resistance profile of K. pneumoniae after biofilm growth on PVC catheter surface is demonstrated and given in Figure 5. The resistance of tested isolates rose dramatically when compared to the antibiotic resistance profile of K. pneumoniae before biofilm formation. The tested strains had 3% DOR resistance (raised to 3% after biofilm formation), 17% ETP resistance (which increased by 4%), 23% MRP resistance (raised to by 8%), 35% GEN resistance (which increased by 8%) and 58% COT resistance (which increased by 7%), 55% IMP resistance (which increased by 15%), 47 % AT resistance (raised to by 3%), 66% CTX resistance (raised to 6%), 69% CPM resistance (which increased by 5%), 82% CXM resistance (which increased by 5%), 86% CT resistance (which increased by 7%) and 97% AMP resistance (raised to by 5 %) after biofilm formation.
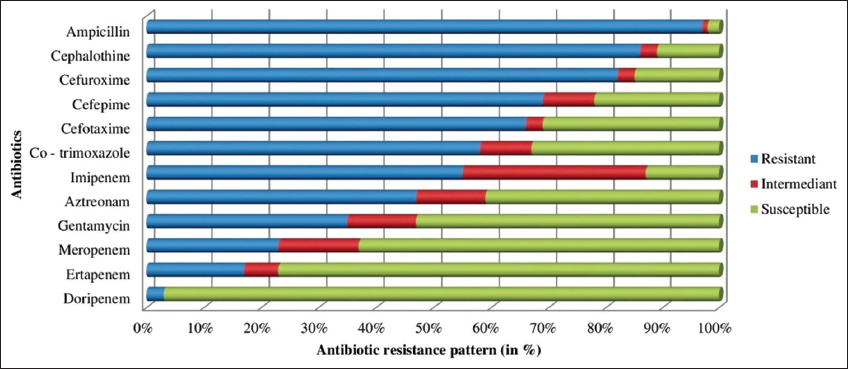 | Figure 5: Antibiotic resistant profile of K. pneumoniae after biofilm formation on PVC. [Click here to view] |
4. DISCUSSION
In the current investigation, around 92% of the tested isolates developed resistance to ampicillin, while 100% of the isolates were responsive to doripenem and 74% of K. pneumoniae isolates expressed as MDR strains which are in agreement with earlier studies of Cepas et al. revealed that 38% of the samples tested positive for MDR, whereas Manjula et al. found that 90.2% of MDR strains tested positive [14,15]. Resistance to penicillin, cephalosporin, fluoroquinolone, amino glycoside and sulfonamide developed rapidly in MDR isolates. Moini et al. demonstrated that 46.6% of MDR bacteria were extremely resistant to ampicillin, third-generation cephalosporins and amino glycosides [16]. Among all the strategies for dealing with the problem of antibiotic resistance, surveillance plays a crucial role. Since its discovery in the mid-1970s, ESBL has been identified as a major contributor to drug-resistant genera in the Enterobacteriaceae family and becoming a worldwide concern in hospitalized patients [13]. Many approaches were tried for the most accurate detection of ESBL. With the use of AMC and TZP as an ESBL inhibitor, MDDST was shown to be a specific approach for identifying ESBL in Klebsiella Spp. The MDDST was capable of detecting ESBL in 97.8% (n = 46/47) ESBL-producing strains which were positively correlated 100% with the study of Modi et al. [17].
The most ESBL-producing strains have been reported using MDDST, followed by IMTDT and DDST. Using MDDST, we were able to determine ESBL in four isolates of K. pneumoniae that had previously tested negative with DDST. The sensitivity of MDDST to K. pneumoniae observed in our investigation is comparable to the report of Modi et al. [17]. MDDST, which detected ESBLs in 97.8% isolates and IMTDT, which disk overed ESBLs in 87.2%, were more sensitive than DDST, which detected ESBLs in only 82.9% of isolates. In this experiment, MDDST and IMTDT were shown to be more sensitive than DDST in this investigation, which is aligned with the study of Khan et al. and Modi et al. used similar techniques to classify ESBL-producing K. pneumoniae isolated from a hospital [12,17]. They claim that the frequency of ESBL-producing clinical strains is so high that it leads to greater fatality rates [17].
