1. INTRODUCTION
Seeing the current scenario of increasing human population, it is estimated that the world population will reach 9 billion by 2050 resulting in higher food demand [1,2]. However, limited availability of fertile land, urbanization, unpredictable weather conditions, and different types of biotic and abiotic stresses emerge as the major constraints in meeting the ever-growing food demand [3]. In the past decade, excessive application of pesticides and chemical fertilizers has been done to increase productivity. But the fact is that only a limited amount is absorbed by the plants and the rest of these nutrients are lost causing environmental pollution [2]. Most of the applied chemicals are non-degradable making them persistent. Additionally, the applied chemicals cause eutrophication of water bodies, disturbance in biogeochemical cycles, and other public health hazards. This highlighted the development of sustainable and eco-friendly technologies to overcome the excessive usage of synthetic fertilizers [4]. Interaction between plants, soil, and microbes greatly influences soil health and plant productivity [5]. Thus, the application of beneficial microbiomes as biofertilizers can work in an eco-friendly manner to improve plant growth and soil fertility [6,7]. Many microorganisms within the plant microbiome play an essential role in the growth and development of crops [7]. Improvement of soil microbial status by application of biofertilizers can stimulate natural soil microbiota affecting accessibility of nutrients and decomposition of organic matter [8]. The ability of biofertilizers to form higher soil microbial diversity can result in increased crop productivity [9]. Microbes isolated from the rhizosphere are screened for plant growth-promoting and effective colonization ability to be used as an effective biofertilizer [2]. Additional properties of plant growth-promoting microbes to be used as biofertilizers include enhancement of nutrient availability, decomposition of organic matter, and role towards mitigation of both biotic and abiotic stresses [4,10]. Biofertilizers comprise living or latent microbial cells that are applied either to soil, seed, or seedlings and improve the availability and uptake of nutrients from soil [7]. Biofertilizers are applied in different forms and prepared using suitable carriers that increase the shelf life and ease of handling microbial inoculants [11]. Biofertilizers hold many benefits including low cost, enhanced nutrient availability and soil fertility, enhanced tolerance towards biotic and abiotic stress, promotion of phytohormone production, improvement in soil health, and lesser environmental pollution [8]. Marketing of biofertilizers is done globally under two categories: organic residue-based biofertilizers and microorganism-based biofertilizers. Organic-based biofertilizers include green manure, crop residues, treated sludge, and farmyard manure whereas microorganisms-based biofertilizers contain beneficial microbes [1]. Biofertilizers contribute to plant growth promotion either through direct or indirect mechanisms. Direct mechanisms include enhanced nutrient availability (nitrogen fixation, phosphate solubilization) and production of phytohormones. Indirect mechanisms include protection from pathogens (through antibiotics, siderophores, and cyanide production), amelioration of abiotic stress, and bioremediation of pollutants [12,13]. Microorganisms-based biofertilizers can be classified as nitrogen-fixing microbes (free-living, symbiotic, and associative), phosphate solubilizing/mobilizing microbes, zinc solubilizing microbes, potassium solubilizing/mobilizing microbes, sulphur oxidizing microbes and plant growth promoting rhizobacteria [2]. A meta-analysis conducted on different varieties of crops for quantification of yield benefits of biofertilizers found nitrogen fixers and phosphate solubilizers as highly effective [14]. Biofertilizers comprising nitrogen fixers or phosphate solubilizers are available either in solid, liquid, or powdered form as inoculants in liquid medium [15]. Liquid biofertilizers use Rhizobium, Azotobacter, Azospirillum, and phosphate solubilizing bacteria (PSB) as inoculants [16]. There are several added advantages of using liquid over solid-based biofertilizers and the most important one is the addition of an adequate amount of nutrients in liquid formulation. Sometimes some cell protectants can also be added to enhance the shelf life of microbial biofertilizers [17]. Additionally, the usage of solid-base inoculants is hindered by certain difficulties that are not commonly found in liquid inoculant formulations. Liquid biofertilizers possess qualities such as longer shelf life, better survival, cost-effectiveness, and easy handling [15,16]. Liquid biofertilizers further possess added advantages compared to carrier-based biofertilizers (CBFs). CBF are somewhat intolerant to temperature fluctuations whereas liquid biofertilizers are tolerant to such changes. CBF has a low moisture-retaining capacity that affects the viability of organisms for longer periods while liquid biofertilizers facilitate enhanced viability. The possibility of contamination is high in the case of CBF as bulk sterilization is not so effective mode to prevent contamination which can be conveniently controlled through appropriate quality control measures and proper sterilization techniques in the case of liquid-based biofertilizers [18,19]. A research study performed by Shravani et al. [15] compared the shelf life between CBF and liquid biofertilizers through the viable count method. Their results revealed a subsequent decrease in microbial population with an increase in contamination in the case of carrier-based biofertilizers compared to liquid biofertilizers. Liquid-based biofertilizers showed constant viable cell count for at least 5–6 months whereas CBF showed constant viable cell count for only the first 3 months. In addition to this, pH fluctuation along with a decrease in moisture content with time was found to be more pronounced in CBF. As a result, liquid-based biofertilizers were found to be more effective compared to carrier-based fertilizers on the studied parameters. Another study estimated the shelf life of liquid biofertilizer inoculants prepared using different cell protectants [16]. For estimation of shelf life, colony-forming units were determined at monthly intervals for liquid-based biofertilizers including Rhizobium, Azotobacter, Azospirillum, and PSB. The study found maximum cell colonies with polyvinyl pyrrolidone in comparison to other cell protectants [16]. Similarly, other studies also performed quality analysis on various parameters among carrier and liquid-based biofertilizers to select the best among them [19]. The Indian government has taken numerous steps to use biofertilizers along with modern agrochemicals to increase crop production [20]. The majority of the current research related to biofertilizers focused on the development of strains, deciphering the mode of action in the rhizosphere, and increasing biological nitrogen fixation. However, the research on inoculant production is limited. The majority of the Indian inoculant producers still adopt the age-old practice of Burton technology rather than using liquid inoculants with high microbial load [21]. The considerate knowledge about the formulation of inoculant quality will develop through wide-field research on inoculants and their effectiveness on crop production. Microbial inoculants are specified multipurpose microorganisms that endorse better plant development or biological control when applied in farming practices [22]. The product quality enhancement will be directly dependent upon the microbial inoculant condition and viability. Therefore, improvement in the overall microbial inoculant structure and function ensures the reliability of biofertilizers as compared to chemical fertilizers to farmers for sustainable agriculture practices in terms of promoting good soil health for the future. Protocols with measurable standards for production and quality assessments of inoculants are not well known for many crops until now. More research is needed to understand the microbial interactions between introduced microbial inoculants and native soil microbial communities. Thus, more focus should be laid on creating awareness among the masses about inoculant production.
