1. INTRODUCTION
The discovery of antibiotics has been one of the most significant milestones in the history of scientific discoveries ensuring the longevity of humankind. Bacterial infections constitute a significant fraction of the total infections caused in nosocomial settings worldwide and antibiotics are the drugs used to prevent and treat them [1]. However, the emergence and spread of bacteria with new antibiotic resistance mechanisms has posed a significant challenge to our ability to treat infections [2]. Even though antimicrobial resistance (AMR) develops naturally over time, a very high burden of infection combined with the overuse and/or misuse of antibiotics, poor health infrastructure, and lack of general awareness has led to a situation where the bacteria have shown resistance against many of the most effective antibiotics such as carbapenems [2-4]. At present, resistance has been observed even against the antibiotics listed under the “reserve” category by the World Health Organization (WHO) (such as colistin) necessitating the search for newer treatment options [5].
In 2017, the WHO released a priority list of pathogens that should be the targets for research and development of new therapeutic interventions [6]. Globally, seven pathogenic bacteria, referred to as the ESKAPEE group denoting Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp., and Escherichia coli are the major cause of concern due to the increase in drug resistance. In the ESKAPEE category, notorious pathogens, namely, A. baumannii (carbapenem-resistant), P. aeruginosa, (carbapenem-resistant), and Enterobacteriaceae (carbapenem-resistant and ESBL-producing) are listed as pathogens of top-most priority by the WHO (Priority 1: Critical) [6]. Murray et al. reported that in 2019, the global burden linked with drug-resistant infections analyzed across 88 pathogen–drug combinations accounted for 4.95 million deaths, among which about 1.27 million deaths were directly linked to drug resistance. Further, their analysis showed that the death rates were highest in low- and middle-income countries (such as sub-Saharan Africa), indicating that AMR bacteria are a cause of serious concern for poor countries in the world [7]. In the Indian context, as per the AMR Surveillance data for the year 2020 published by National Centre for Disease Control, Ministry of Health and Family Welfare, E. coli is the predominant strain (31%) followed by Klebsiella spp. (21%), S. aureus (17%), Pseudomonas spp. and Enterococcus spp. (both 11%), and Acinetobacter spp. (9%).
Between July 2017 and June 2021, globally, 12 antibiotics received approvals for clinical use, but only one molecule belonged to a new class of drugs (boronic acid-based β-lactamase inhibitors) [8]. Until November 2021, <20 traditional molecules were in Phases 1 and 2 and <10 molecules reached Phase 3 of the clinical development pipeline for the treatment of the WHO-priority pathogens [8]. It is noteworthy that these numbers are far lower than the number of molecules being developed for cancer treatment [9]. Further, after factoring in the failure rates of the clinical trials, and the lack of sufficient novelty in the antibiotics currently in the clinical development pipeline, at present, enough traditional small molecule-based interventions are not available to keep up with the ever-increasing burden of drug-resistant bacterial strains [10]. Therefore, there is an immense need to develop alternative intervention strategies to combat infections caused by emerging drug-resistant bacterial strains. Non-traditional strategies may include the development of monoclonal antibodies (MAbs), antibiotic potentiators, immune system modulators, phage-based therapies, lysins, antimicrobial or anti-biofilm peptides, and probiotics-based therapies [8,9]. This review discusses the strategies available for the discovery of MAbs and discusses antibodies that are already approved or are at various stages of development for the treatment of bacterial infections.
2. MABs AS THERAPEUTICS
Antibodies are protein molecules that specifically recognize and bind to their target and aid in its elimination from the system. Ever since the discovery of antibodies as a key component of the humoral immune response mediated by B-cells, they are widely recognized as an important class of molecules for both diagnostic and therapeutic applications. A typical antibody molecule contains 4 polypeptide chains linked to each other through disulfide bonds [Figure 1]. Polyclonal antibodies are derived from the blood of immunized animals or humans recovered from a disease condition and have been used for the treatment of numerous disease conditions such as tetanus, diphtheriae, snakebite, and COVID-19 [11,12]. However, the production of polyclonal antibodies relies on the availability of immunized animals or human donors and is subjected to batch-to-batch variations [13]. These issues can be addressed by the development of MAbs, which are derived from single B-cell clones and are homogenous. In 1975, George Kohler and Cesar Milstein described Hybridoma technology to produce MAbs, wherein B-cells obtained from immunized mice are fused with immortal myeloma cells to obtain immortal hybridoma cells that serve as a continuous source of the antibody encoded by the parent B-cell [14]. This technology revolutionized the production of MAbs and made them a popular choice for diagnostic and therapeutic applications. OKT-3 MAb was the first murine antibody approved for clinical use as an immunosuppressant to prevent organ rejection after transplant in 1986 [15]. However, it was withdrawn later due to the severe side effects caused by its murine origin [16,17]. Antibodies of murine origin have been known to cause human anti-murine antibody response and, consequently, are unsuitable for therapeutic applications. Such MAbs require further engineering before they can be considered suitable for administration in humans. This engineering largely involves the development of humanized antibodies, wherein the complementarity-determining regions (CDRs) of murine antibodies that contribute to the target binding are implanted into the human antibody framework [18]. This reduces the immunogenicity profile of the antibodies while retaining their specificity.
