1. INTRODUCTION
Rice (Oryza sativa) a cereal generally famous because of its rich starch content and high dietary calories is widely cultivated throughout the world and it has become a major staple food because of its low price [1]. On the other side, a huge quantity of paddy fields and starchy material crops like corn and potatoes yielded are spoiled every year in India untimely due to natural disasters, insufficient storage, transport, and lack of technology. From 2015 to 2020 about 0.002–0.014 percent of food grains like rice and wheat were wasted according to the reports by India Today. Mismanagement along with scarcity of resources such as polythene covers for protection against winter dew as well as moisture and most of the time the grains being already damaged due to rice bags being torn while piled up on each other as well as spillage and so on, contribute to wastage are some of the main causes of damage to rice grains. The spoilage includes discoloration, breaking down, splitting, fungal and bacterial spoilage, insect infestation, becoming dusty, grain being soggy and bad smelled, and so on [2]. These damaged cereals are used as renewable biomass because of the high starch suitable for bioethanol production.
The United States of America is the world’s most leading producer of bioethanol with 15.77 billion gallons of production in 2018 as reported by the U.S. Grains Council. The global ethanol production of 84% is occupied by the United States and Brazil. The large majority of Brazil predominantly uses sugarcane while corn ethanol is produced in the USA. Rice (Oriza sativa) being the third most staple grain crop which is an alternative and abundant in the world against be 530 million gallons, with additional ethanol consumption of 730 million gallons’ reported in 2019 [3]. To reduce the import dependency of petro-based fuels, the Indian government has executed National Policy on Biofuels-2018, with an ambition of 10% ethanol blending by 2022 [3,4].
Starch is a polysaccharide and during the heating process, it undergoes gelatinization and hydrolysis to convert into simple sugars like glucose [5]. Rice starch can be hydrolyzed using enzymes, secreted by several fungal species [6] bacteria [7], and some yeast [8] producing amylase and glucoamylase. Recombinant modified tetraploid Saccharomyces cerevisiae can express amylase and glucoamylase enzymes during fermentation to produce ethanol [9]. Three steps are practically involved in extracting glucose from starch which include Gelatinization, liquefaction (at 80°C to 125°C), and saccharification (at 55°C to 65°C).
In the first step of Gelatinization, the starch grains along with excess water are heated which increases the accessibility to enzyme and amorphous amylopectin region. Amylases carry out liquefaction by hydrolyzing the α-(1-4) chemical bond of starch which produces Maltose, Dextrin, Maltotriose, and Maltopentoses with a less than 30 dextrose equivalent [10,11]. Saccharification by amylase enzymes in the fermentation (at 30°C to 35°C) is used to produce ethanol [9].
Multi-step processes can be merged up to carry out in a single-step process which includes pretreatment, hydrolysis, and fermentation during the biomass bioconversion to bioethanol. A mixture of two or more enzyme combinations as cocktails in a determined ratio can be applied to lignocellulose biomass for conversion into fermentable sugars [12,13]. The synergistic action of enzymes enhances biomass conversion and liberates excess fermentable sugars under moderate conditions reducing the extremity of hydrolysis when compared methods involving to Physico-chemical techniques [14,15].
Bio-catalytic methods show significant benefits such as they are highly specific, have no toxic/inhibitors generation, a limited equipment, limited demand for costly and sophisticated equipment, environmentally favorable methods, and bear the potential to be commercially exploited. The enzyme cocktail employment helps in enhancing the yield of sugar upon action by two or more enzymes synergistically and helps in preserving the enzyme hydrolysis can be carried out at more economical quantities of the enzyme, when compared to the large quantities of single enzyme being employed in excess [16]. Most biological methods depend on starchy material conversion into glucose from grains and then into bioethanol which includes three steps as follows: liquefaction of starch at 80°C to 125°C, Saccharification at 55°C to 65°C, and Fermentation at 32°C to 35°C to convert sugar into bioethanol [2].
