1. INTRODUCTION
Rice (Oryza sativa L.) is a vital global crop that feeds over 3.5 billion people, especially in Asia, Africa, and Latin America. Therefore, increasing rice production is critical to tackle the growing food shortages [1,2]. The global demand for rice continues to rise due to population growth, urbanization, putting immense pressure on agricultural systems to enhance productivity while tackling challenges such as climate change, water scarcity, and land degradation [3,4]. A sudden change in the environment, such as a change in temperature or water level (due to floods and droughts), an affects the development of plants, yield, and grain quality, thereby affecting the world’s food security. Rice plants undergo three distinct stages of development in their life cycle.
The vegetative phase includes germination, seedling establishment, tillering (the formation of lateral shoots), and stem elongation. The reproductive stage begins with panicle initiation (pre-booting), continues through booting (when the panicle is enclosed in the leaf sheath), heading, and ultimately, grain filling and maturity [5,6]. According to Yoshida [7] and Ashikari et al., [8] the heading is vital because it influences flowering time and pollination, as well as grain formation and filling, all of which directly affect the quantity, size, and quality of harvested grains. The yield of rice is influenced by three primary factors: The number of panicles, the number of spikelets per panicle, and the grain-filling rate. Rice cultivars characterized by larger panicles demonstrate greater yield potential due to a higher spikelet count per panicle. Therefore, shortening flowering time should also be considered one of the options to finish the harvest before bad weather arrives. However, scientists have found that when shortening the maturity period, rice yield and grain quality are also affected [9].
In rice, phytohormones are essential for growth and yield regulation, particularly during the heading period, affecting grain filling, floret development, and panicle exsertion [10]. Phytohormones coordinate important cellular functions such as division, elongation, differentiation, and senescence, acting as crucial regulators of the development stage [11]. According to Zhang et al., maturation is sensitive to environmental factors such as temperature and water availability [12,13]. Imbalances in hormone levels at this stage can lead to poor yield outcomes, including incomplete panicle emergence, poor fertility, and poor grain production [14]. During the heading stage, hormonal changes redirect the plant from vegetative growth to reproduction. Gibberellins play a key role in stem elongation and panicle exsertion, which are important for pollination and self-pollination in rice [15]. Mutants that lack gibberellin biosynthesis, such as the semi-dwarf1 (sd1) genotype, show reduced height and incomplete panicle emergence, highlighting the hormone’s necessity during heading [16]. Studies using high-performance liquid chromatography (HPLC) have shown that gibberellin levels peak during stem elongation and decline as the plant approaches panicle initiation, suggesting a temporal regulation that aligns with reproductive priorities [17]. Auxins regulate apical meristem activity and vascular development, influencing heading, panicle formation, and nutrient transport [18,19]. The level of indole-3-acetic acid (IAA) decreases, signaling the cessation of vegetative growth and the onset of reproductive differentiation at the transition from tillering to panicle initiation [20]. According to Kieber and Schaller, cytokinins play a crucial role in heading and grain filling by promoting cell proliferation and maintaining sink activity in the panicle [10]. Zeatin concentration rises during stem elongation and peaks around heading, which facilitates floret development and prevents premature senescence of cells in the reproductive stage [21]. This surge in cytokinins is vital for determining grain number, a key component of yield, as it ensures the viability of spikelets [8]. However, insufficient research has been conducted on the precise timing of plant growth regulator (PGR) treatment.
Therefore, we investigated the effects of PGRs applied at different developmental stages to shorten the maturation period of rice. In addition, the analysis of correlations between morphology, agronomy, physiology, and biochemistry in this study contributes to sustainable agriculture and global food security in other crops, especially in climate change conditions.
2. MATERIALS AND METHODS
2.1. Plant Material
The seeds of Oryza sativa L. cv. OM5451 was sourced from the Mekong Delta Rice Research Institute in Vietnam.
2.2. Cultivation Conditions
Seeds were treated by soaking in warm water (50°C) for 2 h, followed by 24 h at 35°C [22]. Then, seeds were placed in wet paper and incubated in the dark for approximately 48 h until seedling roots reached 1–2 mm in length. After germination, seedlings were transplanted into boxes measuring 40 cm in width, 60 cm in length, and 40 cm in height. Each box was filled with 50 dm3 of soil (pH 6.0–6.5), supplied by Saigon Green Biotechnology Company (Ho Chi Minh City). The soil texture comprised 63.4% sand, 28.5% silt, and 8.1% clay. The soil had an organic matter content of 24,91 g/kg, available phosphorus of 0.062%, and potassium of 0.93%. Each box accommodated 24 plants, spaced 10 cm apart.
Plants were fertilized with a total of 100 kg/ha nitrogen (N), 40 kg/ha phosphorus (P2O5), and 30 kg/ha potassium (K2O). Fertilizer application was split across growth stages: all P2O5 was applied pre-sowing; 50% of N and 50% of K2O were applied 7 days after sowing (seedling stage); 25% of N and the remaining 50% of K2O were applied 22 days after sowing (tillering stage); and the final 25% of N was applied 38 days after sowing (panicle initiation stage). Water levels were maintained at 6–7 cm above the soil surface during vegetative growth, reduced to 4–5 cm during the reproductive phase, and discontinued 15 days before harvest.
The experiment was conducted in conditions with a 12-h photoperiod (light intensity 150 ± 20 klux at noon), daytime temperatures of 33 ± 3°C (noon), nighttime temperatures of 24 ± 3°C (20:00), and relative humidity of 65 ± 5% (noon).