1.1. Rhizobium Biofertilizer And Its Specification
Biological nitrogen fixation is the process of conversion of atmospheric nitrogen into ammonia by diazatrophs. This process replenishes total nitrogen content and the fixed nitrogen regulates the growth and yield of the crops. This biologically fixed nitrogen is more sustainable and less available for leaching and vitalization [2]. Microbial genera that can form symbiotic associations with roots of legumes include Mesorhizobium, Azorhizobium, Allorhizobium, Rhizobium, Sinorhizobium, and are collectively termed Rhizobium [2]. The taxonomy of Rhizobium underwent frequent changes and was later classified as fast-growing (Sinorhizobium) and slow-growing rhizobia (Bradyrhizobium) depending upon their growth rate. Sequencing through 16s rRNA further classified rhizobia into 10 genera. It is a nitrogen-fixing bacterium that colonizes the roots of leguminous plants. Rhizobium helps plants to use atmospheric nitrogen by converting it into ammonia. The use of nitrogen-fixing bacteria like Rhizobium will reduce the dependence on chemical fertilizers and hence decrease the environmental risks posed by them. Rhizobia carries nod genes that code for Nod factors responsible for the formation of root nodules [23]. Rhizobium within the formed root nodules reduces nitrogen which is then incorporated into plants in various forms [24]. Rhizobium further triggers inter-chemical responses. Flavonoid molecules released as a signal by the host induce the expression of nodulation genes which in turn produces lipochitooligosaccharide signals to initiate mitotic cell division within roots to form nodules. Rhizobium-based biofertilizers were first tested in India in the early seventies. A successful trial led to the application of similar treatments in other legumes and cereal plants [25]. Symbiotic association between Rhizobium bacteria isolated from the tree legume such as Acacia mangium has also been studied with soybean [26]. Rhizobia species can even form symbiotic relationships with non-leguminous host species such as Parasponia [27]. The nitrogen-fixing ability of Rhizobium is affected by legume species and the prevailing environmental conditions. Rhizobium fixes nitrogen in the range of 50–100 kg N/hectare. Rhizobium biofertilizers further enhanced the yield of leguminous crops by 10%–35% [24]. Rhizobium and its species are highly effective as biofertilizers since they significantly increase nodulation in plant roots, enrich soil rhizosphere, and increase plant growth. A specific strain of Rhizobium biofertilizer (Rhizobium sp. CCNWYC119) is highly effective for three different peas [28].
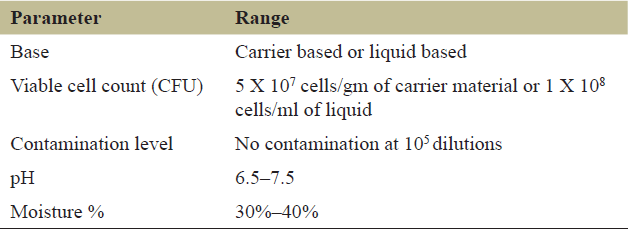 | Table 1. Specifications for Rhizobium based fertilizers determined by Govt. of India. [Click here to view] |
Production of Rhizobium sp. based biofertilizer can be done by using molasses as a principal carbon source in a growth medium [29]. Strict quality assessment measures were implemented by the government of India under a fertilizer control order (FCO) for the production of quality biofertilizers [21]. Mandatory specifications for Rhizobium-based biofertilizers are summarized in Table 1. The Rhizobium has the potential to be used as an industrially important biofertilizer and can have applications at a mass scale. Three additives were evaluated for their role in the growth and survival of liquid-based Rhizobium inoculants in the seed of Mungbean (Vigna radiata L.) [30]. Other carriers that have been reported for bio-inoculant preparation of biofertilizers include vermicompost, lignite, sodium alginate [31], and coal powder [32]. Collected bacterial isolates were then characterized and screened for growth-promoting properties such as mineral solubilization, phytohormone, and siderophore production. Isolate showing the best result was used for the production of rhizobial biofertilizer using different carrier materials. The shelf life of vermicompost, lignite, and sodium alginate-based rhizobial inoculants was studied at monthly intervals after storing them at either 28°C ± 2°C or 4°C storage temperature. A gradual decrease in rhizobial population was observed at both storage temperatures from the first to eight months but the survival rate was better at 4°C in comparison to 28°C ± 2°C. Thus, experimental results revealed 4°C as the best storage temperature for carrier-based inoculants and sodium alginate as the best carrier material for the preparation of Rhizobium CBF [31]. The usage of suitable media for the production of Rhizobium-based biofertilizers further enhances the quality and quantity of food. Inoculants of Rhizobium developed with coal powder as a carrier were found to be more effective for the development of plants compared to activated charcoal powder [32]. In another study Rhizobium was isolated from the roots of Pisum sativum and quality control analysis of Rhizobium biofertilizer was performed using coal ash and multani mitti as inert solid carrier along with shelf-life studies using plate pour and CFU count methods. Results showed good growth for around 2 months when coal ash was used as an inert solid carrier compared to multani mitti [33].