Since the availability of resources or utilities or energy being factors of concern to the industries, the saccharification step has been decreased recently as the above-mentioned factors directly influence the production costs. Liquefaction as well as simultaneous saccharification and fermentation (SSF) are the enhanced steps in the starch conversion process where the enzyme carrying out saccharification hydrolyses the liquefied starch into the fermentable sugars followed by bioethanol production [17].
The novelty of this study compares the two different commercial enzymes, S1 and S2, on damaged rice starch and identifies the maximum glucose-liberating enzyme and ethanol conversion by the fermentation process, comparing it with mesophilic yeast GSR (NCIM 3640) and thermotolerant yeast OBC 14 [3,15]. Novozymes commercial amylases have shown effective glucose liberation and better ethanol yield by thermotolerant yeast OBC14. This is accomplished by accounting for the improvement in the conversion rate from total sugar to ethanol and further gives ideology for the biorefinery concept to utilize spent slurry for other byproducts. Unlike previous studies on the production of bioethanol from rice grain flour, this study solely focuses on single and double digestion of two different enzyme sources with thermotolerant yeast fermentation for bioethanol production.
2. MATERIAL AND METHODS
2.1. Rice Flour
Damaged rice grains of variety BPT 5204, a summer crop harvested from agriculture fields of Suryapet district, Telangana, India, were obtained in 2017, ground to fine powder of 90–120 µm particle size, oven dried at 45°C and the chemical composition of rice flour was analyzed by the method of McCleary et al. [18] and it was found to have total solids (0.73 g/g), total starch (0.54 g/g), reducing sugars (0.11 g/g), total nitrogen (58.0 mg/g), and ash content (49.7 mg/g), respectively [19,20].
2.2. Commercial Enzymes
Two common commercial enzymes Alpha-amylase and Glucoamylase were obtained from two different companies S1 and S2. Alpha-amylase 15,000 units/g and Glucoamylase 1,50,000 units/g from S1 company, Alpha-amylase 240 units/g, glucoamylase 1,000 units/g in S2 company were compared with two different companies enzyme activity on rice flour.
2.2.1. Enzyme acquired from local market (S1)
Dilute the stock enzyme solution (1,50,000 units) with acetate buffer pH 4.6 and add 25 units to alpha amylase to 50 ml digested flask.
2.2.2. Novozymes (S2)
Dilute stock enzyme 1,000 units to 25 units supplement to a pretreated flask, before adjustment with acetate buffer pH 4.6 and incubation at 60°C for 1 hour at 150 rpm.
2.3. Medium and Culture
The two glucose fermenting yeast strains (Saccharomyces cerevisiae) NCIM 3640 and OBC14 thermotolerant yeasts were selected and maintained in the YEPD agar (Yeast extract 1%, Peptone 2%, Dextrose 2%, Agar-Agar 2.5%) [21]. A loop full of culture inoculated in YEPD broth from overnight culture the growth was monitored at different time periods by measuring OD at 600 nm and the inoculum was adjusted to 10% culture after diluting it to 0.6 OD at 30°C at 150 rpm.
2.4. Single Digestion (Two step digestion)
2.4.1. Alpha-amylase
Sodium acetate buffer was prepared at a concentration of 0.1 M, and to that, 20 g of rice flour was added to make a slurry with pH 6. S1 enzyme solution contains around 15,000 units/gm and S2 enzyme contains 240 KNU/gm dilute two enzymes up to 50 units using buffer. 50 units of diluted enzyme subjected into 50 ml of substrate containing 40% substrate load. Two milliliters of aliquot were taken in Eppendorf’s for 0 hour sample. Incubate at 90°C for 4 hours shaking at 150 rpm. Sugar was estimated by dinitrosalicylic acid (DNS) and high performance liquid chromatography (HPLC) at 0 hour & after 4 hours incubation along with glucose standard.
