1. INTRODUCTION
Wheat (Triticum aestivum L.), a member of the Poaceae family, is an allohexaploid species with 21 chromosome pairs arranged into three sub-genomes; A, B, and D. It has a BBAADD genome composition and a chromosome number of 2n = 6x = 42 [1]. It was developed through natural hybridization between Emmer wheat (AABB) (Triticum dicoccon), also known as "farro," and Goat grass (DD) (Aegilops tauschii), and commonly referred to as hard grass [2]. Wheat is a self-pollinating plant with spike-type inflorescences that contains three anthers connected to the base by slender filaments, enclosed within bract-like structures called the lemma and palea that enclose its fruit called caryopsis [3].
1.1. Productivity of Wheat in India and Nigeria
The data (2021–2022) from the Farmers Welfare unit, Ministry of Agriculture and, of Indian Government and Federal Ministry of Agriculture and Rural Development of Nigeria, reveals a significant difference in wheat productivity per hectare between India's and Nigeria's top wheat-producing states. In India, states like Punjab and Haryana achieve much higher productivity, with yields ranging from 4.8–5.2 to 4.5–4.9 tons/ha, respectively. Other Indian states, like Western Uttar Pradesh and Gujarat, also produce more than 3 tons/ha. In contrast, Nigeria’s leading wheat-producing states, such as Borno and Kano, have considerably lower productivity, with yields ranging from 1.5 to 2.0 tons/ha. The lowest yields in India, like in Jharkhand (1.5–1.8 tons/ha), are comparable to some of the highest yields in Nigeria [4]. Thus, India's wheat productivity per hectare is significantly higher than Nigeria's across most states, indicating more advanced agricultural practices for wheat cultivation in India (Table 1).
1.2. Concept of Genetic Variance
The line into tester is a powerful mating design to find the best combiner to be used for further crop improvement. Thus, the study of gene action that controls the expression of different characters could aid selection of good parents. When a genotype exhibits high mean yield and little variability over a range of environmental conditions, it is said to be a stable genotype [5]. Choice of consistent genotypes that are suited for wider environmental conditions required sufficient knowledge of components of genetic variance [6–8], reported that knowledge of the correlation between yields and yield influencers aids the selection of desirable/superior parents and best cross combinations for the commercial exploitation of high yielding parent in a conventional breeding programme. Therefore, understanding the relationship between the two is essential for yield improvement in wheat. [9] stated that the degree of genetic determination which is considered as the ratio of genetic variance over phenotypic variance, can be used to estimate potentially high-yielding populations in relation to yield influencing traits and is useful for any successful breeding programme. Thus, the breeder must understand some behaviors on how genes are expressed in the inheritance of all traits under studies. Thus, crop yield is the sum of all individual yield components operating together with small and cumulative effects on the final yield. Yield is polygenically controlled and breeding for high-yielding genotypes requires knowledge of the mode of gene expression [8]. The study of heterosis has become essential, not optional, due to the challenges involved in developing commercial hybrid seeds in wheat. Exploiting heterosis is economically effective in the wheat breeding programme [8,10].
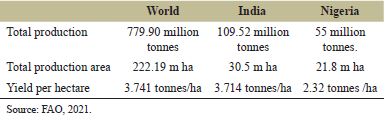 | Table 1. Area, production, and productivity of wheat. [Click here to view] |
Sprague and Tatum [11] this concept described general and specific combining abilities. General combining ability (GCA) reflects the mean yield/performance of a line across different cross combinations, while specific combining ability (SCA) represents the deviation from GCA, showing whether the performance exceeds or falls short of expectations. It was noted that genes with additive effects play a more significant role in GCA, whereas SCA is influenced by dominance genes and epistatic effects (inter-allelic interactions). Combining ability serves as an effective tool for selecting desirable parents capable of producing crosses with high genetic value. The level of hybrid vigor depends on the combining ability of the genotypes used in the hybridization. Genotypes capable of transmitting favorable traits or hybrid vigor to their offspring are considered to have high combining ability (good combiners). Combining ability helps in identifying parental lines that contribute favorable traits to their offspring. This allows researchers to choose the best parental genotype of wheat, leading to the development of high-yielding and stress-tolerant varieties. For developing countries, where resources for extensive breeding programs may be limited, this approach can be used to maximize the chances of success with fewer inputs [12].
1.3. Constraint and Study's Outcome
Despite the fast and steady increase in the population of India and Nigeria (being ranked as 55th among wheat-producing countries), wheat production currently encounters many constraints that affect its yield and quality like salinity, heat stress, and insufficient breeding information such as genetic variances, GCA, SCA, adaptability, and exploitation of heterosis. Previous studies used very limited number of genotypes for their experiments which can lead to inconsistency of research findings due experimental errors. The present studies used 67 wheat genotypes that are enough to increase precision and reduces experimental errors. Most of existing studies were conducted either on India or Nigeria but not both, leaving a serious gap between these two diverse agro ecological zones. Thus by including Nigerian locations, the present study adds valuable data on how wheat genotypes perform in African climates, contributing to relatively under-researched region with sufficient breeding information such as genetic variances, GCA, SCA, adaptability, and exploitation of heterosis that will be used for yield optimization and provides breeders greater opportunity for further wheat improvement. The present investigation also provides valuable insights into inheritance patterns of yield-related traits that underpin the development of advanced breeding techniques, including hybrid breeding and selection of superior parent that aimed at improvement of wheat production to ensure resilience against different environmental stresses that ultimately contributes to sustainable agricultural practices that aimed at wheat improvement to mitigate food insecurity for the growing population.
To boost wheat yield, plant breeders have explored commercial hybrid seed production through genetic/cytoplasmic male sterility, and the use of some chemicals that induce sterility in wheat. However, due to the polyploidy of wheat and the technical difficulties involved in producing hybrid seeds on a commercial scale, these efforts have little to no practical significance. Therefore, an amicable remedies to this scenarios is the exploitation of heterosis and identification of superior parent, high yield potential crosses and effective selection is quite necessary for wheat improvement [13]. Heterosis breeding is one of key option for wheat improvement due to challenges associated with the introduction of large scale hybrid seeds in Wheat production, hybrid breeding is rewarded [8]. Reif et al. [14], reported that, heterosis breeding is an eco-friendly and non-transgenic method of breeding programme.
1.4. Some Limitations of the Study
The limitations of the current study are as follows; it did not incorporate molecular markers or genomic tools to explore the genetic basis of the observed traits. To address this challenge, future research could integrate marker-assisted/accelerated selection (MAS) or genome/genomic selection to identify or track beneficial alleles associated with high yield and stress tolerance. This strategy would streamline the breeding process and increase the precision in selecting desirable traits.
2. MATERIAL AND METHODS
2.1. Material and Experimental Site
Experimental materials consisted of 67 wheat genotypes, including 15 lines, three testers, four checks, and 45 F1s crosses/hybrids were generated through the hybridization of fifteen female lines with three male testers to conduct stability analyses for yield and yield components. The planting material comprised 15 lines. BHU 25, WB-02, BHU 31, HD 3721, PBW 725, CRD GEHNU1, PBW 550, PBW 677, PBW 822, HD 3117, DBW 173, HD 3086, DBW 222, CSW 18, and PBW 757. The three testers were PBW ZN1, PBW 343, and HD 3326, with four checks HD 2967, DBW 187, Norman, and Borlaug-100. Other materials included Breeder's kit. Soil plant analysis development (SPAD) handheld meter, meter rule, electric balance (Compax-Cx-600), seed counting machine, digestion apparatus, sodium hydroxide, hydrochloric acid, and more. Weather report 2023-2024 Rabi seasons across three locations on climate variability during evaluation period (Table 2)
2.2. Methods
In Phase I (2022-2023 Rabi season), an Augmented Design was employed to generate 45 F1 hybrids through the hybridization of 15 lines/females with three testers/males, through line × tester mating design as described by Kempthorne [15]. In Phase II, during the Rabi season of 2023–2024, Randomized complete block design with three replications was used. Standard agronomic practices were followed as recommended. The research took place at three locations; Lovely Professional University, India; Kebbi State University of Science and Technology, Aliero, Nigeria; and the Lake Chad Wheat Research Institute, Nigeria. Emasculation and pollination procedures were performed, and quantitative data were collected based on guidelines from the International Board for Plant Genetic Resources and the International Crops Research Institute for the Semi-Arid Tropics [16] for wheat descriptors. Qualitative data, such as chlorophyll/green pigment content, were measured using a SPAD meter, and protein/crude content was assessed using the micro Kjeldahl method. Data were analyzed using ANOVA [17], line × tester analysis, combining ability [15], and heterosis estimation [18].