Additionally, Abdallah et al. [34] analyzed attributes of co-inoculants under different conditions. Co-inoculants were constructed using charcoal and packed for storage at either room temperature (25°C–30°C) or refrigeration (4°C). Random samples were taken after 3 months for viability test of Rhizobium and PSB. The study revealed positive results where viable counts were maintained for both cultures in charcoal-based co-cultured inoculants. Similar to this, the efficiency of co-inoculation of Rhizobium and PSB on yield and seed quality of leguminous crops has also been reported by other studies [35]. A consortium of microbes in which more than one microbe is used also showed satisfactory shelf life with no antagonistic effect confirming the co-culturing of multiple inoculants as biofertilizers [36].
1.2. Factors Determining the Specificity and Efficacy of Different Strains of Rhizobium as Biofertilizers
Application and optimization strategies of biofertilizers in sustainable agriculture include suitable strain selection. Choosing a suitable strain is one of the major optimization strategies of biofertilizers. Environmental stress tolerance is also an additional and important factor in the practice of suitable strain selection [2]. For example, Rhizobium biofertilizer-based technology and practice have been developed for the southern area of Rajasthan. ICAR- All India Network Project on Soil Biodiversity Biofertilizers (ICAR-AINP/SBB) ensured the isolation and characterization of 397 competent rhizobial strains for black gram, green gram, soybean, cluster bean, pea, cowpea, chickpea, pigeon pea, groundnut, methi, and bersim [37]. It includes the identification of the best strain based on genetic screening through amplified ribosomal DNA restriction analysis and other specified methods. Besides this, the specificity and efficacy of Rhizobial strains for various leguminous crops depend upon the following crucial factors:
- Host specificity: The rhizobial specificity against legumes has a wide range of hosts. Each Rhizobium strain has specific nodulation-affecting genes (nod, nol, and noe) which decide the specific plant root to form nodules. The genome of many rhizobium species such as Bradyrhizobium japonicum, Sinorhizobium meliloti, Mesorhizobium loti, and so on, has already been sequenced. Rhizobial species such as Rhizobium loti and Rhizobium etli have different host choices (Phaseolus spp. for Rhizobium etli and Lotus spp. for Rhizobium loti), but produce identical Nod factors [38]
- .
- Nodulation capability: The nodule formation by different strains of rhizobium on the legume of the host plant is a very crucial factor for the overall nitrogen fixation process. Efficient nodulation determines the nitrogen uptake ability of the plant [39].
- N2 fixation efficiency: Nitrogen fixation efficiency is one major factor that determines the specificity and efficiency of a particular rhizobium [38]. Various environmental factors viz. soil types, soil pH, water holding capacity, soil moisture content, and temperature are crucial for the ability of rhizobium to fix nitrogen.
- Environmental stress tolerance: Various stresses such as drought, flood, salinity, and extreme low and high temperatures can influence the nodulation, nitrogen fixation capability, growth, and overall survivability of Rhizobium strains.
2. QUALITY ANALYSIS OF RHIZOBIUM-BASED BIOFERTILIZER
The quality analysis is a very crucial and mandatory step in Rhizobium-based final biofertilizer production in terms of soil fertility and crop production. The quality analysis of Rhizobium-based biofertilizer involves different procedures including serial dilution, plating, and counting of viable cells for bacterial enumeration in a given sample. In addition to this, quality control analysis of overall processes such as examination of mother culture, broth examination, and peat examination is also necessary (Fig. 1).
2.1. Biological Strain Standardization
Biological strain is the original microbial strain in terms of accurate genus and species identification and standardized under the controlled conditions used for the synthesis of biofertilizer. Rhizobium strain can be procured from a microbial culture collection center and revived by standard protocols. Mother culture examination performed by selection of suitable raw material is done to screen for accurate strain. The test includes maintenance, regular inspection of growth, and purity of samples by streak plate and staining methodology. Gram staining method is performed to check the mother culture where rhizobial cells retain the color of safranin stain and appear red under microscopy indicating gram-negative culture [40].
 | Figure 1. Basic steps involved in quality control of biofertilizers. [Click here to view] |
Broth examination is accomplished by checking the pH of the broth (6.1–6.2) [36]. Rhizobial cells are stained either by Gram stain or Fuchsin stain and appear as rod-shaped under a microscope. Media Performance Testing is done to ensure media performance by inoculating the media with known microbial cells. The measured turbidity of broth culture having efficient rhizobial cells will show optical density in the range of 0–1.0 O.D value. Counting of total cells by Petroff-Hausser counter and of viable cells by spread plate procedure is followed by serial dilution.
Peat examination is performed by analysis of moisture content. The optimum moisture content of standard peat inoculant should range from 40% to 50%. Low and high moisture content is not suitable for active rhizobial growth. Counting of viable cells is done by spread plate method similar to the examination of broth culture [40].
2.2. Shelf-Life Studies Through Colony Forming Unit (CFU)
The shelf life of any biofertilizer determines the period beyond which the activity of the biofertilizer expires. It represents the deadline before which the product needs to be consumed. The most commonly employed method for shelf-life studies of rhizobium-based culture is by counting CFU obtained after serial dilution of biofertilizer. From the serially diluted samples, a small fraction (around 100 µl) is plated onto the plates and the plates are then incubated at 37°C for overnight. This is followed by counting of CFU for each dilution to determine the bacterial count [41]. Counting of CFU is done at different time intervals such as 15 days, 30 days, and 90 days, respectively, and change is determined by comparing it with the previously calculated data. There will be a progressive decrease after each interval and the observation is made for the least microbial count to determine the shelf life of the biofertilizer.