2.4.2.Gluco amylase
The pH of the above samples was adjusted with acetic acid to pH 4.6 with pH meter. S1 and S2 gluco amylase enzymes were added to the pH-adjusted samples. Initially, 2 ml of samples were collected at 0 hour and incubated at 60°C for 1 hour with shaking at 150 rpm, and sugars liberated from both 0 & 1 hour samples were estimated using both DNS and HPLC methods.
2.5. Double Digestion (One Step Digestion)
In a double digestion method take two flasks with 20 g rice flour each and add 50 ml of Na-acetate buffer with pH.5.0 add the same amount of enzymes as mentioned in single digestion of both enzymes for each of S1 and S2. Take 2 ml of sample after adding enzyme at 0 hour and incubate at 70°C for 4 hours with shaking. Estimate 0 and 4 hours samples for sugars release by DNS method and further save all the samples for HPLC analysis. You will have two 0 hour and two after incubation samples for each digestion and for each company. 4 samples for amylase, 4 for gluco amylase, and 4 for double digestion, a total of 12 samples for 1 experiment. While estimating by DNS, you have to dilute each sample ten times and take 0.05 and 0.100 ml for analysis in duplicate. Accordingly, you can dilute further or reduce dilution so that your OD will be in between 0.200 and 0.500 approximately.
2.6. Statistical Analysis
Experiments were conducted in triplicates for three times (n = 9) and the average of these values was noted. The standard deviation was calculated as ≤5%.
3. RESULTS AND DISCUSSIONS
In India, as a staple food, an abundance of rice straw is available even after 50% of it is used as fodder. Among feed stocks, rice straw yields more bioethanol production (230 l/ton) than others [22]. Rice straw is more available worldwide, particularly in Asian countries which is around 668 million metric ton (MT) yielding 282 billion liters theoretically if technology available [23]. But Bioethanol production needs more fossil energy 27% to 118% than ethanol produced from different feedstocks [24]. Therefore, the use of physical and enzymatic methods can lower these costs particularly if powerful enzymes at a cheaper rate are available.
3.1. Hydrolysis Performance of Enzymes on Brewers Rice (Single Digestion and Double Digestion)
The studies were conducted using α-amylase and glucoamylase for hydrolysis of the starch from the flour to liberate glucose which can be used to extract bioethanol. Preliminary studies were conducted to optimize the concentrations of the amount of flour, the enzymes, and the optimum time needed for maximum liberation of the sugar. It was found that up to 40% of the flour concentrations can be easily used without gelling for easy slurry. Favaro et al. [25] also reported good digestion of up to 30% of flour. Studies were conducted with optimal conditions using the enzymes on rice flour of broken rice. The hydrolysis of starch to glucose by two-step digestion is 81% to 94% which is higher than that of cassava as reported by Papathoti et al. [26] in the case of saccharification efficiency and that of one-step digestion is 52% to 72% which is lower (Table 1). S1 enzymes produced more glucose (20%–22%) than S2. These results are consistent with starches from sweet potato and cassava [25,26]. The glucose liberated from both sources was better utilized (95%–98%) during fermentation (Fig. 1a–c). The fermentation efficiency in terms of ethanol yield is from 89% (S1) and 96 % (S2). The sugar produced by S2 is better utilized than S1. When S1 glucoamylase is added to the fermentation medium along with the strain OBC 14, it is observed that glucose was utilized fast in 24 hours (96%) and 99% in 36 hours (Fig. 1d). In one-step digestion, both glucose utilization and ethanol liberation were good but two-step utilization was better when compared. The results show that commercial enzymes yielded more amount of fermentable sugars even at low temperatures 65°C–90°C compared to heat and acid treatments at high temperatures (121°C–140°C). Thus, the commercial enzyme activities of S1 and S2 were better than those of enzyme activities studied by Ntaikou et al. [27]. When compared to the conventional processes which include greater temperatures for Liquefaction, theoretically, 56.7 g of bioethanol should be generated from 100 g of starch at maximum yields considering that starch is converted into glucose completely. With 89% to 96% fermentation efficiency, the ethanol production is around 504 to 544 l per MT which is comparable to other studies.