Biofilms are grouping of microbial cells adhering to a living or inert surface by a self-produced exo-polymeric matrix containing polysaccharides, proteins and extracellular DNA (eDNA). Biofilms obstruct antibiotic penetration, slow bacterial growth, enhance the formation of persister cells and permit for genetic exchange [18,19]. As a consequence, an explicit understanding of biofilm production may facilitate the development of biofilm combating approaches. Despite the fact that K. pneumoniae biofilm development has been intensively explored, the severity of biofilm formation on alternative catheter materials has been understudied. Moreover, the mechanism of supreme biofilm growth is observed in the catheter employed for the diabetic patients due to the glycosuria condition where their urine contains glucose substrates remains unclear. The present study established the quantification of biofilm development over two distinct catheter materials and the impact of glucose supplementation in the biofilm formation of K. pneumoniae over the catheter surfaces tested. The growth of K. pneumoniae biofilms on the surface of a silicone elastomer bonded catheter was assessed. According to this finding, roughly 12.7% had highly substantial biofilms, which increased to 20% with the addition of glucose to the media (TSBG) used; around 44.6% of the tested strains showed medium biofilm development, with a more than 20% increase and 42.5% of the tested strains revealed the weakest biofilm formation, with a <10% increase. The data of this experiment is in complete agreement with the earlier report of Lee et al.; Bharathy et al. and Monisha et al. [6,20,21].
The formation of K. pneumoniae biofilms on the surface of a PVC based catheter was examined with the glucose supplement to the media (TSBG), approximately 36% of the strains formed extremely high biofilms, increasing to 60%; roughly 36% of the tested strains showed the greatest biofilm growth, with a 40% rise. Around 17% of the isolates showed a 20% increase in biofilm growth when glucose was added to the media and 8.5% of the strains showed the weakest biofilm formation, with <20% increase. As a result, silicone elastomer bonded catheters should be favored since they had a lower impact on biofilm growth and were reported to preserve flexibility and physical qualities, as well as having less sepsis, a longer life and fewer insertions in patients. Furthermore, silicone has been shown to reduce urinary mucosa damage and allergies. Hence, silicone is recommended for long-term use in catheter-dependent patients [22].
Numerous research conducted over the past 20 years has produced contrasting findings. For instance, Abidi et al. analyzed 22 Pseudomonas aeruginosa isolates and found that MDR isolates produced considerably more biofilm, Eyoh et al. have unable to detect a significant difference between MDR and non-MDR Staphylococcus aureus biofilm development which is positively in agreement with the present findings [23,24]. Insufficient studies have examined the quantitative relationship between antibiotic resistance and biofilm forming capacity as well as the increase in resistance following biofilm formation.
5. CONCLUSION
K. pneumoniae, which are MDR and produces ESBL, is steadily growing in India. The study’s susceptibility data revealed that the tested K. pneumoniae strains exhibited a significant level of resistance to distinct antibiotic classes tested. To assess the true burden of antibiotic resistance and provide effective sickness treatment in the clinical setting, comprehensive monitoring of their susceptibility patterns is essential. Furthermore, from this study, silicone-based catheters are suggested for long-term use due to their features, which include lower impact of biofilm formation in the presence of sugar substrates, flexibility, reduced CAUTIs and fewer incisions in patients. Consequently, biofilm functions as a strategy for bacterial survival. The observations raise concerns about the mechanisms by which bacteria balance their association with biofilms and their resistance to antibiotics. By simplifying these mechanisms could offer novel perspectives that might progress the creation of treatments and preventative measures for biofilm-related illnesses.
6. ACKNOWLEDGMENTS
The authors wish to express their profound gratitude to the Department of Biomedical Science, Bharathidasan University, Tiruchirappalli, for providing the necessary facilities to carry out the experiment.
7. AUTHORS’ CONTRIBUTIONS
The work was designed by Dr. KS, Dr. KP supervised and designed the part of work. LSB performed the experiments, collected the data and prepared the manuscript. BA helped in generating data and preparing manuscript. Dr. KS corrected the manuscript and granted his approval.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors declare no conflict of interest.
10. ETHICAL APPROVAL
This article does not contain any human participants or animals performed by any of the authors.
11. DATA AVAILABILITY
This manuscript contains all of the data collected during the study.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Janda M. The genus Klebsiella:An ever-expanding panorama of infections, disease-associated syndromes, and problems for clinical microbiologists. Clin Microbiol Case Rep 2015;1:1-7.
2. Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014;3:743-58. [CrossRef]
3. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae:A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 2017;41:252-75. [CrossRef]
4. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Disk Susceptibility Testing. 7th ed. Wayne PA, USA:Clinical and Laboratory Standard Institute;2000.
5. Desai S, Sanghrajka K, Gajjar D. High adhesion and increased cell death contribute to strong biofilm formation in Klebsiella pneumoniae. Pathogens 2019;8:277. [CrossRef]
6. Lee JS, Bae YM, Lee SY, Lee SY. Biofilm formation of Staphylococcus aureus on various surfaces and their resistance to chlorine sanitizer. J Food Sci 2015;80:M2279-86. [CrossRef]
7. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms:From the natural environment to infectious diseases. Nat Rev Microbiol 2004;2:95-108. [CrossRef]
8. Hoyle BD, Costerton JW. Bacterial resistance to antibiotics:The role of biofilms. Prog Drug Res 1991;37:91-105. [CrossRef]
9. Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011;63:1055-60. [CrossRef]
10. Hansen DS, Aucken HM, Abiola T, Podschun R. Recommended test panel for differentiation of Klebsiella species on the basis of a trilateral interlaboratory evaluation of 18 biochemical tests. J Clin Microbiol 2004;42:3665-9. [CrossRef]
11. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Disk Susceptibility Testing. 28th ed. Wayne PA, USA:Clinical and Laboratory Standard Institute;2018.
12. Khan MK, Thukral SS, Gaind R. Evaluation of a modified double-disc synergy test for detection of extended spectrum beta-lactamases in AMPC beta-lactamase-producing Proteus mirabilis. Indian J Med Microbiol 2008;26:58-61. [CrossRef]
13. Menon T, Bindu D, Kumar CP, Nalini S, Thirunarayan MA. Comparison of double disc and three dimensional methods to screen for ESBL producers in a tertiary care hospital. Indian J Med Microbiol 2006;24:117-20. [CrossRef]
14. Cepas V, Lopez Y, Munoz E, Rolo D, Ardanuy C, Marti S, et al. Relationship between biofilm formation and antimicrobial resistance in gram-negative bacteria. Microb Drug Resist 2019;25:72-9. [CrossRef]
15. Manjula NG, Math GC, Patil SA, Gaddad SM, Shivannavar CT. Incidence of urinary tract infections and its aetiological agents among pregnant women in Karnataka region. Adv Microbiol 2013;3:473-8. [CrossRef]
16. Moini AS, Soltani B, Ardakani AT, Moravveji A, Erami M, Rezaei MH, et al. Multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolated from patients in Kashan, Iran. Jundishapur J Microbiol 2015;8:e27517. [CrossRef]
17. Modi D, Patel D, Patel S, Jain M, Bhatt S, Vegad MM. Comparison of various methods for the detection of extended spectrum beta lactamase in Klebsiella pneumoniae isolated from neonatal intensive care unit, Ahmedabad. Natl J Med Res 2012;2:348-53.
18. Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health 2020;17:6278. [CrossRef]
19. Mahto KU, Das S. Bacterial biofilm and extracellular polymeric substances in the moving bed biofilm reactor for wastewater treatment:A review. Bioresour Technol 2022;345:126476. [CrossRef]
20. Bharathy LS, Monisha BA, Sathiyamurthy K. Antibiotic resistant pattern of Klebsiella pneumoniae and their biofilm development on diverse surfaces. J Pure Appl Microbiol 2022;16:1990-7. [CrossRef]
21. Monisha BA, Bharathy LS, Sathiyamurthy K, Premkumar K. Antibiotic resistance and biofilm development of Escherichia coli on different surfaces. J Pure Appl Microbiol 2022;16:1884-92. [CrossRef]
22. Huang WY, Wei LP, Ji YG, Xu DX, Mo JK. Effect of silicon and latex urinary catheters:A comparative study. Di Yi Jun Yi Da Xue Xue Bao 2005;25:1026-8.
23. Abidi SH, Sherwani SK, Siddiqui TR, Bashir A, Kazmi SU. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol 2013;13:57. [CrossRef]
24. Eyoh AB, Toukam M, Atashili J, Fokunang C, Gonsu H, Lyonga EE, et al. Relationship between multiple drug resistance and biofilm formation in Staphylococcus aureus isolated from medical and non-medical personnel in Yaounde, Cameroon. Pan Afr Med J 2014;17:186. [CrossRef]