To assess changes in microbial activity and viability of biofertilizers over time comprehensively, several complementary methods and biochemical assays can be employed apart from CFU based method of calculating cell viability in biofertilizers. One such method is the Most Probable Number (MPN) assay which estimates the microbial population density based on dilution series and positive growth in liquid media. MPN method is a carbohydrate fermentation method that gives accurate results for finding the number of viable cells in the biofertilizers [42]. Another method is the fluorescence-based viability staining method. In this method, fluorescent dye is used to differentiate between live and dead cells under a fluorescence microscope. Enzyme activity profiling is also used to measure the activity of specific enzymes which is produced by microbes in biofertilizers. Thus enzyme activity helps to assess the functional capacity aspects of the microbe. Rhizospheric soil of Chilean avocado (Persea americana Mill.) analyzed using the Biolog EcoPlate™technique revealed that the microbial diversity in three commercial biofertilizers is highly different [43]. Thus, by integrating these complementary methods and assays, researchers and practitioners can gain deeper insights into the shelf-life dynamics of biofertilizers, optimize storage conditions, and enhance their efficacy in agricultural applications.
2.3. Mixing of Rhizobium-Based Biofertilizers and Analysis of Data Gathered After Different Time Intervals
The Rhizobium-based biofertilizer must be mixed with an appropriate carrier to maintain the microbial activity of the biofertilizer for reasonable time intervals. Liquid broth of rhizobium-based biofertilizer is mixed with a suitable carrier and anti-caking agent in aseptic conditions. It is then packed properly and the anti-caking agent prevents the formation of clumps by maintaining the moisture level. The quantity of carrier and anti-caking agent is fixed to optimize the growth of microbes. Commonly used carrier and anti-caking agents for the rhizobium-based biofertilizer are aluminum silicate and tri-calcium phosphate, respectively. After the mixing of rhizobium fertilizer with the appropriate anti-caking agent and carrier, the quality of the biofertilizer is checked after different time intervals. The viability of the microorganism (Rhizobium) is checked by counting CFU up to 6 months duration starting from day zero. Careful analysis of results provides an estimate regarding the shelf life of biofertilizers.
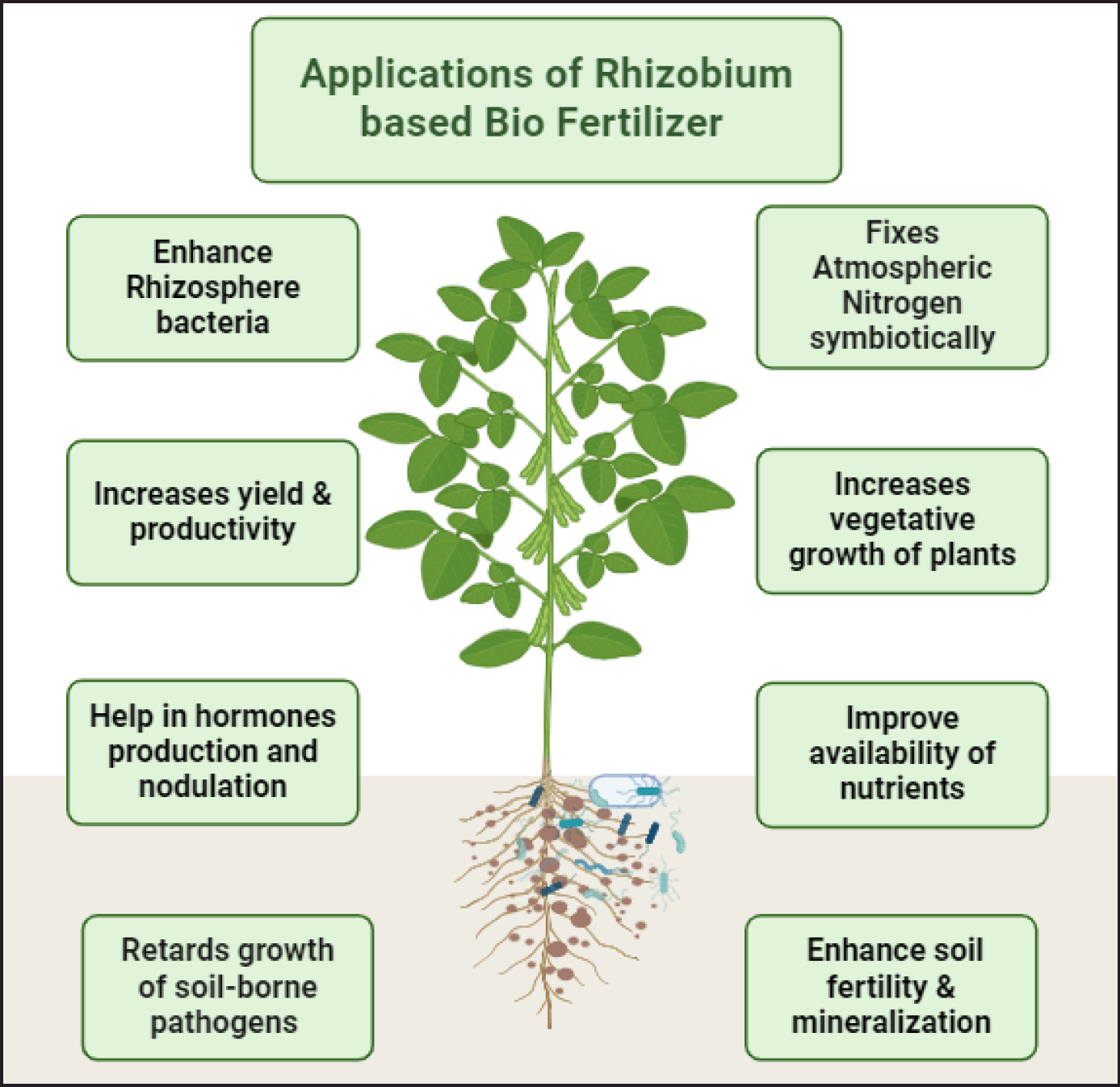 | Figure 2. Diagram showing beneficial effects of Rhizobium based biofertilizers. [Click here to view] |
2.4. Key Quality Assessment Parameters Outlined by the Government Regulations for Rhizobium-Based Biofertilizers
In India, the recommendation for the use of biofertilizers for all crops comes under the organic farming and Integrated Nutrient Management (INM) plans. It is estimated that the market for biofertilizers will increase by 10.1% between 2021 and 2026 with the improvement of organic farming and overall soil structure [37]. The quality assessments of Rhizobium and other notified biofertilizers have been reported in the Fertilizer (Control) Order, 1985 and the quality standards of these bio-fertilizers have been specified under the FCO, 1985. Several key quality assessment parameters outlined by the government for rhizobium-based biofertilizers are:
- Instructions and labeling: Proper instructions and information about strains of Rhizobium and host plants, their method of application, expiry, storage, and maintenance condition should be mentioned on the product. Appropriate labeling affects the correct use and overall results of applied biofertilizer.