3.2. Fermentation of Brewers Rice Into Ethanol Using Meophilic and Thermotolerant S. cerevisiae
Technology development in the biofuel sector must take industrial viability into account. Noteworthy, both strains exhibited fermenting performance on total sugars into ethanol, a benchmark of up to 36 hours of fermentation. The HPLC analysis revealed the concentrations of glucose utilization with bioethanol production at starting of fermentation in the case of NCIM 3640 where initial concentrations of glucose and bioethanol were 89.5 and 0.28 g/l at 0 hour, respectively, and the glucose consumption increased with an increase in bioethanol production exponentially up to 24 hour after which the bioethanol production slowed down due to scarcity of glucose in the media leaving 0.31 g/l of glucose and 40.72 g/l of bioethanol concentrations by the end of 36 hours (Table 2). For OBC 14, the efficiency was 96% with 25.5 g/l bioethanol production with glucose utilization of 52 g/l initially to 0.83 at the end of fermentation. The bioethanol production by S2 digested rice flour is shown in Table 3. Here in the case of NCIM 3,640, the bioethanol concentrations increased from 0 to 34.59 g/l with glucose utilization from 71 to 0.034 g/l initially from 0 to 36 hours of the fermentation process and the fermentation efficiency was 96% at the end of the process. For OBC 14, the glucose utilization and bioethanol production ratio with an increase in time acted upon by S1 enzyme. Initially, glucose and bioethanol concentrations were 59.55 and 0 g/l correspondingly and by the end of fermentation, the bioethanol production was up to 30.75 g/l with decreasing concentrations of glucose to 0.1 g/l due to rapid utilization. The bioethanol production efficiency was 98% at the end of the process. Treatments like washing-off the acid can be avoided by this method thus lessens costs and pollution. These studies are encouraging for more utilization of damaged, broken rice for more production of bioethanol provided these grains are available at lower costs around Rs.10 to 15/kg if purchased in bulk. So the overall fermentation efficiency of S. cerevisiae strains NCIM 3640 (GSR) and OBC14 showed 89%–96% and 96%–98% with S1 and S2 enzymes, respectively, which were higher than reports shown by Ntaikou et al. [27], Mandade and Shastri [28] and Hashem et al. [18,19]. Also, the two strains were better in the utilization of glucose and production of bioethanol when compared to other strains such as Pichia anomala [27] S. cerevisiae, Pichiabarkeri, Candida intermedia [19] Kluyveromyces marxianus MTCC 4139 strain [26].