2.2.1. Experimental sites
The environmental conditions at the three experimental sites were as follows; location one was the Teaching and Research Farm, Department of Genetics and Plant Breeding, Lovely Professional University, Phagwara, Punjab, India, located at a latitude of 31.2245° N and longitude of 75.7711° E, at an altitude of approximately 243 m above sea level, with an annual rainfall of 527.1 mm. Location two was the Teaching and Research Farm of Kebbi State University of Science and Technology Aliero, Kebbi State, Nigeria, situated in the Sudan Savanna agro-ecological zone at latitude 13°08' N and longitude 50°15' E, with an altitude of around 250 m above sea level and annual rainfall ranging from 1,500 to 1,700 mm. The third location was the Lake Chad Wheat Research Institute in Borno State, Nigeria, located at latitude 11.8467° N and longitude 13.1571° E, at an altitude of approximately 325 m above sea level, with annual rainfall between 900 and 1,500 mm. In Punjab, India, temperatures ranged from 19°C (January) to 36°C (April), with lows between 8°C (January) and 21°C (April). In Kebbi State, Nigeria, temperatures varied from 17.1°C (January) to 27°C (April), and in Borno State, Nigeria, temperatures ranged from 14°C (January) to 24°C (April). Regarding soil types, Location I (India) has fertile, alluvial soils with low organic matter content. Location II (Kebbi State) features generally sandy or loamy soils with lower organic matter content, while Location III (Borno State) has sandy or loamy soils (entisols or aridisols) that are nutrient-depleted. Punjab experiences cooler winters during the two Rabi seasons, while Kebbi and Borno States have drier climates, with Borno being the driest site out of the three locations.
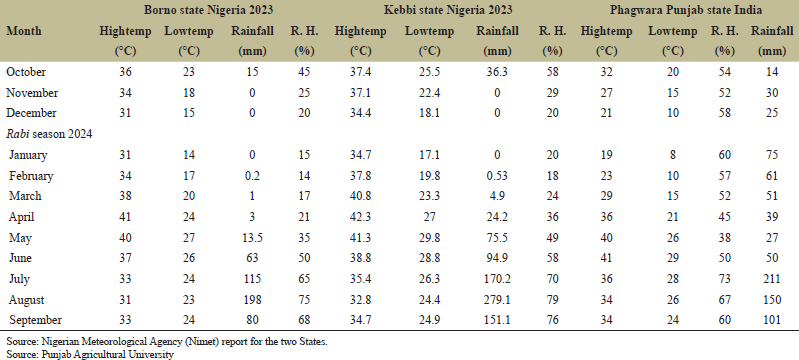 | Table 2. Weather report 2023–2024 Rabi seasons across three locations on climate variability during evaluation period. [Click here to view] |
2.2.2. Statistical analysis
Analysis of variance (ANOVA) was one of the statistical methods employed to analyze the data [17], line × tester analysis [15], and ANOVA for combining ability [15], and heterosis estimation [18].
2.2.2.1 Estimation of CGA and SCA variances and their effects.
The estimate of GCA and SCA variances and their effects were carried out by line × tester method using data sourced from F1 generation and its parents.
The method for analysis were described below:
Xijk = is the submission of µ + gi + gj + sij + eijk
Where:
µ = Mean
gi = for estimation of GCA effects on ith T, i = 1,2,…T.
gj = GCA effect estimation, jth= T, j = 1,2,…L.
sij = submission the effect of SCA with ith= T and jth = L crosses
eijk = total error in relation with ijkth data, where k = stand for 1,2,…replications
Individual estimated effects as follows
Where:
X... = stand as submission of cross combinations
Where:
Xi.. = submission of ith T across all L with their replicates.
Where:
X.j. = submission of jth L divided by T and their replicates.
- For individual estimation:
- gi = Also stand as
- gj = stand as submission of
- And sij = submission of
Where:
Xij. = Submission of ijth in all crosses like (L and T) divided by replicates.
Standard of error on GCA was found through:
Standard error for GCA of F = √(Me ÷ rm)
Standard error GCA in T = √(Me ÷ rf)
Standard error for SCA = √(Me ÷ rf)
Standard Error (gm – gf) line = √(2Me ÷ rm)
Standard Error (gm –gf) tester = √(2Me ÷ rf)
Standard Error (Sij - Skl) = √(2Me ÷ r)
Where:
Me = Error MS
gm = genotype of male
gf = genotype of male
Critical Differences Calculation:
Critical differences (C.D) = t at 5% × SED; 1% probability levels at error d.f.
 | Table 3. ANOVA for estimation of SCA and GCA variances and their effects is given below. [Click here to view] |
Where, L = female (f), T = male (m), r = replication, SV = source of variation, and E = error
x..k = total of LXT in kth rep.
Xij = total of ijth combination of crosses for all replicates,
xi = submission of jth T across lines with replicates,
x2.j. = lines submission divided by L and replicates,
xij… = total number of all LXT crosses over replicates,
σ2e = sum of error variation,
R = sum replicates,
M = tester,
F = lines,
Mm = Mean square as a result of tester .S. due to males,
Mf = Mean square as a result of lines,
Mmf = Mean square as a result of LXT,
Me = Mean square as a result of error' submission
2.2.2.2 Genetic components.
Covariance of half − sib (F) =
Covariance of half − sib (T) =
Covariance of half − sib (mean)
Covariance of full − sib (mean) =
Where:
Mf = Females mean squares
Mm = Males mean squares
Mfm = Females × Males mean squares
Me = Mean squares error
r = Replications number
f = Females number
m = Males number
σ2gca = Covariance of half-sib (mean) =
Therefore:
σ2A = 2 Covariance of half-sib (mean); if F = 1 and
σ2A = 4 Covariance of half-sib (mean); If F = 0
σ2sca =
Therefore:
σ2D (sca) = σ2 ; if F = 1, and
σ2D (sca) = 4 × σ2 ; if F = 0
Where:
F = Inbreeding coefficient
Mean degree of dominance
Calculated using formula given by Kempthorne and Curnow [19].
Average degree of dominance =
Where, σ2sca = Estimation of variance due to sca.
σ2gca = Estimation of variance due to gca
2.2.2.3 Heterosis estimation over better parent (heterobeltiosis) and standard variety.
Heterosis was calculated based on an increase or decrease in mean values of the crosses (F1’s) compared to better parent (BP) (Heterobeltiosis) and standard variety (SV) (Standard Heterosis) (%).
- The heterosis over better parent (Heterobeltiosis) =
- The heterosis over standard variety (Standard heterosis)=
Where:
F1 = Mean of F1
BP = Better - parent mean
SV = Standard variety mean
The test of significance was determined by using the following formulae:
't' (The heterobeltiosis) =
't' (Standard heterosis) =
Standard error of the hybrid vigor over better - parent and the standard variety =
Where:
Me = Variance of mean error
r = Replications numbers
Critical Differences (C.D) = t × SE; 5 or 1% p-value at error d.f.