- Carrier materials: The sterile carrier material must be used for the Rhizobium cell. It is necessary for its activity and survivability at the time of application on seeds or soil.
- pH: It is also an important parameter that determines the effectiveness and survival of Rhizobium cells. It should be usually between the 6.0 and 7.5 pH range for optimal results.
- Moisture content: The moisture content of applied biofertilizer also ensures the survivability of Rhizobium cells at the time of transportation, application, and storage. Disproportionate moisture will ultimately cause reduced shelf life and spoil overall practices.
- Cell viability and Shelf life: Different storage conditions have different impacts on the Rhizobial cells. A minimum percent of viable Rhizobial cells as well as a specific shelf life is necessary for efficient nodulation of the host plants. It should be necessary to check the viability and stability before use. The mathematical measurements for different field conditions are given by various technical bulletins developed by ICAR [37].
By applying these parameters, regulating agencies ensure the reliability of rhizobium-based biofertilizers for enhancing crop productivity.
3. FORMS AND APPLICATIONS OF RHIZOBIUM-CONTAINING BIOFERTILIZER
The biofertilizer formulation is an important multistep process that includes mixing of suitable carrier with the inoculant. The biofertilizer can be mixed either in liquid or in solid form carrier. The type of bioformulation (liquid or solid) affects the application of biofertilizer. Liquid formulation consists of microbial culture with water and other substances that enhance adhesion in the formulated product [44]. Solid formulation also known as carrier-based formulation contains either organic or inorganic carrier prepared as granules or powder [45].
In some studies, aluminum silicate is used as a solid inert carrier in combination with tri-calcium phosphate as an anticaking agent in Rhizobium-based biofertilizers. A good CFU count was seen in those biofertilizers even after 3 months [46]. Biofertilizers can be applied through seed inoculation, root dipping, and soil application [47]. These steps play an important role in crop production leading to agricultural sustainability. Seed inoculation involves mixing carrier biofertilizers with water to form a slurry. Seeds are then mixed within a slurry to uniformly coat the seeds with a mixture of inoculants before sowing. Root dripping of biofertilizer application is mostly suitable for transplanted crops. This involves dipping the root of the plant in a biofertilizer-water mixture before transplanting. The soil application method covers the spreading of biofertilizer onto the soil surface before sowing seeds or application as a foliar spray [48].
3.1. Advantages of Rhizobium-Based Biofertilizer
Biofertilizers are helpful in enormous ways as they are a key source in enhancing plant growth and development (Fig. 2). Application of Rhizobium increases the growth of crops significantly by affecting major growth and development factors such as height, size, seed germination, chlorophyll, nitrogen content, and so on [38,49]. Inoculation of rice seeds with rhizobial strains increased straw and grain yield [50]. They either act directly by nitrogen fixation and affecting plant hormones or indirectly by minimizing the pathogenicity [51]. Higher yield and soil fertility are also maintained with the application of Rhizobium [52]. Rhizobium-based biofertilizer is engaged in plant growth-promoting activities that are essential for the development and nourishment of plants. The positive effect of rhizobial inoculation has also been observed in non-leguminous crops. Rhizobium further improves growth through the production of phytohormones and siderophores. Improvement in yield along with other beneficial effects has been observed in cereal grains inoculated with Rhizobium. Rhizobium also holds the potential to be used as a crucial biocontrol agent [53]. Additional effects of rhizobium include mobilization of nutrients, increased stress tolerance, and induction of disease resistance [54]. Biofertilizers increase the organic content of soil hence improving the exchange of nutrients, water retention, and buffering capacity of soil. It helps in fighting different plant and soil-borne diseases. It also enhances the biological activity of soil along with the decomposition of toxic substances [55]. In one field study, the effect of liquid and solid-based Rhizobium was studied in urdbean. Due to Rhizobium inoculation, it was observed that there is good nodulation in the roots which in turn increases nitrogen fixation leading to better crop growth as compared to the uninoculated control sample [55]. Rubio-Canalejas et al. [56] reported in E. sativa plants that inoculating the roots of E. sativa with Rhizobium sp. resulted in secondary root development at 6–8 days as compared to uninoculated treatment. The use of Rhizobium biofertilizer will reduce our necessity for chemical fertilizers which makes them excellent environment-friendly resources in terms of crop improvement and soil fertility. Furthermore, the formulation of biofertilizer is a relatively easy and less time-consuming process with enhanced shelf-life. Rhizobium leguminosarum strain PEPV16 which was isolated from the root nodules of Phaseolus vulgaris has been shown to colonize the roots of Lactuca Sativa and Daucus carota thereby increasing both the roots and shoots of the plants [57]. Rhizobium biofertilizers increase grain yields especially in leguminous crops by root stimulation for nutrient uptake, increasing the quantity and quality of important enzymes, and hormones such as auxins which are required for the growth and development of plants [58]. Sometimes Rhizobium strain gives the best results when used in combination with other important microbial strains such as Pseudomonas and Bacillus species. Rhizobium strain inoculation in combination with either Pseudomonas putida, Pseudomonas fluorescens, or Bacillus cereus resulted in increased root nodulation, enzyme activity, and growth of the pigeon pea plant in comparison to only Rhizobium inoculated pigeon pea plant and uninoculated control sample [59]. Thus, Rhizobium biofertilizer can be applied in consortium with other microbial strains for increased efficiency.