2.3. PGR Treatments
Exogenous PGRs, including IAA, GA3, and benzyladenine (BA) (Merck, Germany) at 25 or 50 mg/L, were applied on three different developmental stages: Tillering (26 days after sowing), panicle initiation (36 days after sowing), or booting (46 days after sowing). Treatments involved spraying 50 mL of PGR solution, containing 0.01% Tween-20 as a surfactant, onto the apical shoots daily for 3 consecutive days at a time between 16:00 and 17:00. Distilled water served as the control. Each treatment was replicated 4 times, each replicate included three containers, each container comprised 24 plants. The spikelets were tagged for subsequent monitoring. The following parameters were assessed:
At the flowering stage: Days to 50% flowering (from sowing), flag leaf length (tip to blade base), and flag leaf area (calculated using LeafByte software, Getman-Pickering).
At the ripening stage: Days to 85% seed yellowing on panicles, plant height (base to panicle tip), panicle length (main stem), percentage of filled grains per panicle, and 1000-grain weight (dried to 13% moisture).
2.4. Determination of Phytohormone Content in the Shoot Tip at Different Development Stages
Endogenous levels of IAA, GA3, GA4, and cis-zeatin riboside (cZR) in the shoot apex were measured on days 23 (tillering), 30 (internode elongation), and 37 (panicle initiation) after sowing, corresponding to the tillering, stem elongation, and panicle initiation stages, respectively. The apical shoots (0.5 cm in length) with mature leaves removed were ground, and extracted in either a methanol: water:formic acid mixture (15:4:1 by volume) for IAA and cZR or pure methanol for GA3 and GA4. Extracts were filtered and analyzed using HPLC-diode-array detection (DAD) (Agilent Series 1200, USA) on a Zorbax Extend C18 column, following the method of Nakagawa et al. [23]. Hormone concentrations were expressed as μg/g fresh weight (FW).
2.5. Experimental Design and Statistical Analysis
The experiment followed a randomized block design with four replicates per treatment, each replicate consisting of three containers (72 plants total per treatment). The data were tested for homogeneity of group variances (Levene test). Then, data were analyzed using one-way analysis of variance in Statistical Package for the Social Sciences 26.0 (IBM Corp., Armonk, NY, USA), with significant differences determined at P < 0.05 using Duncan’s test. Results are reported as means ± standard deviation.
3. RESULTS
3.1. Changes in Phytohormone Content in the Shoot Apex at Different Development Stages
The level of phytohormones in the shoot apex of O. sativa cv. OM5451 exhibited distinct patterns across the tillering, internode elongation, and panicle initiation stages, as determined by HPLC-DAD analysis according to Table 1. Content of IAA decreased progressively from 1.45 μg/g FW at tillering to 0.91 μg/g at internode elongation, and further to 0.40 μg/g at panicle initiation, with statistically significant (P < 0.05) differences across all stages. GA3 experienced a decline of 18.29 μg/g from tillering to internode elongation, followed by a decrease of 0.56 μg/g from internode elongation to panicle initiation, with both changes differing significantly (P < 0.05). GA4 was detected exclusively at tillering (8.15 μg/g) and was absent in subsequent stages. In contrast, cZR was undetectable at tillering. Still, it emerged at internode elongation and panicle initiation, with no significant difference between these latter stages. These shifts indicate a transition from vegetative to reproductive priorities, with auxins and gibberellins dominating early growth and cytokinins supporting later reproductive development.
Table 1: Changes in phytohormone content in the shoot apex at different development stages.
| Development stages | Content of phytohormone (μg/g FW) | |||
|---|---|---|---|---|
| Zeatin | IAA | GA4 | GA3 | |
| Tillering | - | 1.45±0.07a | 8.15±0.66 | 19.33±0.37a |
| Internode elongation | 0.45±0.08ns | 0.91±0.13b | - | 1.04±0.05b |
| Panicle initiation | 0.55±0.04ns | 0.40±0.08c | - | 0.48±0.33c |
Different letters in each column indicate significant differences according to the Duncan test (P≤0.05). (–) Not present in the sample; (ns) no significant difference at P≤0.05. IAA: Indole-3-acetic acid, FW: Fresh weight, GA: Gibberellic acid.
3.2. Effect of PGRs Applied at the Tillering Stage
Treatments with PGRs at the tillering stage (day 26) affected reproductive and yield traits [Figures 1 and 2]. Treatment with IAA at 50 mg/L significantly reduced (P < 0.05) the time to 50% flowering by 2.19 days compared to controls, increasing the 1000-grain weight to 27.58 g compared to the control 26.21 g. The flag leaf length (32.59 cm), area (41.47 cm2), and panicle length (23.47 cm) remained unchanged [Figure 2]. In contrast, the application of GA3 at 25 mg/L extended flowering by 2 days, whereas GA3 at 50 mg/L delayed flowering by 5.94 days. In addition, GA3 at 50 mg/L increased plant height to 97.16 cm, compared to 93.05 cm in the control, and prolonged maturity by 2.81 days, resulting in a total of 86.44 days compared to 83.63 days in the controls. However, there was no significant effect on grain weight, which remained at 26.08 g. BA at 25 mg/L (62.06 days) and 50 mg/L (62.19 days) had no significant impact on flowering time, maturity, or yield parameters. These results suggest that IAA promotes early reproductive onset and grain filling, whereas GA3 at this stage prioritizes vegetative growth.