3. RESULTS AND DISCUSSION
ANOVA recorded significant different (p < 0.001) among female, male and female × male reported that, no single genotype was significant for all traits across three locations; however, some genotypes that consistently exhibit high GCA values across different environments were PBW-757 and DBW-222. Some crosses that consistently exhibit high SCA like PBW-677 × PBW-343, PBW-757 × PBW ZN1, CRDGEHNU1 × PBW ZN1 and HD-3721 × HD-3326 (Tables 4 and 5). Since no single genotype was significant for all traits, selection of individual traits might overlook the importance of combined traits. Thus, breeder can develop selection indices that combine multiple prioritized traits, adjusting their weights based on the breeding objective(s) and environmental conditions. This method enables the simultaneous improvement of multiple traits and genotypes by focusing on critical traits that directly affect yield. The result was commemorated research conducted by Barot et al. [20] and Gami et al. [13], who noted that none of the genotypes showed significant differences across all traits studied. Estimates of genetic variance components revealed that certain characters, such as "GYP" (374.5) and "BY" (219.8) (Table 5), had high genotypic variance, suggesting strong gene action over these traits. As mentioned by Barot et al. [20] and Kumar et al. [21], genetic variance was highly related to additive gene effect. The relative contributions of additive and non-additive gene effects for yield and its components showed that the additively of gene for most of yield related traits accounted for relatively low proportions of the total genetic variance. In contrast, non-additive gene action made the largest contribution to the overall genetic variation. As a result, heterosis breeding is highly effective for improving yield. For instance, the additive contribution to protein content was 0.0%, while non-additive 8.8% in spikelets number per main spike, additive contribution was 0.74%, and the non-additive contribution was 1.78%; while for productive' tillers number additive contribution recorded 1.78%, while non-additive was 2.17%. The additive contribution for biological yield was 1.76%, while non-additive was 1.34%; for harvest index (HI), the additive contribution was 2.83%, and non-additive was 1.34%; and the relative contributions of grain yield per plant (GYP) were 1.43% for additive and 5.51% for non-additive (Table 5). Certain traits, such as "CLC" and "GYP," exhibited moderate additive variance (10.57 and 5.35, respectively), indicating that these traits can be selected for breeding programs since they are influenced by additive genetic effects (Table 5). These findings was in conformity with results of Hajer et al. [5] and Fellahi et al. [12], reported additivity gene effects can be leveraged for genotype improvement in wheat.
For GCA, WB-02 was identified as the best line for grain filling period (GFP 2.33, Rank 1). Gami et al. [13] also noted that some male parents showed significant differences in their GCA values for traits such as days to 50% heading and GFP. PBW ZN1 was the top tester for nearly all yield-related traits, including the number of productive tillers (0.02, Rank 1), GYP (0.53, Rank 1), and the number of spikelets per spike (0.06, Rank 1) (Table 6).
Four parents were identified as promising combiners for GCA in relation to yield-related characters in wheat, suggesting the parent could serve as potential promising candidates for further breeding programs, such as the development of synthetic varieties or for enhancing grain yield. These parents are listed hierarchically as follows: PBW ZN1, PBW-757, PBW-822, and DBW-173.
The best identified parents with high-GCA (like DH-3086 and PBW-757) could be cross with other genotypes with high-GCA to combine favorable traits using pedigree breeding (to develop synthetic variety) and pure line selection. Can also can be used for recurrent parent selection using backcross breeding programs to improve the performance (consistent performance) of other lines. High GCA parents can serve as ideal foundation parents for large-scale breeding programs (Table 6). It is suggested that additive gene actions predominated in the observed traits, implying that the environment had little to no impact on these characters. Gupta et al. [22], Kumar et al. [21], and Kumar et al. [8], also reported that environmental factors have minimal or no effect on additive gene action. No single genotype recorded high GCA and SCA values across different environments. However, some genotypes that consistently exhibit high GCA values across different environments were PBW-757 and DBW-222. Some crosses that consistently exhibit high SCA like PBW-677 × PBW-343, PBW-757 × PBW ZN1, CRDGEHNU1 × PBW ZN1, and HD-3721 × HD-3326.
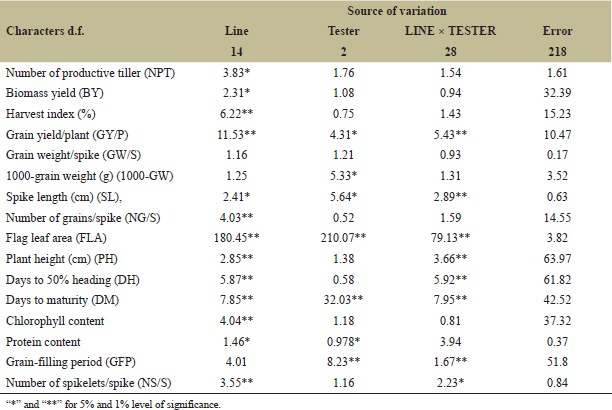 | Table 4. Analysis of variance for combining ability for 16 characters in L × T mating design in wheat. [Click here to view] |
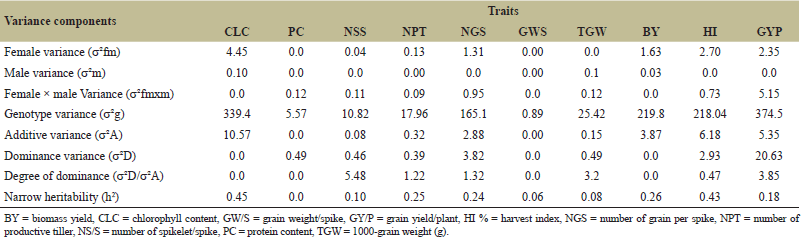 | Table 5. Estimates of components of genetic variance for various yield contributing traits in wheat. [Click here to view] |
Due to some mechanisms naturally possessed by wheat, some genotypes like BHU-25, PBW-550, BHU-31, HD-3721, and PWB-725 recorded high tolerance for multiple environmental stresses as evidenced showed by their relatively higher GCA estimates across most of their traits and environments.
For SCA, the positive values indicates better than expected, while negative values indicates lower than expected [11]. Highest SCA value (ranked 1st) recorded in DBW-222 × PBW ZN1 (0.49) for a number of productive tiller; PBW-822 × PBW ZN1 for GYP (3.79); DH-3086 × PBW-343 for HI (0.67). However, poor SCA recorded in CRD GEHNU 1 × HD-3326 (ranked 45th for spike length) (Table 7). According to Askander et al. [23] and Fellahi et al. [12] stated that, high SCA is used for hybrid vigor and superior performance in specific crosses while low-SCA crosses can be stably inherited and improved over time. Six crosses revealed significant positive SCA effects for yield and yield related characters, listed hierarchically as follows: DBW-222 × PBWZN1, DH-3086 × PBWZN1, PBW-677 × PBW-343, HD-3721 × HD-3326, and CRD GEHNU1 × PBWZN1.
Conclusively for GCA and SCA, the information on GCA effect could be used in the selection of superior parents; estimation of half-sib families; and for pure line selection method. While the information for SCA could be used useful in the selection of superior cross combinations for heterosis breeding; an estimation of full sib families and the information on both GCA and SCA can be applied for the reciprocal recurrent selection.