4. DEVELOPMENT AND APPLICATION OF BIOFERTILIZERS IN SUSTAINABLE AGRICULTURAL PRACTICES ESPECIALLY LEGUMINOUS CROPS
Biofertilizers represent a sustainable approach to enhance agricultural productivity, particularly in leguminous crops, through nitrogen fixation, phosphorus solubilization, and improved nutrient uptake. The rhizome helps in root nodule formation in leguminous plants. Field experiments were conducted to assess the effect of co-inoculation of Rhizobium leguminosarum bv. viciae, a phosphorus-solubilizing bacteria, and plant growth-promoting rhizobacteria on certain parameters such as symbiotic association, growth, and yield of lentils [60]. The result showed a synergistic effect with improvement in nodulation, growth, and yield of lentils compared to individual application. Similarly, the inoculation of field-grown chickpeas with nitrogen-fixing and phosphate-solubilizing rhizobacteria revealed that the plant growth and grain yield can be increased [61]. Multiple inoculations of Mesorhizobium, phosphate solubilizing Pseudomonas and Bacillus spp. increased the uptake of N and P by field-grown chickpeas in a significant manner. The development, application, and ongoing research on multiple consortia usage will help sustainable agriculture practice significantly by reducing environmental impact, improving soil health, and supporting economic viability for farmers. With the evolvement of technology and understanding, biofertilizers will play a vital role in achieving global food security goals while minimizing ecological footprint.
4.1. Advantages and Limitations of Different Biofertilizer Application Practices
There are different methods through which biofertilizers can be used such as seed inoculation, root dipping, and direct mixing with soil. Each of these methods has its own distinct advantages and limitations that should be considered when deciding on the most suitable approach for a particular farming system or crop. The advantages and disadvantages of different applied methods are the following:
- Seed Inoculation method: It is very cost-effective as fewer amounts of biofertilizers are required thus reducing the overall usage. When biofertilizers are directly applied to the seeds it ensures early colonization of the rhizosphere. Limitations of this method are that it is not suitable for all types of crops. Its effectiveness can be affected by environmental factors such as the pH of the soil and temperature.
- Root dipping: Direct contact of biofertilizer with root ensures rapid colonization in the rhizosphere. It enhances the root’s nutrient uptake capacity. This method is suitable for all types of crops. The limitation of this method is that it requires careful handling and improper dipping techniques can damage the roots thus affecting the plant growth.
- Direct soil application: Direct soil application of biofertilizer treats a larger volume of the soil. It has long-lasting effects thus providing sustainable benefits over time. The limitation of this method is that environmental factors like soil conditions (pH, moisture, and temperature) affect the activity of biofertilizers. As compared to seed inoculation and root dipping this method is more time-consuming to become fully effective.
4.2. Targeting Application of Biofertilizers for Sustainable Agriculture
The application of microbial fertilizers enhanced soil health, increased crop productivity, and thus contributed a lot to sustainable agriculture. The following are the strategies for addressing identified shortcomings in the application of microbial fertilizer:
- Research and development: More investment in research should be done to develop more robust strains of biofertilizers that are compatible with different soil and environmental conditions.
- Effective quality control: Rigorous quality measures during the production of microbial strains ensure the long-lasting effectiveness of biofertilizers.
- Improvement in application techniques: Automated seed inoculators can be used for precise farming and other automated machines such as root dipping machines ensure uniform distribution of biofertilizers and maximize the impact of biofertilizers.
- Environmental monitoring: The use of environmental sensors and other monitoring systems can assess the soil conditions and thus biofertilizer application timing can be optimized.
- Educational training and awareness camps: Training should be provided to farmers about the proper application of biofertilizers, optimal timing of usage, and other application techniques.
- Government support and certification: Implement government policies that incentivize the use of biofertilizers, such as subsidies to farmers for the purchase of biofertilizers. Certification programs should be established to maintain the standard of biofertilizers and ensure high quality of biofertilizers.
- Developing suitable carrier: To develop a suitable carrier that is non-toxic to bacterial inoculants, the carrier should be free-flowing with minimum lump formation. It should be autoclavable and also non-toxic to plants as well.
- Improving storage facility: The long-term stability of biofertilizers is dependent on how it is stored and proper quality control of biofertilizers during storage over time.
5. CONCLUSION
Excessive use of chemical fertilizers to increase crop yield has caused serious environmental hazards and toxicity to human health. These chemical fertilizers further caused eutrophication, leaching of pollutants, and soil pollution leading to various human health concerns. Compared to chemical fertilizers, biofertilizers are eco-friendly, inexpensive, and non-toxic with almost similar potential in increasing the yield. Research related to the agricultural sector has come ahead a long way to make biofertilizers a better replacement for chemical fertilizers. Biofertilizers play an important role in meeting the demands of food supply by increasing soil fertility and crop productivity. Being organic, it does not cause any damage to nature. Application of microbial fertilizers currently faces certain problems like inadequate promotion, lack of suitable carriers for product formulation, lack of storage facilities for the prevention of biofertilizers from contamination, and inconsistency in biofertilizer efficacy. These shortcomings can be overcome by increasing awareness about microbial fertilizers and their benefits among the farmer community.
6. AUTHOR’S CONTRIBUTIONS STATEMENT
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. ACKNOWLEDGEMENT
The authors are thankful to affiliated institutions for providing the requisite facilities.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
13. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chaudhary P, Singh S, Chaudhary A, Sharma A, Kumar G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front Plant Sci 2022;13:930340.
2. Kumar S, Diksha, Sindhu SS, Kumar R. Biofertilizers: an eco-friendly technology for nutrient recycling and environmental sustainability. Curr Res Microb Sci 2021;3:100094.
3. Glaser B, Lehr VI. Biochar effects on phosphorous availability in agricultural soils: a meta-analysis. Sci Rep 2019;9(1):9338.
4. Zhang J, Cook J, Nearing JT, Zhang J, Raudonis R, Glick BR et al. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol Res 2021;245:126690.
5. Harman G, Khadka R, Doni F, Uphoff N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front Plant Sci 2021;11:610065.