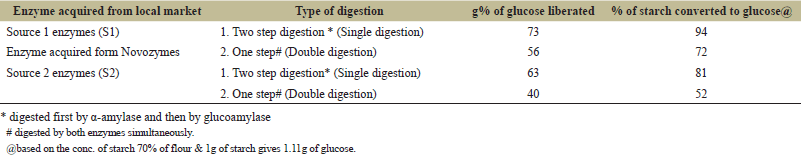 | Table 1. Effect of commercial amylase & gluco-amylase on hydrolysis of starch from flour of broken rice. [Click here to view] |
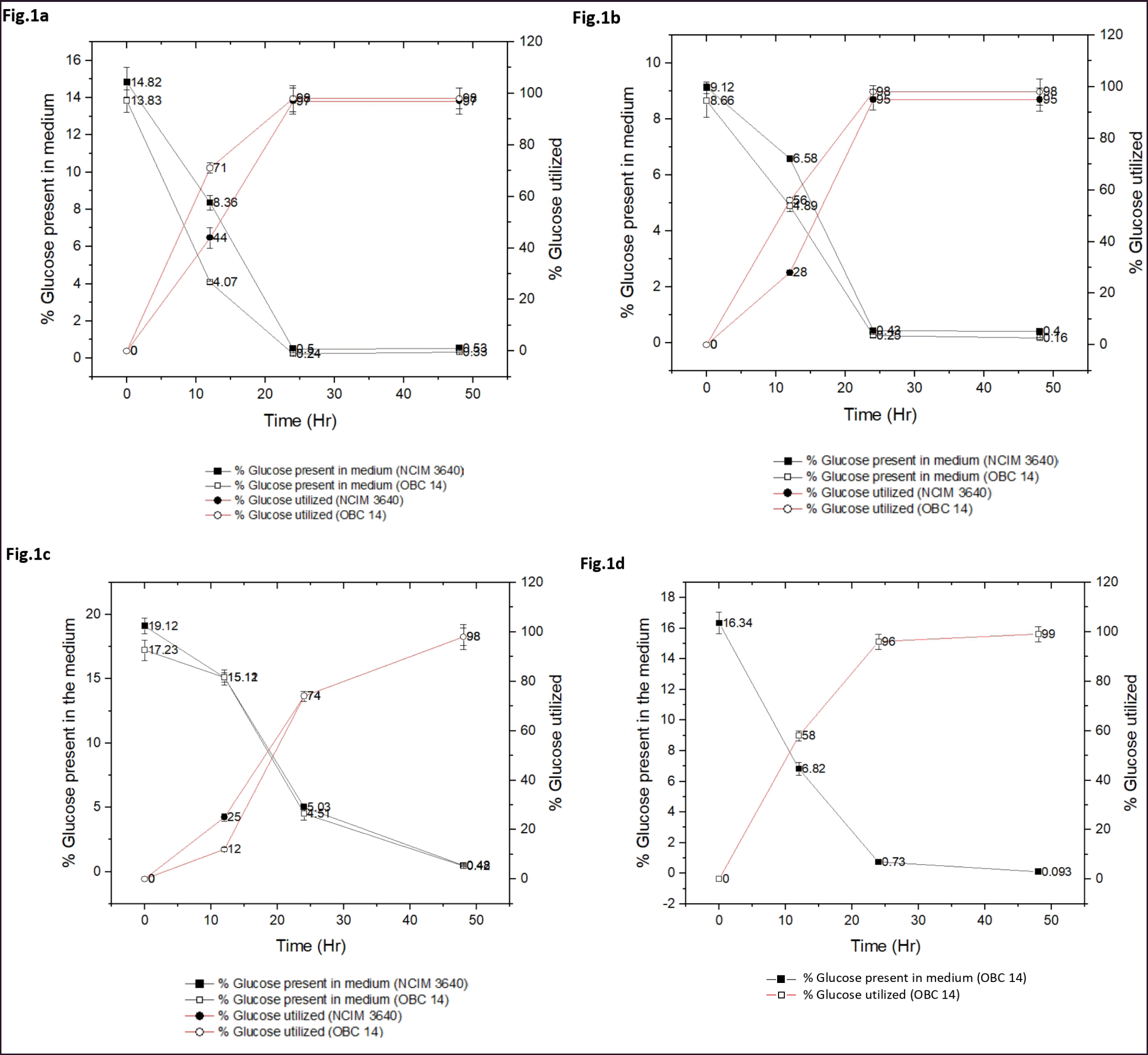 | Figure 1. (a) Effect of fermentation on production of bioethanol from hydrolysed rice flour (S1 source). Saccharomyces cerevisiae NCIM 3640 (GSR) versus S. cerevisiae OBC 14 (DNS). (b) Effect of fermentation on production of bioethanol from hydrolysed rice flour (S2 source). Saccharomyces cerevisiae NCIM 3640 (GSR) versus S. cerevisiae OBC 14 (DNS). (c) Source S1 double digestion fermentation by S. cerevisiae NCIM 3640 (GSR) versus S. cerevisiae OBC 14 (DNS). (d). Addition of additional Gluco-amylase to the fermentation medium fermented by S. cerevisiae OBC 14 (DNS). [Click here to view] |
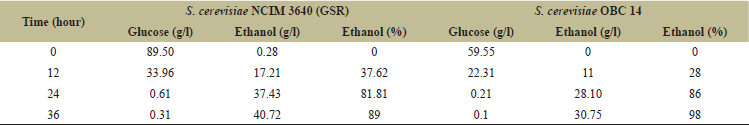 | Table 2. Effect of fermentation on production of bioethanol for S1 digested rice flour by S. cerevisiae NCIM 3640 (GSR) & S.cerevisiae OBC 14 [HPLC]. [Click here to view] |
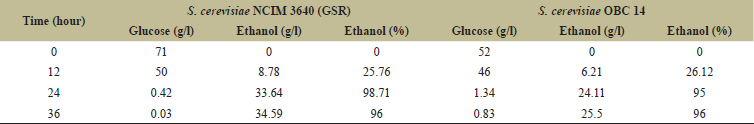 | Table 3. Effect of fermentation on production of bioethanol for S2 digested rice flour by S. cerevisiae NCIM 3640 (GSR) & S. cerevisiae OBC 14 [HPLC]. [Click here to view] |
With the increasing demand for fossil fuel, the concept of gasoline blending with ethanol is being aggressively promoted by the Government of India to decrease petrol dependency. Each year approximately 500 Million liters of ethanol would be required even considering the 10% ethanol blending with Gasoline. (http://www.gujagro.org/agro-foodprocessing/molasses-base-alcohol-34.pdf). Eco-friendly and highly sustainable energy resources have turned out to be a requirement for developing countries like India which is presently the fourth highest energy end-user worldwide with a 5.6% annual growth rate in the energy demand aspect. The market involving refined items increases to a greater degree. By 2030, with a growth rate of 5.8% demand for diesel would increase which may touch 65 MT, respectively. Employing bioethanol and biodiesel instead of Gasoline and Diesel is being promoted by transport sectors considering the environmental impacts. The blending of bioethanol into gasoline has been suggested for the Indian context considering various aspects of blending requirements like the feedstock availability as well as the effect in data variation and impact of increased demands for gasoline are assessed keeping in view various purposes [28].