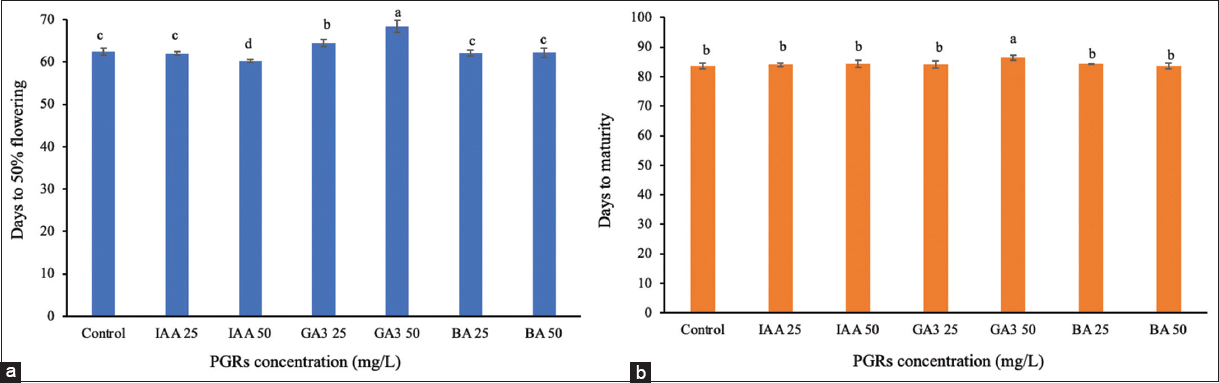 | Figure 1: Effect of plant growth regulators treatment at the tillering stage on flowering and maturity time. (a) Days to 50% flowering, (b) days to maturity. [Click here to view] |
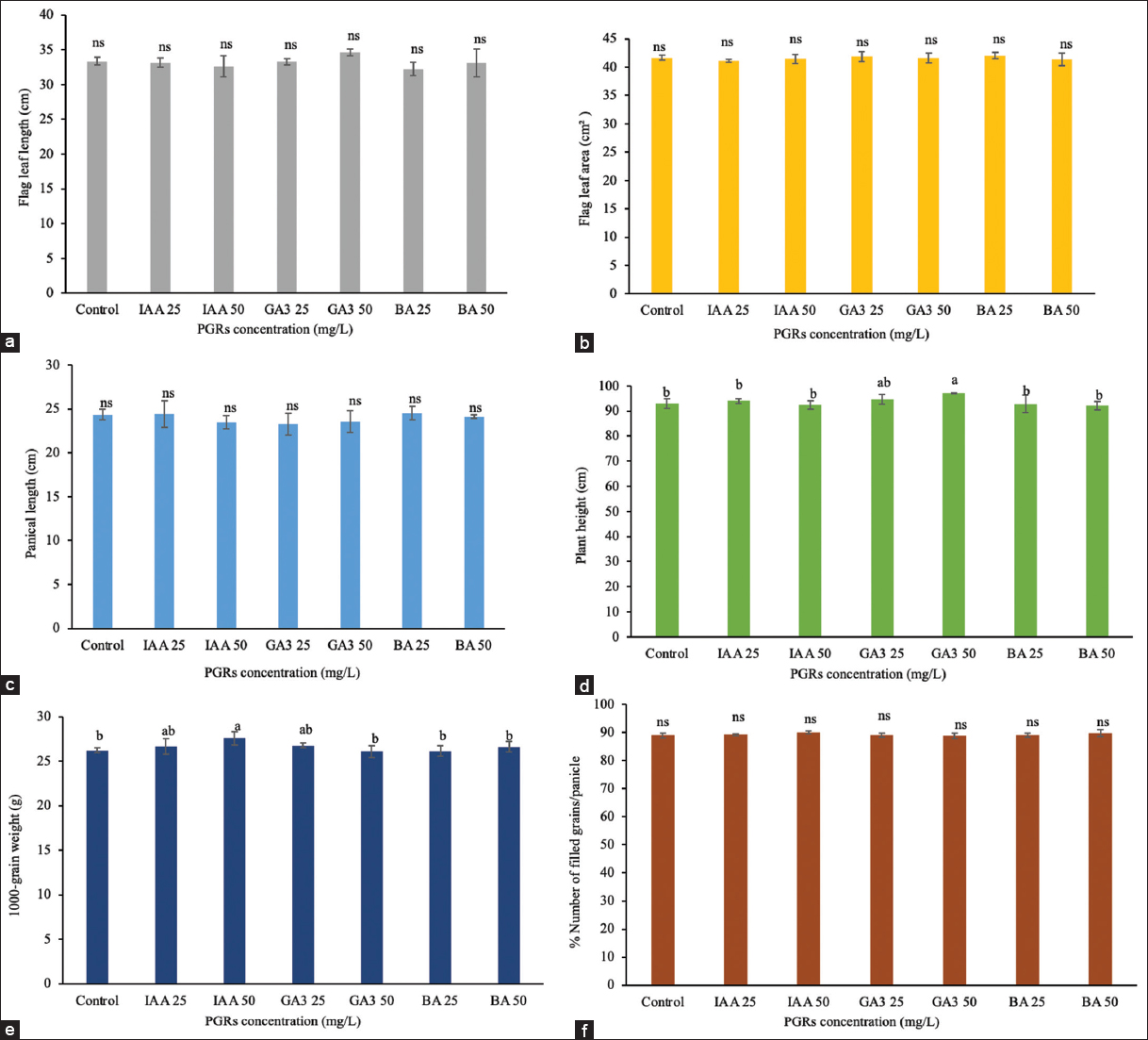 | Figure 2: Effect of plant growth regulators on the development and grain yield during tillering stages. (a) Flag leaf length, (b) flag leaf area, (c) panicle length, (d) plant height, (e) 1000-grain weight, (f) the number of filled grains per panicle. [Click here to view] |
3.3. Effect of PGRs Applied at the Pre-booting (Panicle Initiation) Stage
During the panicle initiation stage (day 36), the treatments had an impact on reproductive development [Figures 3 and 4]. IAA at 50 mg/L reduced the time to 50% flowering by 1.68 days (60.88 days compared to the control at 62.56 days), with no significant changes in flag leaf traits, maturity (83.81 days), or 1000-grain weight (26.20 g). GA3 at 50 mg/L decreased flowering time by 2.87 days, lowered maturity by 4.37 days (84.50 days in the control), increased flag leaf length to 35.58 cm, and raised plant height to 99.02 cm, whereas 1000-grain weight (26.16 g) remained unchanged. GA3 at 25 mg/L and BA at both 25 and 50 mg/L concentrations showed no significant differences from the control group in most traits. These findings suggest that GA3 enhances both reproductive development and vegetative growth traits such as flag leaf length and plant height at this stage, whereas IAA primarily accelerates the onset of flowering without affecting yield-related traits.