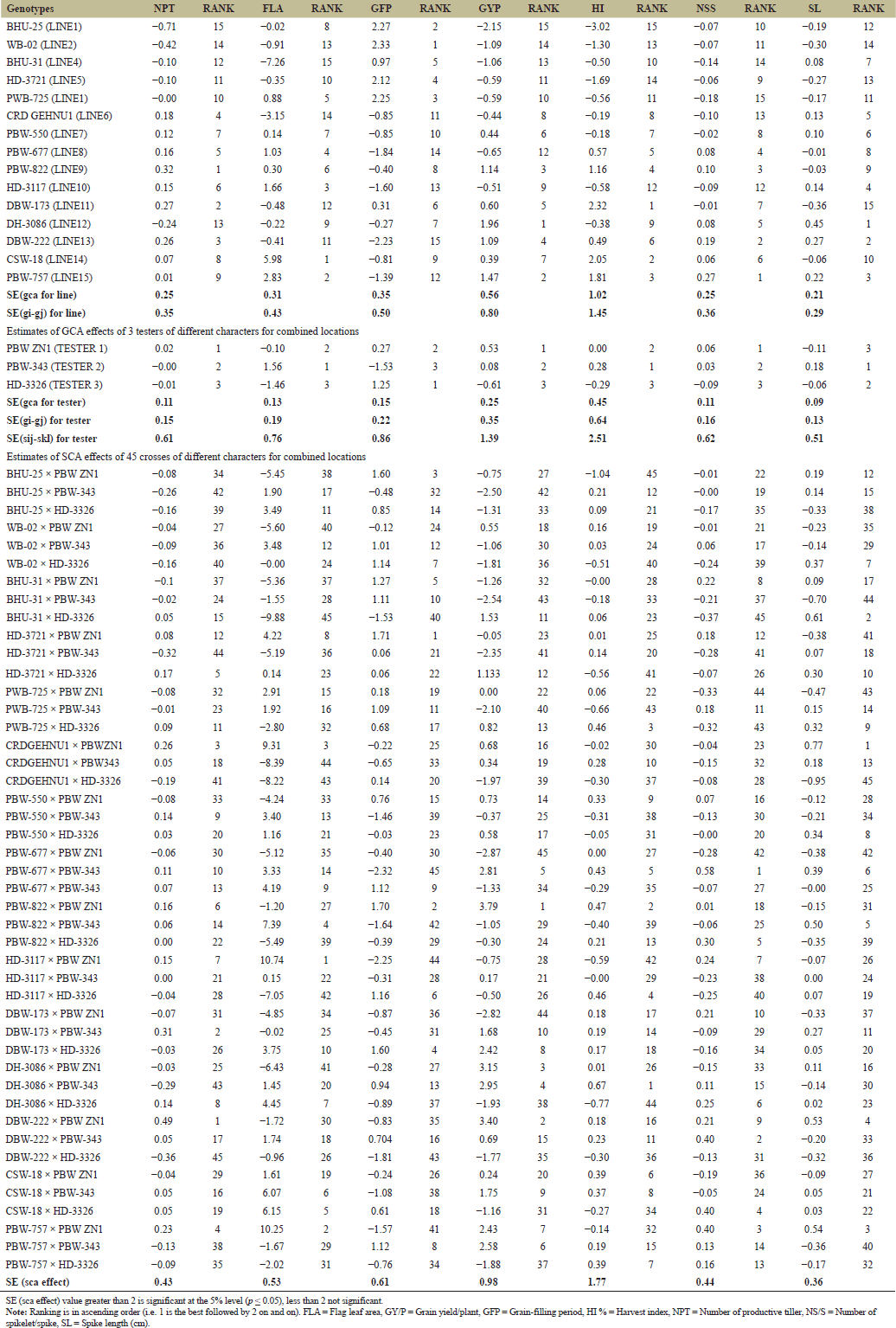 | Table 6. Estimates of GCA effects of 15 lines of different characters for combined locations. [Click here to view] |
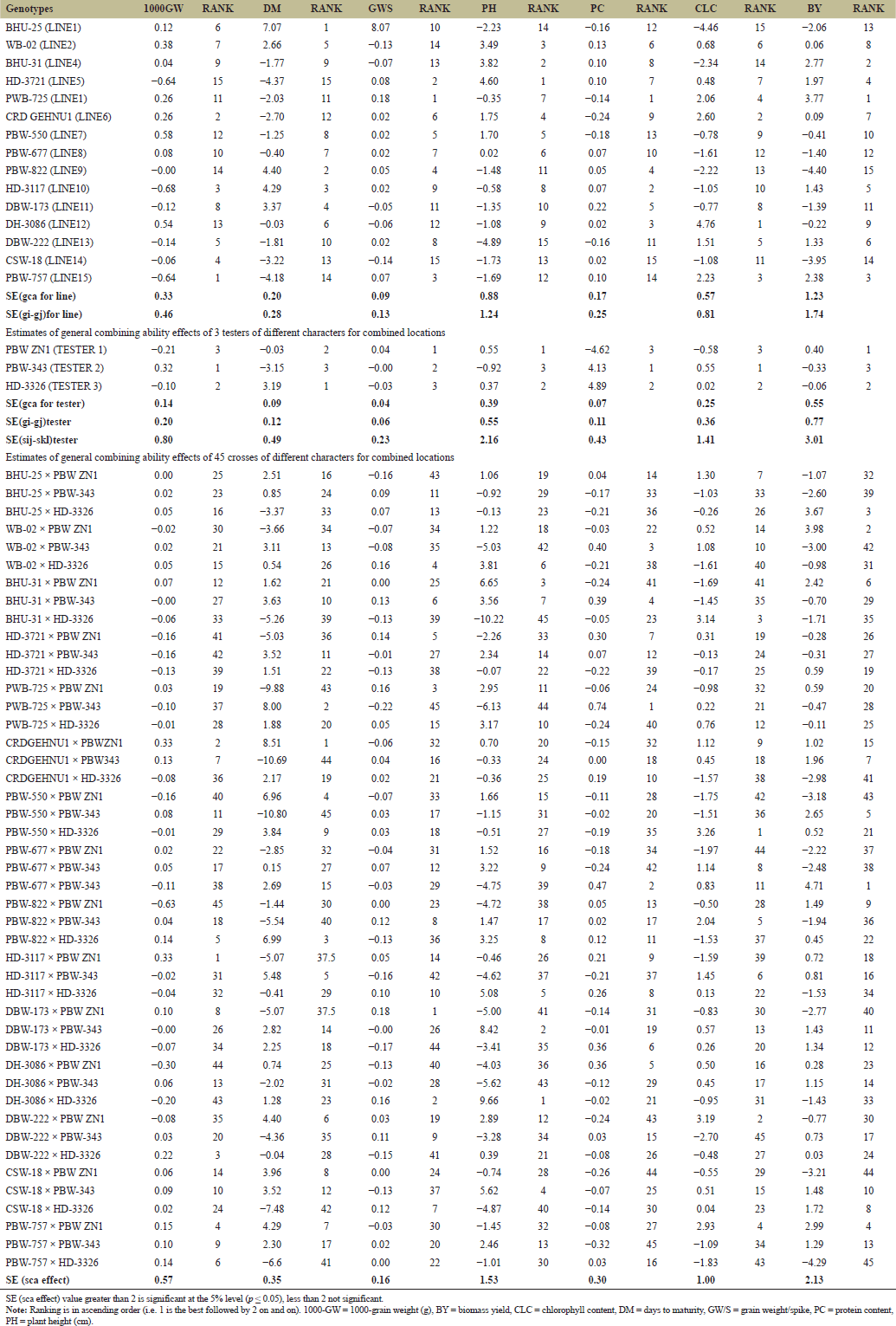 | Table 7. Estimates of general combining ability effects of 15 lines of different characters for combined locations. [Click here to view] |
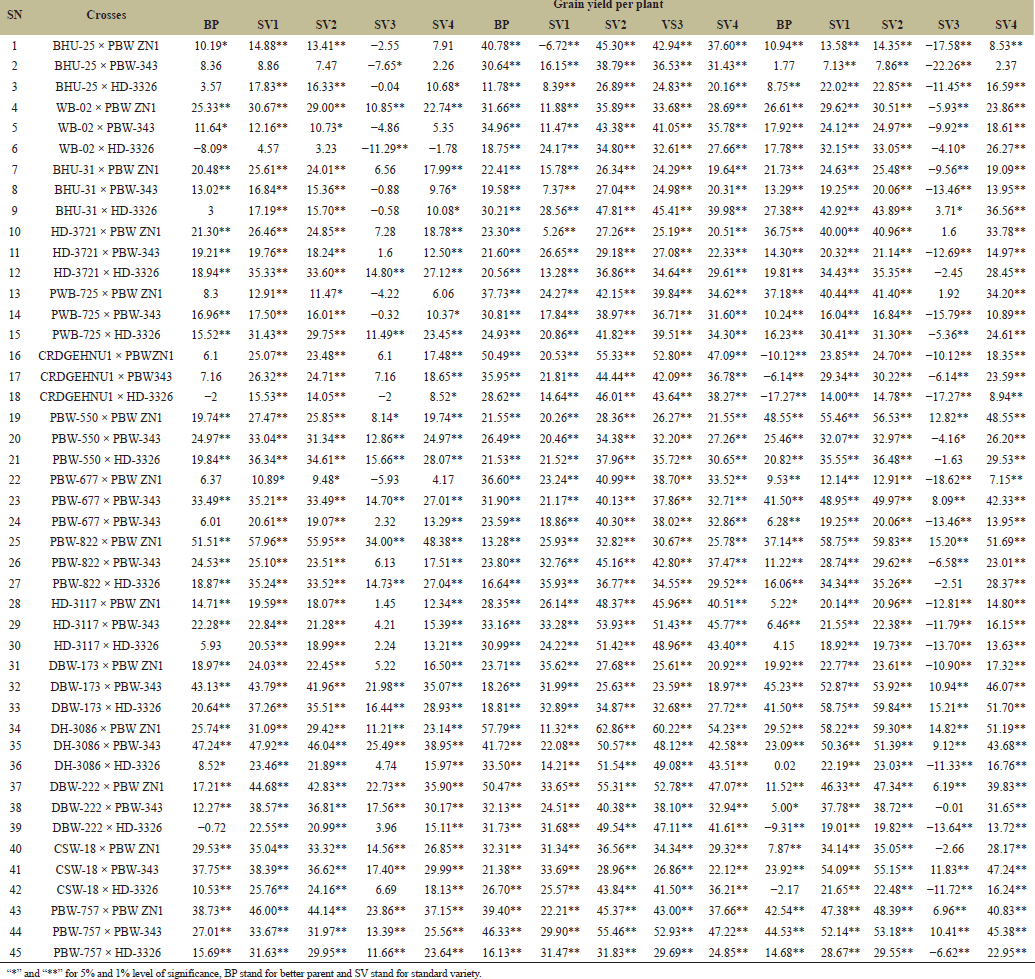 | Table 8. Estimation of heterosis over better parent and four standard varieties in wheat across three locations. [Click here to view] |
The magnitude of GCA varies across different traits due to additively effects while SCA also varies due to non-additive effects. Thus, GCA is typically more stable across environments because the additive genetic effects are less influenced by environmental variability. However, SCA being non-additive, is more susceptible to environmental changes, making it more variable across the traits that are sensitive to environmental conditions [9].
Heterosis is highly preponderance in cross-pollinated crops whereas, very limited in self-pollinated species like wheat Thus, progeny showing additive effects indicated that, the plants cannot take advantage of heterosis, however, dominance and over dominance gene action is essential for heterosis [23]. Therefore, standard heterosis (SV1–SV4) for grain yield per plant recorded across four varieties and three locations revealed the highest and positive BP heterosis in PBW-822 × PBW ZN1 with 51.51 while lowest and negative BP heterosis WB-02 × HD-3326 with −8.09. Highest standard heterosis was recorded in DH-3086 × PBW ZN1 with 62.86 in SV2 while the lowest standard