The raw rice straw is cheaper at Rs.600 to 700/ton and the theoretical ethanol cost will be Rs.700/430 l = Rs.1.62/l. To this, we have to add for transportation, chemicals, and other requirements of water, energy, pollution-causing treatments, and so on. The value employing Grain-based technology for ethanol production is INR 23 to 28 /liter against methods involving molasses here molasses-based technology needs high pretreatment and saccharification costs. Currently, Grain based technology is near to molasses-based technology, INR 20 to 23/l is the production cost from molasses (1.0 INR = 0.0225683 USD), which is a little higher when compared to Brazil employing molasses, i.e., INR 14 to 16/l [29].
Reports show that in conventional processes, the Indian ethanol producers with capacities of 110–130 MT of broken rice (68% starch, 28% dry solids) consume 49.5 MT of steam during liquefaction steps to cook the Indian broken rice followed by saccharification simultaneously (SSF). Yeast fermentation processes yielded 410 l of Bioethanol /MT of broken rice at 10% V/V at 20°C with a fermentation efficiency of 86% [30]. As far as enzymes are concerned, the total cost of enzyme per liter of alcohol produced is 45 to 50 paisa as per manufacturer. A number of plants having distilleries involving molasses have converted to cereal grains-based plants for ethanol production.
4. CONCLUSION
The present study shows the feasibility of using industrial enzymes which can reduce the costs of bioethanol production. In our study, very small quantities of enzymes yielded a good amount of fermentable sugars 81%–94% conversion in the case of broken rice. Without giving up food demands, alternatives such as grasses, woods, agricultural wastes, damaged rice grains as well as unconventional raw materials like micro-algae remain as reliable solutions for economically feasible ethanol production. The present study suggests that two different amylolytic enzyme sources (α-amylase and glucoamylase) of two different companies improved to liberate maximum sugars from rice flour hydrolysate during fermentation. The data suggest that among two different fermentations (Single and double digestion) two step single digestion showed good saccharification with 98% ethanol fermentation by thermotolerant S. cerevisiae strain OBC14 with the highest ethanol yield as compared to double digestion.