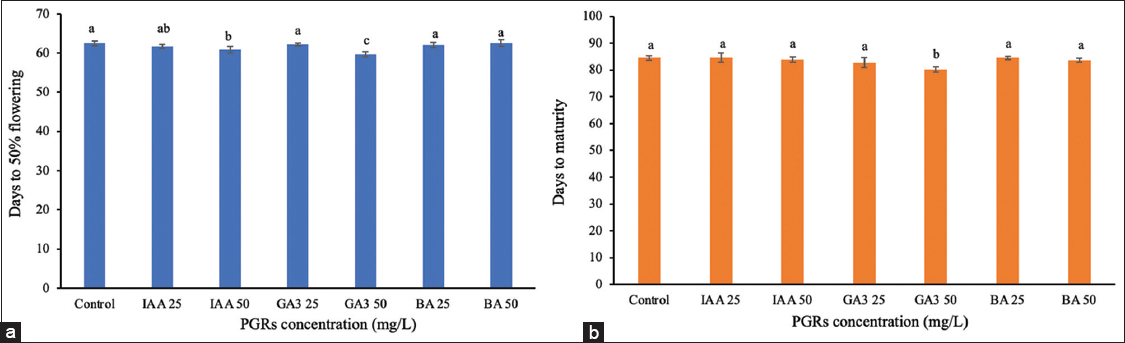 | Figure 3: Effect of plant growth regulators treatment at pre-booting stage on flowering and maturity time. (a) Days to 50% flowering, (b) days to maturity. Data are presented as the mean ± standard deviation, according to Duncan’s multiple range test. [Click here to view] |
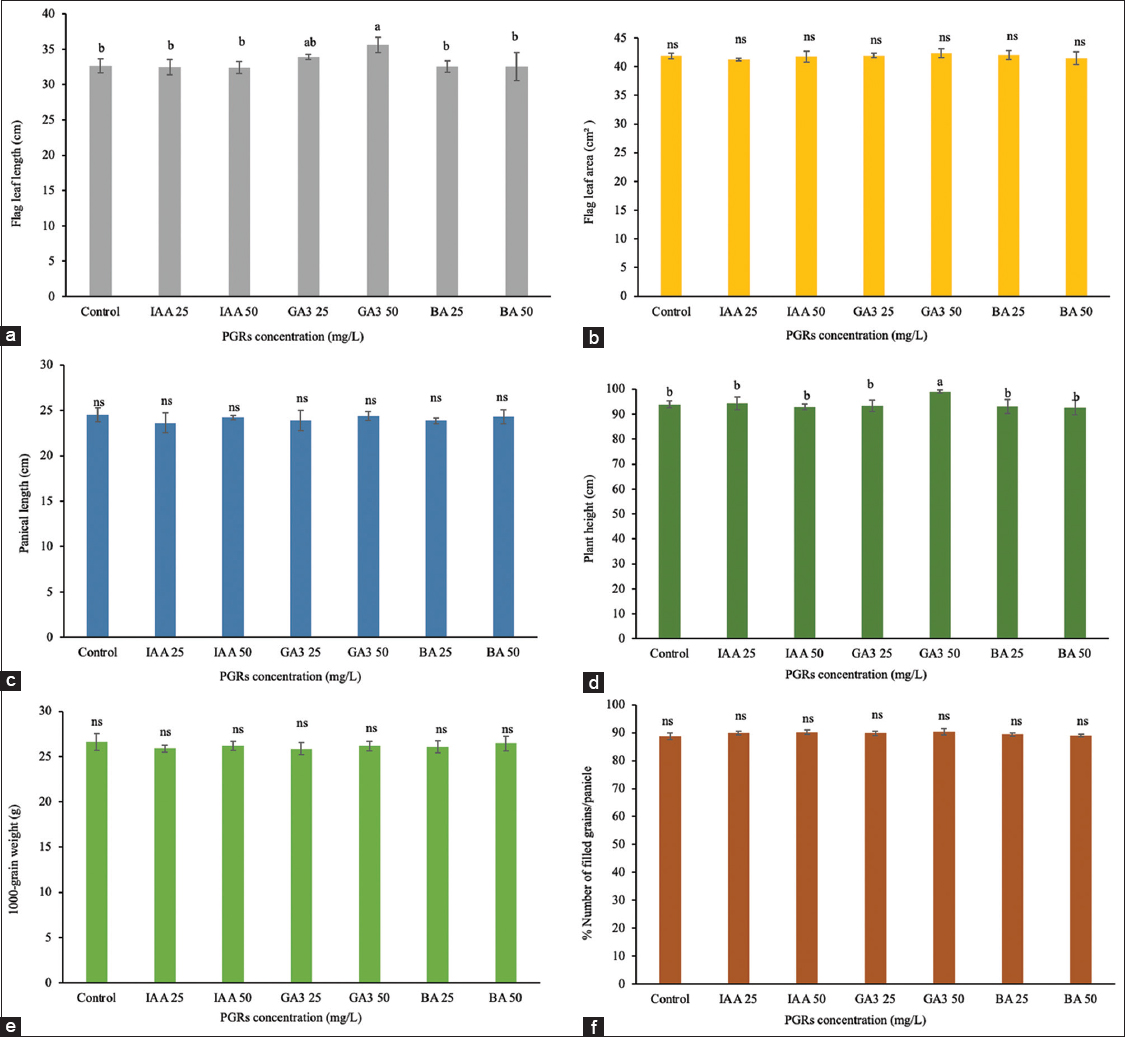 | Figure 4: Effect of plant growth regulators treatment at pre-booting stage on the development and grain yield. (a) Flag leaf length, (b) Flag leaf area, (c) panicle length, (d) plant height, (e) 1000-grain weight, (f) the number of filled grains per panicle. Data are presented as the mean ± standard deviation, according to Duncan’s multiple range test. [Click here to view] |
3.4. Effect of PGR Treated at the Booting Stage
The most significant effects were observed with treatments at the booting stage (day 46) [Figures 5 and 6]. The GA3 at 25 mg/L treatment showed significantly impacted plant development, reducing the time to 50% flowering by 4.25 days (58.13 days for treated plants and 62.38 days for controls) and shortening maturity by 6 days (77.50 days for treated and 83.50 days for controls) [Figure 7]. The treatment resulted in increased flag leaf length (37.27 cm), flag leaf area (47.41 cm2), panicle length (26.21 cm), and 1000-grain weight (28.82 g). GA3 at 50 mg/L reduced flowering time by 5.88 days and maturity by 9.06 days, while also enhancing growth parameters, such as flag leaf length (increased by 7.35 cm) and flag leaf area (increased by 10.27 cm2). The plant height rose by 14.08 cm, and panicle length grew by 3.01 cm. The percentage of filled grains increased to 91.33% from 88.59% in the control group (P ≤ 0.05). In addition, the weight of 1000-grain grew from 26.43 g to 30.40 g. Treatment with IAA at 25 mg/L and 50 mg/L, as well as BA at both concentrations, showed no significant effects on any measured traits.
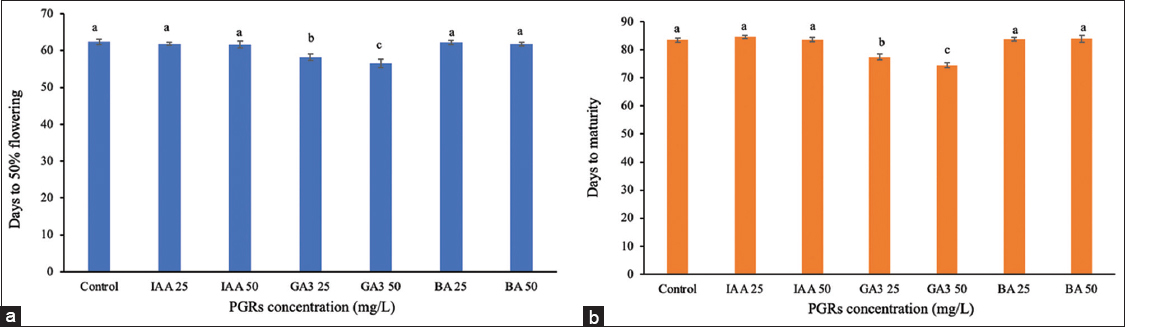 | Figure 5: Effect of plant growth regulators treatment at the booting stage on flowering and maturity time. (a) Days to 50% flowering, (b) days to maturity. Data are presented as the mean ± standard deviation, according to Duncan’s multiple range test. [Click here to view] |