heterosis recorded in BHU-25 × PBW-343 with −7.65 in SV3 (Table 8) this indicates the presence of both additive and dominance genetic variances. Askander’s et al. [23] study showed that, the tested attributes had both positive and negative heterosis for all the traits, therefore, dominant genetic variance is more preponderance than additive genetic variance. PBW-677 × PBW-343 shows the highest BP heterosis (26.12%) indicating that this cross exceeds its BP in terms of HI. While PBW-822 × PBW ZN1 Shows very high values across standard heterosis (61.99%) in SV1 and (30.56%) in SV3. This cross is likely very adaptable across multiple environments or standards (Table 9). Summarily, the results can be used to improve yield; parent selection, broad adaptability and identification of best crosses. Heterosis for inferior and superior BP and standard varieties were identified. For number of productive tillers PBW-822 × PBW ZN1 and PBW-757 × PBW ZN1 outweighed the performers of SV1 (118.53), SV2 (78.08 ), SV3 (57.75 ), and SV4 (73.58) while WB-02 × HD-3326 and BHU-25 × HD-3326 show the least heterosis for both in BP and standard heterosis (SV2 −8.34, SV3 −18.8) (Table 10). It is therefore proved that, heterosis in cross-pollinated crops is highly preponderance, whereas, very limited in self-pollinated species like wheat [18]. Days to 50% Heading for various wheat crosses across three locations (Tables 11 and 12