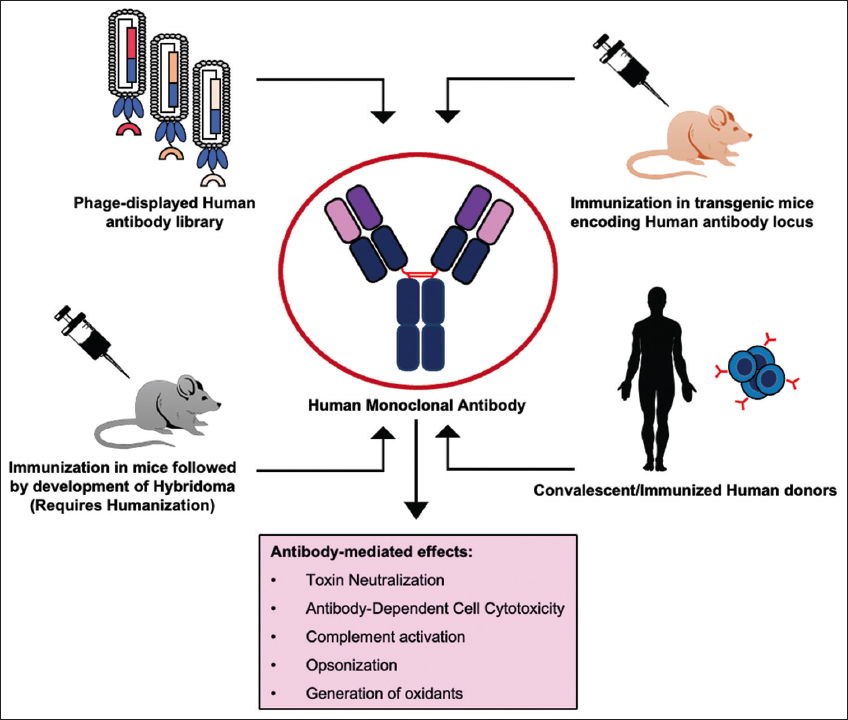 | Figure 1: Strategies available for the development of human monoclonal antibodies. [Click here to view] |
MAbs of human origin alleviate most of the issues posed by murine antibodies. Human MAbs have been successfully used as therapeutics for the treatment of various diseases including cancer, autoimmune disorders, organ transplantation, and most recently COVID-19 [18,19]. Over time, MAbs have emerged as an important class of protein-based biologics with estimated sales of USD 300 billion by 2025 [18]. After the approval of OKT-3, it took almost 29 years to approve the 50th therapeutic MAbs in 2015, whereas the 100th MAb was approved in 6 years in April 2021 emphasizing the advancement in the field and wide recognition of MAbs as therapeutic molecules [20]. Therapeutic MAbs act through multiple modes of action including neutralization of the target, antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and targeted drug delivery using antibody-drug conjugates [Figure 1] [21]. Further, once a specific antibody is obtained, it can be engineered to produce versions with improved properties including better PK-PD profiles, improved effector functions by Fc engineering, etc. [22].
3. STRATEGIES FOR HUMAN MAB DISCOVERY
Over the past few decades, technological advances have enabled the production of fully human MAbs. Techniques such as the use of phage-displayed human antibody libraries, transgenic animals, single B-cell cloning, computational antibody engineering, and next-generation sequencing are now available for discovering human MAbs against any target in principle [Figure 1] [18,23-26].