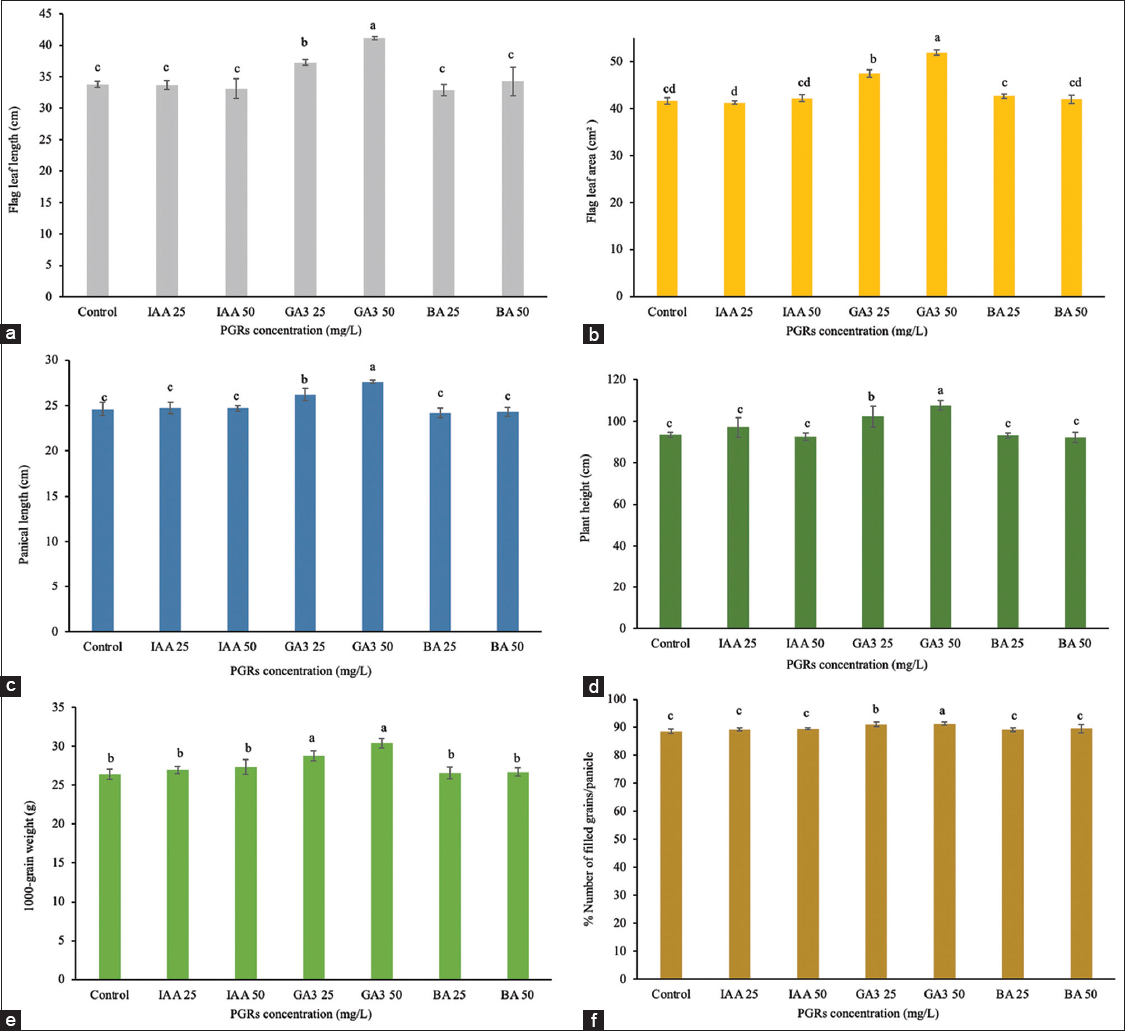 | Figure 6: Effect of plant growth regulators treatment at the booting stage on the development and grain yield. (a) Flag leaf length, (b) flag leaf area, (c) panicle length, (d) plant height, (e) 1000-grain weight, (f) the number of filled grains per panicle. Data are presented as the mean ± standard deviation, according to Duncan’s multiple range test. [Click here to view] |
 | Figure 7: Effect of gibberellic acid (GA) treatment at the booting stage on the development, 58 days after sowing. (a) Rice plants without flowers are in control, (b) rice plants with newly emerged spikelets when treated with 25 mg/L GA3, (c) rice plants with fully opened spikelets when treated with 50 mg/L GA3. (Bar: 20 cm). [Click here to view] |
4. DISCUSSION
In O. sativa cv. OM5451, IAA concentration gradually decreased through the stages from 1.45 μg/g FW at tillering to 0.91 μg/g FW at internode elongation and continued to decline at panicle initiation (0.40 μg/g FW) [Table 1]. IAA levels decreased significantly from tillering to flowering initiation, suggesting a key role for auxin in growth processes such as shoot formation and stem elongation. This decrease may facilitate the diversion of essential nutrients to reproductive structures, supporting the growth of the cotton shoot meristem, as observed in previous studies [24,25]. Similarly, GA3 exhibited the highest concentration at tillering (19.33 μg/g FW), followed by a marked decline to 1.04 μg/g FW at internode elongation and 0.48 μg/g FW at panicle initiation. GA3 showed a significant decrease during these stages, whereas GA4 was present only at tillering (8.15 μg/g FW) and was absent in later stages [Table 1]. High levels of gibberellins during tillering promote stem elongation and vegetative growth, consistent with their established functions in rice [15]. The decrease in gibberellin levels indicates the transition from the vegetative to reproductive growth phase, consistent with the timing regulation of gibberellins [21]. GA4 at tillering suggests a role in early vegetative growth