). “Days to 50% heading” measures the time from the emergence of heads to 50% of the plants produces heads, it is also an indicator of length of days to reach maturity level. Different wheat crosses, represented by combinations of plant varieties, were tested under various environments in three distinct locations. The dataset includes different types of crosses (BHU-25 × PBW ZN1, PBW-757 × PBW ZN1) tested against standard checks, labeled BP, SV1, SV2, SV3, and SV4, with maturity times varied significantly across locations. For example, the cross PBW-757 × PBW ZN1 took an additional 35.85–52.38 days to reach 50% heading at location 2 compared to other crosses. At location 1, very few crosses showed delayed heading than in location 3, suggesting that location 3 may have had conditions that either delayed or varied maturation times more than the other locations, as reported by Gami et al. [13] that maturity period had been influenced by environmental factors. While some crosses showed large variation for grain’ number per spike across location (Table 13). For example, the cross “BHU-25 × PBW ZN1” shows a relatively low grains’ number per spike in location 1 (ranging from 9.4 to 27.05 across the lines) compared to location 2, where the number of grains per spike ranges much higher (from 31.78 to 68.96). This suggests that certain crosses may be better adapted to specific locations like SV4 in cross “BHU-31 × PBW ZN1” at location 1 has a high grain count per spike of 38.05, indicating that SV4 contributes positively to grain number in this cross. This is also seen across other crosses where SV4 consistently produces higher counts compared to BP and other lines. The results was supported by Askander et al. [23] reported that some wheat genotypes reproduced their yield faithfully across different environmental conditions. The hybrids that recorded significant positive hybrid vigor yield components recorded the top six (6) hybrids namely; PBW-822 × PBW ZN1, DH-3086 × PBW-343, PBW-757 × PBW ZN1, DBW-173 × PBW-343, WB-02 × PBW ZN1, and DH-3086 × HD-3326.