5. ACKNOWLEDGMENT
Authors are grateful to the Rashtriya Uchchatar Shiksha Abhiyan (RUSA-2.0), for providing financial assistance to the Centre for Microbial and Fermentation Technology (CMFT), Department of Microbiology, University College of Science, Osmania University, Hyderabad.
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zhang H, Wu F, Xu D, Xu X. Endogenous alpha-amylase explains the different pasting and rheological properties of wet and dry milled glutinous rice flour. Food Hydrocoll 2021;113:106425; CrossRef
2. Mapiemfu-Lamare D, Tang EN, Douksouna Y, Ngome AF, Suh C, Tatah BN, et al. Post-harvest constraints: fungi and insects responsible for rice (Oryza spp) losses during storage in Cameroon. Agri Sci 2023;14:785–803; CrossRef
3. U. S. Grains Council. Global ethanol supply & demand. 2019. Available via https://grains.org/wp-content/uploads/2020/09/Global_ethanol_consumption_gallons.pdf (Accessed 06 April 2021)
4. Das S. The national policy of biofuels of India-a perspective. Energy Policy 2020;143:111595; CrossRef
5. Jasim A, Al-Jassar S, Thomas LA. Comparison in rheological, thermal, and structural properties between Indian Basmati and Egyptian Giza rice flour dispersions as influenced by particle size. Food Hyd 2015;48:72–83; https://doi.org/10.1016/j.foodhyd.2015.02.012
6. Braia M, Cabezudo I, Barrera VL, Anselmi P, Meini MR, Romanini D. An optimization approach to the bioconversion of flour mill waste to α-amylase enzyme by Aspergillus oryzae. Process Biochem 2021;111(1):102–8; CrossRef
7. Rakaz MA, Hussien MO, Ibrahim HM. Isolation, extraction, purification, and molecular characterization for thermostable α-amylase from locally isolated Bacillus species in Sudan. Biochem Res Int 2021;2021:6670380; CrossRef
8. Al Halim LRA, Hemeda NF, Serag AM. Isolation, characterization, and screening of yeast biodiversity for multi- hydrolytic enzymes. J UmmAl-Qura Univ Appll Sci 2024;10:474–84; CrossRef
9. Gronchi N, De Bernardini N, Cripwell RA, Treu L, Campanaro S, Basaglia M, et al. Natural Saccharomyces cerevisiae strain reveals peculiar genomic traits for starch-to-bioethanol production: the design of an amylolytic consolidated bioprocessing yeast. Front Microbiol 2022;12:768562; CrossRef
10. Katanski A, Vucurovi? V, Vucurovic D, Bajic B, SaranovicZ, Seres Z, et al. Bioethanol production from A-starch milk and B-starch milk as intermediates of industrial wet-milling wheat processing. Fermentation 2024;10(3):144; CrossRef
11. Wangpor J, Prayoonyong P, Sakdaonnarong C, Sungpet A, Jonglertjunya W. Bioethanol production from cassava starch by enzymatic hydrolysis, fermentation and ex-situ nanofiltration. Energy Procedia 2017;138:883–8; CrossRef
12. Yeye Lu, Chae M, Vasanthan T, Bressler DC. The potential of fiber-depleted starch concentrate produced through air currents assisted particle separation of barley flour in bio-ethanol production. Bioresour Technol 2020;303:122942; CrossRef
13. Banoth C, Sunkar B, Tondamanati PR, Bhima B. Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 2017;7:334. CrossRef
14. Keshav PK, Banoth C, Ananthapagudem A, Linga VR, Bhima B. Sequential acid and enzymatic saccharification of steam exploded cotton stalk and subsequent ethanol production using Scheffersomyces stipitis NCIM 3498. Ind Crops Prod 2018;125:462–7; CrossRef
15. Chamoli P, Jhildiyal S, Agrawal P, Kumar N, Singh P. Recent advances in the processing of Napier grass (Pennisetumpurpureum Schumach) as a potential bioenergy crop for bioethanol production. J App Biol Biotech 2024;12(4):144–57; CrossRef
16. Bhardwaj N, Kumar B, Agrawal K, Verma P. Bioconversion of rice straw by synergistic effect of in-house produced ligno-hemicellulolytic enzymes for enhanced bioethanol production. Bioresour Technol Rep 2020;10:100352; CrossRef
17. Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, et al. Applications of microbial enzymes in food industry. Food Technol Biotechnol 2018;56(1):16–30; CrossRef
18. McCleary BV, Gibson TS, Mugford DC. Measurement of total starch in cereal products by amyloglucosidase-amylase method: collaborative study. Inter J AOAC 1997;80:571–9.