as previously studied [26]. In contrast, zeatin was undetectable during the tillering stage but emerged at 0.45 μg/g FW during internode elongation, increasing slightly to 0.55 μg/g FW at panicle initiation, with no significant difference between these stages. Zeatin was not detected during tillering but increased during internode elongation and panicle initiation, suggesting an important involvement of cytokinin in reproductive development, especially in maintaining panicle formation. This increase supported flower development, as shown in a previous study [10,14]. Decreased IAA and gibberellin, and increased cZR highlight a coordinated hormonal transition from a vegetative to a reproductive priority, with implications for yield-enhancing.
Treatments with PGRs provide deeper insight into their hormonal functions and their ability to boost crop productivity. When 50 mg/L of IAA is applied during the tillering phase, flowering is hastened by 2.19 days, and the 1000-grain weight increases to 27.58 g from 26.21 g observed in untreated plants [Figures 1 and 2]. Auxin likely increases meristematic activity, triggering panicle formation, which accelerates flowering and promotes grain growth [18,20]. According to Yu et al. [27], IAA increases grain filling in young rice panicles by improving sugar transport. Auxins play a significant role in the development of vascular tissues, which function as essential conduits for the long-distance transport of assimilates [28,29]. The absence of notable changes in flag leaf dimensions or panicle length indicates that IAA mainly influences developmental timing and grain filling. Conversely, applying 50 mg/L of GA3 at the tillering stage postpones flowering by 5.94 days and maturity by 2.81 days, whereas elevating plant height to 97.16 cm [Figure 1]. This postponement stems from GA3 prioritizing vegetative growth over the start of reproduction, as elevated gibberellin levels during tillering encourage stem lengthening. Thus, our results corroborate those of Nagai et al. [30]. Treatments possibly interfering with the processes that initiate flowering, such as the results of Sun and Gubler [31].