6. Murgese P, Santamaria P, Leoni B, Crecchio C. Ameliorative effects of PGPB on yield, physiological parameters, and nutrient transporter genes expression in Barattiere (Cucumis melo L.). J Soil Sci Plant Nutr 2020;20:784–93.
7. Fasusi OA, Cruz C, Babalola OO. Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture 2021;11(2):163.
8. Chaudhary A, Chaudhary P, Upadhyay A, Kumar A, Rani A. Effect of gypsum on plant growth promoting rhizobacteria. Environ Ecol 2022;39:1248–56.
9. Agri U, Chaudhary P, Sharma A, Kukreti B. Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil 2022;474:451–68.
10. Sehrawat A, Sindhu SS. Potential of biocontrol agents in plant disease control for improving food safety. Def Life Sci J 2019;4(4):220–5.
11. Bhattacharjee R, Dey U. Biofertilizer, a way towards organic agriculture: a review. Afr J Microbial Res 2014;8(24):2332–42.
12. Glick BR, Gamalero E. Recent developments in the study of plant microbiomes. Microorganisms 2021;9(7):1533.
13. Sehrawat A, Sindhu SS, Glick BR. Hydrogen cyanide production by soil bacteria: biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022;32(1):15–38.
14. Praveen KV, Singh A. Realizing the potential of a low-cost technology to enhance crop yields: evidence from a meta-analysis of biofertilizers in India. Agric Econ Res Rev 2019;32:77–91.
15. Shravani K, Triveni S, Latha PC, Damodara CK. Evaluation of shelf life and quality of carrier and liquid based biofertilizers. Int J Microbiol Res 2019;11(6):1598–601.
16. Santhosh GP. Formulation and shelf life of liquid biofertilizer inoculants using cell protectants. Int J Res Biosci Agric Technol 2015;2(7):243–7.
17. Mahdi SS, Hassan GI, Samoon SA, Rather HA, Dar SA, Zehra B. Biofertilizers in organic agriculture. J Phytol 2010;2(10):42–54.
18. Patel N, Patel Y, Mankad A. Bio Fertilizer: a promising tool for sustainable farming. Int J Innov Res Sci Eng Technol 2014;3(9):15838–42.
19. Bhavya K, Reddy RS, Triveni S. Comparative study on quality parameters and viability of carrier and liquid biofertilizers. Int J Pure Appl Biosci 2017;5(4):1702–9.
20. Mazid M, Khan TA. Future of bio-fertilizers in Indian agriculture: an overview. Int J Agric Food Res 2014;3(3):10–23.
21. Yadav AK, Chandra K. Mass production and quality control of microbial inoculants. Proc Indian Natl Sci Acad 2014;80(2):483–9.
22. O’Callaghan M, Ballard RA, Wright D. Soil microbial inoculants for sustainable agriculture: limitations and opportunities. Soil Use Manage 2022;38:1340–69.
23. Raychaudhuri S, Yadav AK, Raychaudhuri M. Changing face of Rhizobial technology. Biofertilizer Newsletter 2007;15(1):3–10.
24. Amat D, Thakur JK, Mandal A, Sahu A, Reddy KKK. Production and utilization of legume inoculants (Rhizobium) in India. Harit Dhara 2020;3(2):28–31.
25. Brahmaprakash GP, Sahu PK. Biofertilizers for sustainability. J Indian Inst Sci 2012;92(1):37–62.
26. Lekatompessy S, Nurjanah L, Sukiman H. Study of cross inoculation of Rhizobium tropici with other potential soil microbes on their ability to support the growth of Soybean. IOP Conf Series Earth Environ Sci 2019;308:012041.
27. Poonia S. Rhizobium: a natural biofertilizer. Int J Eng Mgmt Res 2011;1(1):36–8.
28. Nushair AM, Saha AK, Mandal A, Rahman MA, Mohanta MK, Hasan MA, et al. “Rhizobium sp. CCNWYC119: a single strain highly effective as biofertilizer for three different peas (Pigeon pea, sweet pea, and Chick pea). Legume Res 2018;41(5):771–7.
29. Garcha S, Kansal R, Gosal SK. Molasses growth medium for production of Rhizobium sp. based biofertilizer. Indian J Biochem Biophys 2019;56:378–83.
30. Sehrawat A, Yadav A, Anand RC, Kukreja K, Suneja S. Enhancement of shelf life of liquid biofertilizer containing Rhizobium sp. infecting mungbean (Vigna radiata L.). Legume Res 2017;40(4):684–90.
31. Thirumal G, Reddy RS, Triveni S, Damodarachari K, Bhavya K. Evaluate the shelf life of rhizobium carrier based biofertilizer stored at different temperatures at different intervals. Int J Curr Microbiol Appl Sci 2017;6(7):753–9.
32. Gomare KS, Mese M, Shetkar Y. Isolation of rhizobium and cost-effective production of biofertilizer. Ind J Life Sci 2013;2(2):49–53.
33. Singh AK, Deepuraj, Masih H, Kumar Y, Peter JK, Mishra SK, et al. Optimization of production parameters and evaluation of shelf life of Rhizobium biofertilizers. Elixir Bio Tech 2014;67:21787–95.
34. Abdallah HA, Elsalahi RH, Mohamed SE. Quality attributes of co-inoculants based on rhizobia and phosphate solubilizing bacteria under different storage conditions. J Agric Vet Sci 2019;12(10):1–7.
35. Osman AG, Rugheim AME, Elsoni EM. Effects of biofertilization on nodulation, nitrogen and phosphorous content and yield of pigeon pea (Cajanus Cajan). Adv Environ Biol 2011;5(9):2742–9.
36. Sangeetha D, Stella D. Survival of plant growth promoting bacterial inoculants in different carrier materials. Int J Pharm Biol Arch 2012;3(1):170–8.