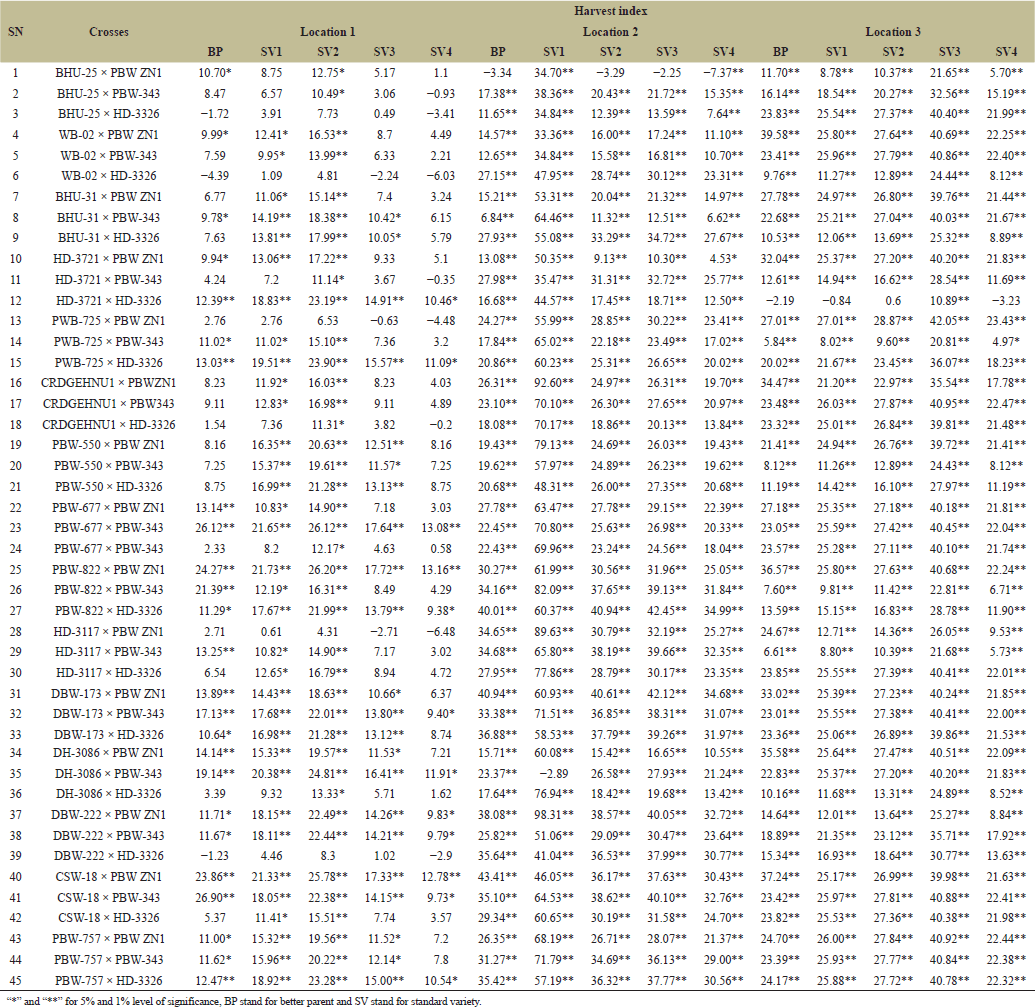 | Table 9. Estimation of heterosis over better parent and four standard varieties in wheat across three locations. [Click here to view] |
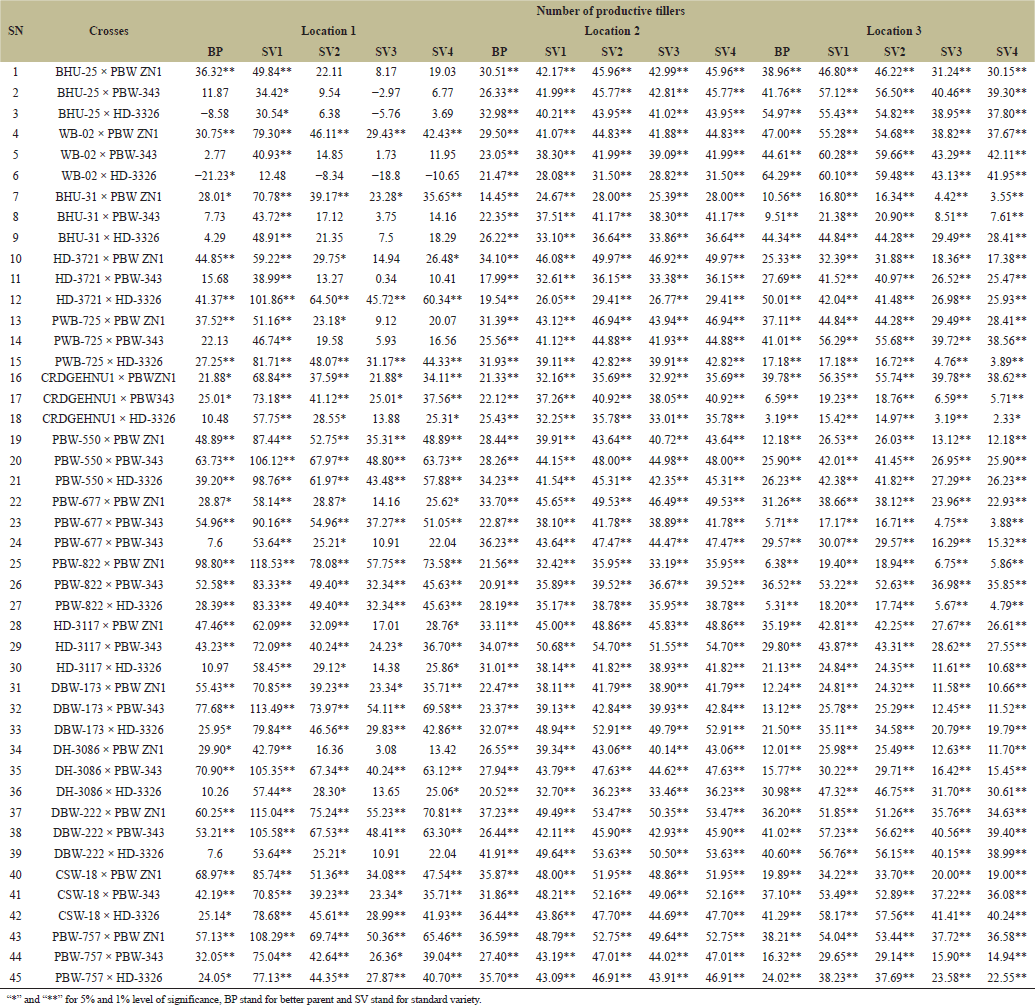 | Table 10. Estimation of heterosis over better parent and four standard varieties in wheat across three locations [Click here to view] |
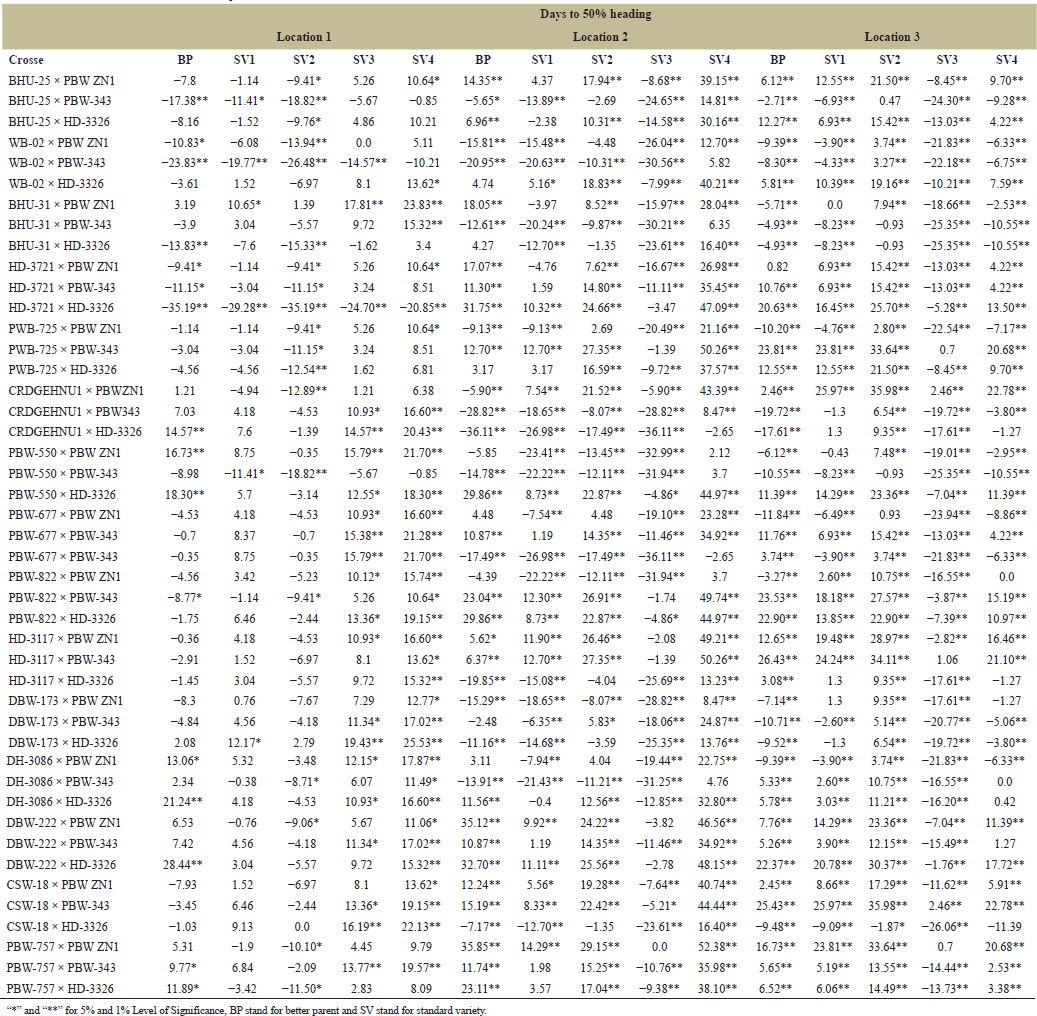 | Table 11. Estimation of heterosis over better parent and four standard varieties in wheat across three locations. [Click here to view] |
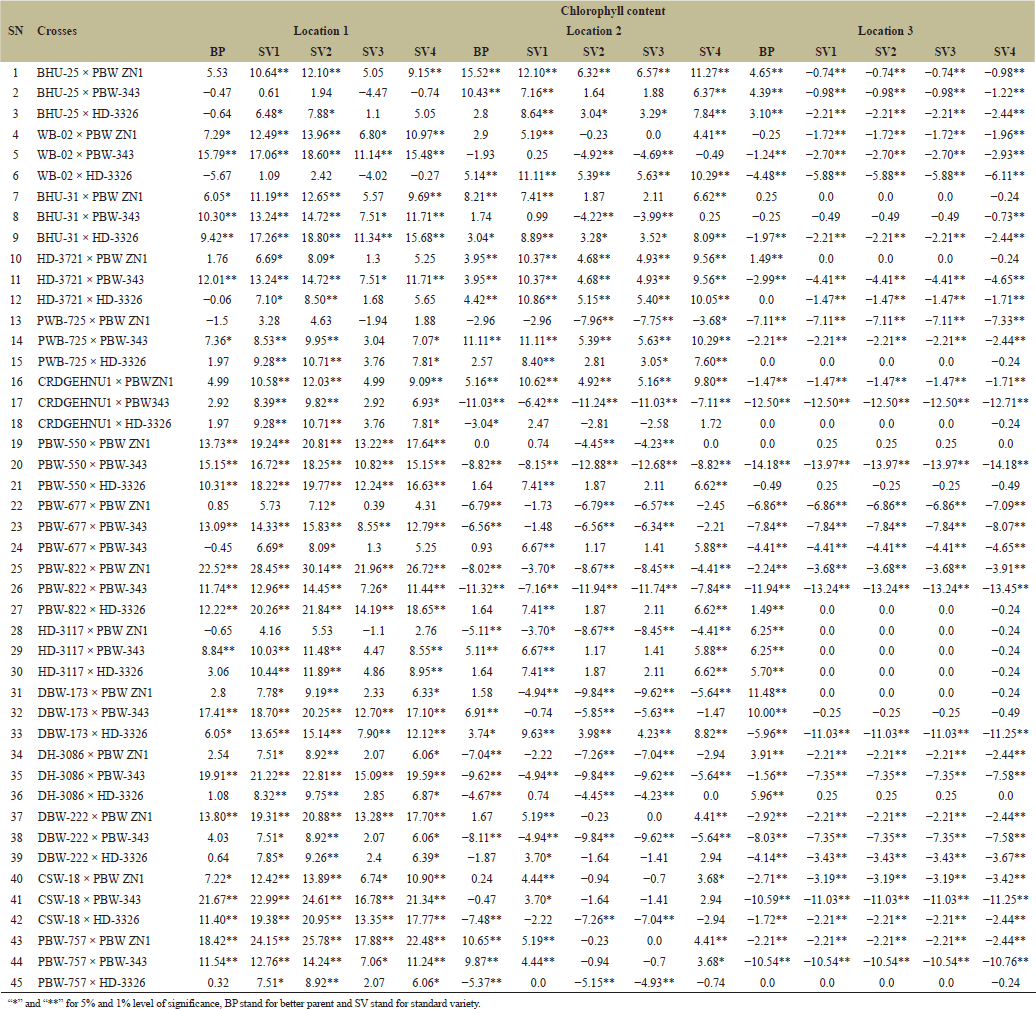 | Table 12. Estimation of heterosis over better parent and four standard varieties in wheat across three locations. [Click here to view] |
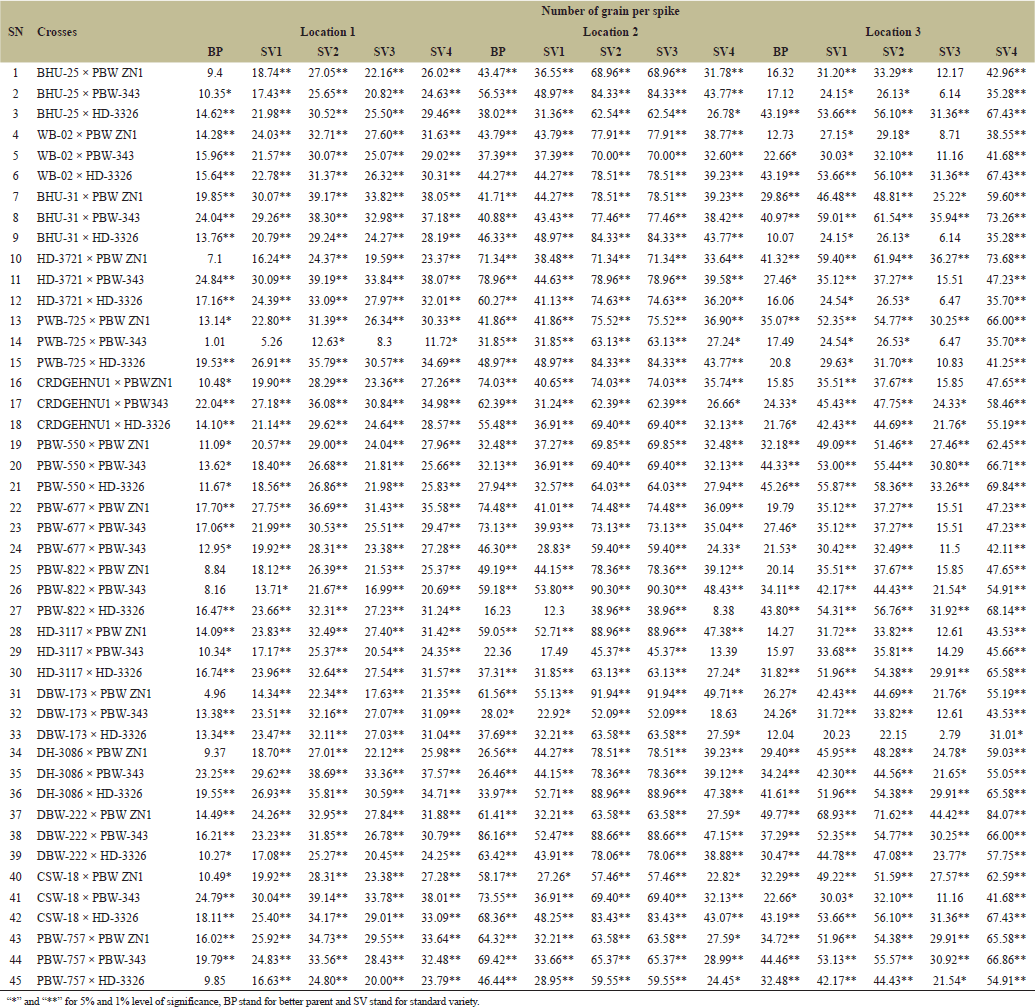 | Table 13. Estimation of heterosis over better parent and four standard varieties in wheat across three locations. [Click here to view] |
The observed level of heterosis in certain crosses served as potential parents to generate high-yielding/performance and resilient wheat couple with incorporating complementary traits, such as stress tolerance, into these high-yielding crosses through methods like heterosis breeding, backcross breeding, or MAS. Followed by conducting multi-location trials to assess the stability of heterosis across environments and can be used as genome editing tools to identify and select favorable alleles for hybrid development [6].