The results of treatment at the initial stage of rice panicle formation showed the effect of PGRs at this stage [Figures 3 and 4]. When 50 mg/L IAA is administered, flowering occurs 1.68 days earlier, whereas the same concentration of GA3 cuts flowering duration by 2.87 days and maturity by 4.37 days. In addition, GA3 induces slight improvements in flag leaf length (35.58 cm) and plant height (99.02 cm). These observations imply that both phytohormones can advance reproductive timing when introduced at panicle formation. Although their effects on grain yield were not as pronounced as those of GA3 treatments applied earlier at the heading stage, these results were also reported by Yang et al. [21]. In contrast, BA at 25–50 mg/L concentrations – tested at multiple growth phases – failed to produce measurable changes in flowering, maturation, or grain weight. This lack of response may stem from inadequate dosage, suboptimal application timing, or divergent activity between synthetic cytokinins and naturally occurring zeatin, which aids panicle development without hastening flowering in rice systems [32].
The greatest improvements in crop yield are observed when GA3 is applied during the booting phase. A 50 mg/L application of GA3 reduces flowering time and maturity while increasing flag leaf length and area, plant height, panicle length, filled grain percentage, and 1000-grain weight [Figures 5 and 6]. These results indicate that GA3 enhances panicle exsertion, photosynthetic capacity through larger flag leaves, and grain filling efficiency, consistent with its documented role in promoting stem elongation and spikelet development during reproductive stages. Comparable results were also identified in a research study focused on Indica hybrid rice [33]. According to previous research, GA3 and IAA treatment at flowering reduced sterility and improved yield by elongating leaf length [34]. The accelerated maturity and higher grain weight suggest improved assimilate partitioning, likely mediated by gibberellin-induced sink strength [35]. In addition, GA3 treatment at 25 mg/L during the heading stage reduced flowering time by 4.25 days and maturity by 6 days, and increased grain weight by 28.82 g, highlighting a clear correlation between concentration and plant response to treatment. The effects of PGRs vary depending on the growth phase of the plants, highlighting their applications in agriculture. In other studies, IAA applied during tillering may enable earlier flowering, whereas GA3 treatment at late tillering stage and 10–30% flowering stage (early reproductive) increased yield by more than 27%, thus GA3 treatment at heading stage increased plant yield [36]. While IAA promotes cell division and elongation, GA3 not only stimulates these processes but also enhances the translocation of photosynthetic assimilates to developing seeds and plays a key role in inflorescence formation. These combined actions accelerate floral initiation and development, resulting in earlier flowering. Therefore, the timely application of GA3 at specific developmental stages serves as an effective strategy to regulate flowering time and improve crop yield. These outcomes corroborate existing evidence identifying auxins and gibberellins as hormones of rice reproduction [37]. This suggests that the PGR treatment was more effective at the pre-flowering stage than previously reported. These findings highlight the intricate correlations among morphological, physiological, and biochemical changes during rice grain development, providing valuable insights for optimizing growth and yield. The application of PGRs at specific developmental stages demonstrates potential in reducing the maturation period without compromising grain quality, which is particularly important in the context of climate variability and changing growing seasons.
5. CONCLUSION
In O. sativa cv. OM5451, the level of endogenous IAA and GA3 decreased from tillering to panicle initiation stage, marking the transition from vegetative to reproductive growth. GA4 levels were only detected during the tillering stage, whereas zeatin appeared during the stem elongation and panicle initiation, supporting reproductive processes. Foliar application of 50 mg/L IAA at the tillering stage advanced flowering by 2.19 days and increased the 1000-grain weight by 1.37 g, hastening reproductive onset. Treatment with 50 mg/L GA3 at booting reduced flowering and maturity durations by 5.88 and 9.06 days, respectively, and enhanced flag leaf size, panicle length, and grain weight, resulting in improved grain filling and yield. These findings demonstrate a strong correlation between the known roles of PGRs and their practical applications in agriculture. Targeted PGR application, based on agronomic analysis, significantly enhanced the development and yield. Nevertheless, further molecular analysis and large-scale field trials are necessary to validate and optimize these effects under changing environmental conditions.
6. ACKNOWLEDGMENT
The authors are grateful to the University of Science at Ho Chi Minh City, Vietnam, for providing the necessary facilities to carry out this study. The authors acknowledge the financial support from the research project (C2023-18-16) by Vietnam National University, Ho Chi Minh City (VNU-HCM), Vietnam.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data are available to the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors, and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliations.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI) tools for writing and editing the manuscript, and no images were manipulated using AI.
REFERENCES
1. Cui Z, Zhang H, Chen X, Zhang C, Ma W, Huang C, et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature. 2018;555(7696):363-6. [CrossRef]
2. Fageria NK. Yield physiology of rice. J Plant Nutr. 2007;30(6):843-79. [CrossRef]
3. Seck PA, Diagne A, Mohanty S, Wopereis MC. Crops that feed the world 7:Rice. Food Secur. 2012;4(1):7-24. [CrossRef]
4. Shafi S, Shafi I, Zaffar A, Zargar SM, Shikari AB, Ranjan A, et al. The resilience of rice under water stress will be driven by better roots:Evidence from root phenotyping, physiological, and yield experiments. Plant Stress. 2023;10:100211. [CrossRef]
5. Matsushima S. Crop Science in Rice:Theory of Yield Determination and Its Application. Tokyo:Fuji Publishing;1970.
6. Dunand R, Saichuk J. Rice growth and development. In:Saichuk J, editor. Louisiana Rice Production Handbook, 2321. Baton Rouge LA, USA:Louisiana State University Agricultural Center;2014. 14.