37. Jain D, Sharma SK, Meena RH, Kollah B, Mohanty SR. Liquid and carrier based Rhizobium biofertilizer technology for Southern Rajasthan of India, MPUAT, Udaipur, India, pp. 1–11, 2022.
38. Bahuguna V, Bhatt G, Maikhuri R, Chandra D. Nitrogen fixation through genetic engineering: a future systemic approach of nitrogen fixation. In: Nath M, Bhatt D, Bhargava P, Choudhary DK, (eds). Microbial metatranscriptomics belowground, Springer, Singapore, pp. 109–22, 2021.
39. Lindstrom K, Mousavi SA. Effectiveness of nitrogen fixation in rhizobia. Microb Biotechnol 2020;13(5):1314–35.
40. Suh JS, Jiarong P, Toan PV. Quality control of biofertilizers. Biofertilizer manual. FNCA Biofertilizer Project Group. Forum for Nuclear Cooperation in Asia (FNCA). Japan Atomic Industrial Forum (JAIF), Tokyo, Japan, pp.112–9, 2006.
41. Brar SK, Sarma SJ, Chaabouni E. Shelf-life of biofertilizers: an accord between formulations and genetics. J Biofertil Biopestici 2012;3:e109.
42. Deaker R, Mijajlovic G, Casteriano A. Estimating the most probable number of bacteria in multistrain biofertilizer inoculants using a multiple-tube fermentation test. In: Kennedy IR, Choudhury ATMA, Kecskes ML, Rose MT (eds). Efficient nutrient use in rice production in Vietnam achieved using inoculant biofertilisers. ACIAR Proceedings, Hanoi, Vietnam, pp. 108–16, 2008.
43. Renganathan P, Andrade-Bustamante G, Martínez-Ruiz FE, Puente EO. Microbial diversity and functional profiles of three commercial biofertilizers and impacts on the bacterial communities of avocado’s soil rhizosphere. Cienc Tecnol Agropecuaria 2024;25(1):e3251.
44. Lee SK, Lur HS, Lo KJ, Cheng KC, Chuang CC, Tang SJ, et al. Evaluation of the effects of different liquid inoculant formulations on the survival and plant growth promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl Microbiol Biotechnol 2016;100(18):7977–87.
45. Sahu PK, Gupta A, Singh M, Mehrotra P, Brahmaprakash GP. Bioformulation and fluid bed drying: a new approach towards an improved biofertilizer formulation. In: Sengar R, Singh A, (eds). Eco-friendly agro-biological techniques for enhancing crop productivity, Springer, Singapore, pp. 47–62, 2018.
46. Matura R, Bahuguna V, Bhandari M, Thapa I, Jain S. Assessment of shelf life and quality of biofertilizers using tricalcium phosphate as an anticaking agent and aluminium silicate as the inert carrier. Ind J Agric Res 2021;57(6):784–7; doi:10.18805/IJARe.A-5650
47. Mahanty T, Bhattacharjee S, Goswami M, Bhattacharya P, Das B, Ghosh A, et al. Biofertilizers: a potential approach for sustainable agriculture development. Environ Sci Pollut Res Int 2017;24(4):3315–35.
48. Lawal TE. Babalola OO. Relevance of biofertilizers to agriculture. J Hum Ecol 2014;47(1):35–43.
49. Safikhan S, Mohammadi M, Reza CM. Effects of seed inoculation by rhizobium strains on chlorophyll content and protein percentage in common bean cultivars (Phaseolus vulgaris L.). Int J Biosci 2013;3(3):1–8.
50. Sammauria R, Kumawat S, Kumawat P, Singh J, Jatwa TK. Microbial inoculants: potential tool for sustainability of agricultural production systems. Arch Microbiol 2020;202(4):677–93.
51. Nosheen S, Ajmal I, Song Y. Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability 2021;13(4):1868.
52. Mabrouk Y, Hemissi I, Salem IB, Mejri S, Saidi M, Belhadj O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. In: Rigobelo EC (ed). Symbiosis. IntechOpen, London, UK, 2018; doi:10.5772/intechopen.73495
53. Baset Mia MA, Shamsuddin ZH. Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr J Biotechnol 2010;9(37):6001–9.
54. Chen J. The combined use of chemical and organic fertilizers and/or biofertilizers for crop growth and soil fertility. International workshop on sustained management of the soil-rhizosphere system for efficient crop production and fertilizer use. Land Development Department, Bangkok, Thailand, pp 1–11, 2006.
55. Biswas PK, Bhowmick B, Bhowmick MK. Effect of liquid and carrier-based Rhizobium inoculants on growth, nodulation and seed yield of urdbean. J Crop Weed 2007;3(2):7–9.
56. Rubio-Canalejas A, Celador-Lera L, Cruz-González X, Menéndez E, Rivas R. Rhizobium as potential biofertilizer of Eruca sativa. In: Gonzalez-Andreas F, James E, (eds). Biological nitrogen fixation and beneficial plant-microbe interaction. Springer, Cham, Swizterland, pp 213–20, 2016.
57. FloresFélix JD, Menéndez E, Rivera LP, MarcosGarcía M, MartínezHidalgo P, Mateos PF, et al. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J Plant Nutr Soil Sci 2013;176(6):876–82.
58. Mnalku A, Demise N, Muleta D, Abera Y, Mitiku G. Manual for rhizobial inoculant development and management. Ethiopian Institute of Agricultural Research, Addis Ababa, 2020, doi:10.13140/RG.2.2.28906.67521
59. Tilak KV, Nandanavanam R, Manoharachari C. Synergistic effects of plant-growth-promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeon pea (Cajanus cajan). Eur J Soil Sci 2006;57(1):67–71.
60. Khanna V, Sharma P. Potential for enhancing lentil (Lens culinaris) productivity by co-inoculation with PSB, plant growth-promoting rhizobacteria and Rhizobium. Indian J Agri Sci 2011;81(10):932.
61. Wani PA, sKhan MS, Zaidi A. Synergistic effects of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on the performance of field-grown chickpea. J Plant Nutr Soil Sci 2007;170(2):283–7.