Some physiological and biochemical mechanisms underlying heterosis in wheat includes; physiological factors like photosynthetic efficiency, resource use efficiency (efficiency in nutrient and water utilization), resistance to environmental stresses (high resistant increase high heterosis), and rate of growth and biomass accumulation rate (high rate increase high heterosis). While biochemical mechanisms of heterosis involves enzymes activities such as nitrogen assimilation, carbohydrate metabolism, and antioxidant defense (often more active in hybrids compared to the parents). Also, heterosis is expressed in genes of hybrids compared to the parents [5].
The magnitude of heterosis varies across different traits and environments due to several factors. According to Shull [24] there are several factors that affect the variation in heterosis such as the presence of additive effect (low heterosis) and non-additive effect (high heterosis), mode of pollination of parents (wheat is self-pollinated, thus, heterosis will be low).
The potential advantages of hybrid breeding in wheat offers several advantages over traditional breeding methods, in that, hybrid breeding allowed systematic improvement of the population by application of pure line selection and exploitation of heterosis simultaneously. Hybrid breeding can lead to faster genetic improvements by effectively utilization of mode of gene action with greater precision and efficiency. Additionally, it allows for the simultaneous combination of complementary traits from various parental lines.
The genetic diversity of the parental lines can be maintained through the prevention of inbreeding depression (breeding within closely related parental line), secondly to perform wide crosses and the use of backcrossing judiciously, and the creation of populations with broad genetic variability. Other methods are olyploidy or double haploid breeding and asexual or apomictic seed production [5].
Some natural mechanisms in wheat includes; Wheat naturally genetic, biochemical, and physiological mechanisms that makes it thrive and produce reasonable yield under different environmental stresses such mechanisms includes; heat shock proteins, superoxide dismutase, and homeostasis for example, osmotic adjustment [2].
4. CONCLUSION
ANOVA recorded significant differences (p < 0.001), for the best lines and testers with high GCA were DH-3086, PBW-757, and PBW ZN1. The top line × tester crosses with high SCA for yield components characters like total grain yield per plant, HI, and spike length were PBW-677 × PBW-343, PBW-822 × PBW ZN1, and DH-3086 × PBW-343. These lines and testers show potential for improving traits controlled by additive genes and can be used for selecting superior parents, developing inbred lines, and ensuring broad adaptability in wheat for GCA. For SCA, the findings can help for identify superior crosses and leverage the mode of gene actions for wheat improvement. With regards to the total grain yield per plant across the genotypes and locations, the highest positive BP heterosis recorded in PBW-822 × PBW ZN1, while the lowest and negative BP heterosis was observed in WB-02 × HD-3326, with a value of −8.09. The highest standard heterosis was recorded in DH-3086 × PBW ZN1, while the lowest standard heterosis was seen in BHU-25 × PBW-343.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. ACKNOWLEDGEMENTS
We would like to express our appreciation to the Tertiary Education Trust Fund (TETFUND), Nigeria, for their funding support. We also appreciate Lovely Professional University, Kebbi State University, and the Lake Chad Wheat Research Institute for providing seeds and granting access to their farms and laboratories.
7. CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. AUTHORS’ DECLARATION
All authors affirm that this article is original and has not been submitted or published elsewhere.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bajaniya NA, Pansuriya AG, Vekari DM, Singh C, Savaliya JJ. Combining ability analysis for grain yield and its components in durum wheat (Triticum durum Desf.) Gujarat, India. Ind J Pure App Biosci 2019;7(4):217–24.
2. Kumar A, Mishra VK, Vyas RP, Singh V. Heterosis and combining ability analysis in bread wheat (Triticum aestivum L.). Indian J Sci Res 2019;6(2):75–9.
3. Dhiwar K, Sharma Dj, Agrawal PA, Pandey D. Stability analysis in wheat (Triticum aestivum L.). J Pharmacogn Phytochem 2020;9(5):295–8.
4. FAOSTAT. Food and Agriculture Organization of the United Nation, Year book. FAOSTAT, Rome, Italy, pp 585, 2021.
5. Hajer SA, H. S, Salih MM, Altaweel MS. Heterosis and combining ability for yield and its related traits in bread wheat (Triticum aestivum L.). Plant Cell Biotechnol Mol Biol 2021; 22(33):46–53.
6. Kumar PS, Verma DK, Tiwari AK, Raji V, Singh G, Raghvendra S. Yield gap analysis and strategy of improving wheat (Triticum aestivum L.) productivity under late sown through front line demonstrations in Eastern Uttar Pradesh. Int J Curr Microbiol App Sci 2020;10:180–84.
7. AbdEl-Hady AH, Zaied KA, Ramadan RA, Lasheen AS. Genetic analysis for yield and its components in bread wheat. J Agric Chem Biotechn 2018;9(4):111–21.
8. Kumar D, Kumar A, Kaur S, Yadav AK. Combining ability for yield attributing traits in wheat (Triticum aestivum L.). J Pharmacogn Phytochem 2018;1:2730–5.
9. Samier K, Ismail A. Heterosis and combining ability analysis for yield and its components in bread wheat (Triticum aestivum L.). Int J Curr Microbiol App Sci 2015;4(8):1–9.
10. Gulzar S, Kamaluddin, Bhatt MA, Yusuf N, Khan MA. Stability analysis for yield, yield component and quality traits in wheat (Triticum aestivum L.) under temperate conditions in Kashmir valley. Plant Arch 2015;15(1):433–40.
11. Sprague GF, Tatum LA. General vs specific combining ability in single crosses in corn. J Amer Soc Agron 1942;34:923–32.
12. Fellahi ZA, Hannachi A, Bouzerzour H, Boutekrabt A. Line × tester mating design analysis for grain yield and yield related traits in bread wheat (Triticum aestivum L.). Int J Agron 2018;10:11–55.
13. Gami RA, Tank CJ, Patel SS, Chauhan RM, Patel CG. Genetic analysis for grain yield and quality in durum wheat (Triticum durum) under late sown condition. Green Fmg 2010;1(6):600–1.
14. Reif JC, Hahn V, Melchinger AE. Genetic basis of heterosis and prediction of hybrid performance. Helia 2012;35(57):1–8.
15. Kempthorne O. An introduction to genetic statistics. John Wiley, New York, pp 302, 1957.
16. IBPGR and ICRISAT. Descriptors for sorghum [Sorghum bicolor (L.) Moench]. Int Board Plant Genet Resour Rome, Italy. – ICRISAT, Patancheru, India, pp 235; 1993.
17. Panse VG, Sukhatme PV. Statistical methods for agricultural workers. 2nd edition, Indian Council of Agricultural Research, New Delhi, India, pp 152–7, 1967.
18. Fonseca S, Patterson FL. Hybrid vigor in a seven-parent Diallel cross in common winter wheat (Triticum aestivum L.). Crop Sci 1968;8(1):85–8.
19. Kempthorne O, Curnow RN. The partial diallel cross. Biometrics 1961;17:229–50.
20. Barot HG, Patel1MS, Sheikh WAL, Patel1 P, Allam CR. Heterosis and combining ability analysis for yield and its component traits in wheat [Triticum aestivum (L.)]. Electron J Plant Breed 2014;5(3):350–9.
21. Kumar P, Nagar SS, Singh YP, Abhishek D, Kumar R. Study of gene action for yield components and gluten content in bread wheat (Triticum aestivum L.). Eco Env Cons 2015:22–5.
22. Kumar P, Nagar SS, Singh YP, Abhishek D, Kumar R. Study of gene action for yield components and gluten content in bread wheat (Triticum aestivum L.). Eco Env Cons 2015;22(2):703–9.
23. Askander HS, Salih MM, Altaweel MS. Heterosis and combining ability for yield and its related traits in bread wheat (Triticum aestivum L.). Plant Cell Biotechnol Mol Biol 2021;22(34):46–53.
24. Shull CH. What is heterosis? Genetics 1914;33:439–46.