7. Yoshida S. Fundamentals of Rice Crop Science. Philippines:International Rice Research Institute;1981.
8. Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309(5735):741-5. [CrossRef]
9. Fang J, Zhang F, Wang H, Wang W, Zhao F, Li Z, et al. Ef-cd locus shortens rice maturity duration without yield penalty. PNAS. 2019;116(37):1?-22. [CrossRef]
10. Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018;145(4):dev149344. [CrossRef]
11. Linh TM, Huong TT, Viet BT. Study on the flowering in rice plant (Oryza sativa cv. OM5451). Sic Technol Develop J. 2023;26(3):2943-9. [CrossRef]
12. Yano M, Kojima S, Takahashi Y, Lin H, Sasaki T. Genetic control of flowering time in rice, a short-day plant. Plant Physiol. 2001;127(4):1425-9. [CrossRef]
13. Zhang H, Li H, Yuan L, Wang Z, Yang J, Zhang J. Post-anthesis alternate wetting and moderate soil drying increases grain yield and reduces cadmium accumulation in rice. Field Crops Res. 2016;196:186-93. [CrossRef]
14. Yang J, Peng S, Visperas RM, Sanico AL, Zhu Q, Gu S. Grain filling pattern and cytokinin content in the grains and roots of rice plants. J Plant Growth Regul. 2000;30(3):261-70. [CrossRef]
15. Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134(4):1642-53. [CrossRef]
16. Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, et al. Green revolution:A mutant gibberellin-synthesis gene in rice. Nature. 2002;416(6882):701-2. [CrossRef]
17. Yang J, Zhang J, Wang Z, Zhu Q, Liu L. Water deficit-induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. J Agron. 2001;93(1):196-206. [CrossRef]
18. Jia L, Dai Y, Peng Z, Cui Z, Zhang X, Li Y, et al. The auxin transporter OsAUX1 regulates tillering in rice (Oryza sativa). J Integr Agric. 2024;23(5):1454-67. [CrossRef]
19. Wang Y, Tao Z, Wang R, Zhao Y. Recent advances in auxin research in rice and their implications for crop improvement. J Exp Bot. 2018;69(2):255-63. [CrossRef]
20. Pal P, Ansari SA, Jalil SU, Ansari MI. Regulatory role of phytohormones in plant growth and development. Plant Horm Crop Improv. 2022;???:1-13. [CrossRef]
21. Yang J, Zhang J, Wang Z, Zhu Q, Liu L. Hormonal regulation of reproductive phase transitions in cereals. Trends Plant Sci. 2022;27(3):280-94. [CrossRef]
22. Islam MM, Kayesh E, Zaman E, Urmi TA, Haque MM. Evaluation of rice genotypes for drought tolerance at germination and early seedling stage. Agriculturists. 2018;16(1):44-54. [CrossRef]
23. Nakagawa K, Kiko T, Hatade K, Asai A, Kimura F, Sookwong P, et al. Development of a high-performance liquid chromatography-based assay for carotenoids in human red blood cells:Application to clinical studies. Anal Biochem. 2008;381(1):129-34. [CrossRef]
24. Patel R, Mohapatra PK. Stimulatory effect of GA3 and IAA on ripening process, kernel development and quality of rice. PJBS. 1996;2(3):410-12. [CrossRef]
25. Mir AR, Siddiqui H, Alam P, Hayat S. Foliar spray of auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol Mol Biol Plants. 2020;26:2503-20. [CrossRef]
26. López-Cristoffanini C, Serrat X, Jáuregui O, Nogués S, López-Carbonell M. Phytohormone profiling method for rice:Effects of GA20ox mutation on the gibberellin content of japonica rice varieties. Front Plant Sci. 2019;10:733. [CrossRef]
27. Yu Y, Xu X, Hu Y, Ding Y, Chen L. Indole-3-acetic acid (IAA) and sugar mediate endosperm development in rice (Oryza sativa L.). Rice. 2024;17(1):66. [CrossRef]
28. Burian A, Raczy?ska-Szajgin M, Pa?ubicki W. Shaping leaf vein pattern by auxin and mechanical feedback. J Exp Bot. 2021;72(4):964-7. [CrossRef]
29. Su J, Cui W, Zhu L, Li B, Li M. Response of carbohydrate metabolism-mediated sink strength to auxin in shoot tips of apple plants. J Integr Agric. 2022;21(2):422-33. [CrossRef]
30. Nagai K, Mori Y, Ishikawa S, Furuta T, Gamuyao R, Niimi Y, et al. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature. 2020;584:109-14. [CrossRef]
31. Sun TP, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004;55:197-223. [CrossRef]
32. Cho LH, Yoon J, Tun W, Baek G, Peng X, Hong WJ, et al. Cytokinin increases vegetative growth period by suppressing florigen expression in rice and maize. Plant J. 2022;110(6):1619-35. [CrossRef]
33. Wang X, Zheng H, Tang Q, Mo W, Ma J. Effects of gibberellic acid application after anthesis on seed vigor of indica hybrid rice (Oryza sativa L.). Agronomy. 2019;9(12):861. [CrossRef]
34. Awan IU, Baloch MS, Sadozai NS, Sulemani MZ. Stimulatory effect of GA3 and IAA on ripening process, kernel development and quality of rice. Pakistan J Biol Sci (Pakistan). 1999;2:410-2. [CrossRef]
35. Marcelis LF. Sink strength as a determinant of dry matter partitioning in the whole plant. J Exp Bot. 1996;47:1281-91. [CrossRef]
36. Haifaa MD, Moses C. Effects of foliar and soil application of gibberellic acid (GA3) at different growth stages on agronomic traits and yield of rice (Oryza sativa L.). J Agric Sci. 2022;14(6):55. [CrossRef]
37. Mohapatra PK, Sahu BB. Hormonal regulation of spikelet development. In:Panicle Architecture of Rice and Its Relationship with Grain Filling. Germany:Springer;2022. 167-89. [CrossRef]