19. Hashem M, Alamri SA, Asseri TAY, Mostafa YS, Lyberatos G, Ntaikou I. On the optimization of fermentation conditions for enhanced bioethanol yields from starchy biowaste via yeast co-cultures: sustainability. 2021;13(4):1890; CrossRef
20. Puncha -arnon S, Uttapap D. Rice starch vs. rice flour: differences in their properties when modified by heat–moisture treatment. Carbohydr Polym 2013;91(1):85–91; CrossRef
21. Banoth C, Kethavath SN, Keshav PK, Bhukya B. Selection of stress tolerant Saccharomyces cerevisiae strains isolated from sloughing off soil for bioethanol production using Prosopis juliflora. Res J Biotech 2020;15:1–10.
22. Azarias GS, Barbosa HS, Rustiguel CB, Rosa JC, Ernandes JR, Guimarães LHS. Proteome and fermentative parameters of Saccharomyces cerevisiae CAT-1 under very high gravity fermentation (VHGF) using sugarcane juice. J App Biol Biotech 2017;5(04):006–13; CrossRef
23. Alengebawy A, Ran Y, Ghimire N, Osman AI, Ai P. Rice straw for energy and value-added products in China: a review. Environ Chem Lett 2023;21:2729–60; CrossRef
24. Devi A, Bajar S, Sihag P, Sheikh ZUD, Singh A, Kaur J, et al. A panoramic view of technological landscape for bioethanol production from various generations of feedstocks. Bioengineered 2023;14(1):81–112; CrossRef
25. Favaro L, Cagnin L, Basaglia M, Pizzocchero V, Zyl WHV, Casella S. Production of bioethanol from multiple waste streams of rice milling. Bioresour Technol 2017;224:151–9; CrossRef
26. Papathoti NK, Laemchiab K, Megavath VS, Keshav PK, Numparditsub P, Thanh TL, et al. Augmented ethanol production from alkali-assisted hydrothermal pretreated cassava peel waste. Energy Sources Part A 2021;1–11; CrossRef
27. Ntaikou I, Antonopoulou G, Lyberatos G. Sustainable second-generation bioethanol production from enzymatically hydrolyzed domestic food waste using Pichia anomala as biocatalyst: Sustainability 2021;13(1):259; CrossRef
28. Mandade P, Shastri Y. Multi-objective optimization of lignocellulosic feedstock selection for ethanol production in India. J Clean Prod 2019;231:1226–34; CrossRef
29. Pohit S, Biswas PK, Kumar R, Jha J. International experiences of ethanol as transport fuel: policy implications for India. Energy Policy 2009;37(11):4540–8; CrossRef
30. Myburgh MW, Cripwell RA, Favaro L, Zyl WHV. Application of industrial amylolytic yeast strains for the production of bioethanol from broken rice. Bioresour Technol 2019;294:122222; CrossRef