Phage display technology is one of the most versatile and popular strategies for the isolation of human MAbs. This technology involves amplification of the genes encoding antibodies followed by cloning in different formats (scFv or Fab) and display of antibody fragments in fusion to one of the coat proteins on the phage particles [27]. The phage-displayed antibody library is then used to select specific antibodies against the desired target. Depending on the source of the antibody gene repertoire, human antibody libraries can be either natural or synthetic. Natural libraries can further be divided into immunized or naïve libraries. The immunized antibody libraries comprise the antibody gene repertoire of immunized/convalescent patients, which are majorly primed against particular antigens and the same have been employed for the isolation of target-specific MAbs [27-29]. However, these libraries are primed against a particular target and cannot be used to isolate binders against other targets. On the other hand, naïve libraries contain antibody gene repertoire encoded by individuals who do not have a recent history of any illness and hence are not biased against any particular antigen [30]. Naïve human antibody libraries provide an excellent opportunity to explore the natural human antibody repertoire, which is highly competent to generate immune response against almost every antigen [29]. Hence, such libraries tend to be universal and in principle encode binders to every possible antigen. Synthetic or semi-synthetic repertoires comprise antibody genes whose CDRs are either fully (synthetic) or partially (semi-synthetic) reconstructed in vitro [31]. These libraries are very distinctive and can be tailored to impart improved expression, stability, and binding characteristics [32-34]. Synthetic libraries hold the potential to yield high-affinity antibodies against a diverse range of targets and have been exploited for the isolation of important antibody leads against clinically relevant diseases, for example, H1N1, and for the study of biomarkers using antibody-based proteomics [35-37]. The first fully human MAb, Adalimumab (Humira), was isolated using Phage display and was approved for the treatment of rheumatoid arthritis in 2002 [18]. Following its success, multiple therapeutic antibodies isolated using phage display have received approvals for use in humans [18].
Another technology involves the use of transgenic animals, which encode human antibody immunoglobulin locus and, hence, are capable of producing human antibodies [25]. This technology allows the exploitation of natural mechanisms of the immune system for the generation of specific and high-affinity antibodies against the desired target. The transgenic animals are immunized with the desired target and B-cells are harvested from immunized animals for cloning antibody genes. Panitumumab was the first MAb that originated from the XenoMouse transgenic system and was approved for the treatment of colorectal cancer [38]. Ever since multiple transgenic antibody platforms have become available; they have been successfully employed to produce human MAbs against clinically relevant targets. The most recent example is the development of neutralizing antibodies against the spike protein of SARS-CoV-2 for the treatment of COVID-19 [18,39,40].
Single B-cell cloning technology involves the separation of the B-cell repertoire into individual cells followed by the amplification and linkage of variable light and heavy genes to maintain the original pairing of the B-cell [41]. Depending on the distinct markers present on the surface of the B-cells, they are sorted as single cells and analyzed for specificity against the target antigen [23]. B-cells can be isolated from transgenic animals (encoding human antibodies) or convalescent humans to isolate target-specific antibodies. Single B-cell technology has seen tremendous advancement in the past decade and is beneficial in many important applications including screening repertoires for neutralizing antibodies that can be employed treatment of infectious diseases [42], studying immune response after vaccination [43], etc. Antibody discovery is also aided by computational strategies, which allow the analysis of the immunogenicity profile, biophysical properties, and developability profile of lead antibody molecules for better chances of success during clinical trials [26].
4. MABs AS NON-TRADITIONAL ANTIBIOTICS FOR THE TREATMENT OF AMR BACTERIA
Due to their diverse modes of action and proven effectiveness and safety in the treatment of several diseases such as cancer, MAbs can be considered a relatively low-risk alternative to traditional antibiotics. Key mechanisms that lead to the generation of AMR include alteration of the target site of antibiotics, production of enzymes that degrade antibiotics, efflux of antibiotics through efflux pumps on the bacterial cell surface, and changes in the membrane permeability to reduce the absorption of antibiotics [44,45]. With their potent effector functions, antibody-based antibiotics can be used to mediate the direct killing of bacteria, neutralization of toxins to control the infection, as well as target the molecules such as efflux pumps or antibiotic degrading enzymes that contribute to AMR [46]. Overall, MAbs can disrupt bacterial growth by mechanisms such as inhibition of biofilm formation, preventing iron acquisition, attachment or adhesion, neutralizing toxins, opsonophagocytosis, and complement activation [47]. Further, they can be administered either as a stand-alone therapy or in combination with traditional antibiotics [47]. As compared to antibiotics, the better half-life of antibodies can provide extended protection against infections. Moreover, due to their high specificity, they are expected not to harm the gut microbiome, thereby mitigating the adverse effects caused by antibiotics. Key pros and cons of using MAbs as therapeutic alternatives to antibiotics for countering AMR are listed in Table 1.
Table 1: Pros and cons of using monoclonal antibodies as potential therapeutic alternatives to antibiotics for countering AMR.
| Pros | Cons |
|---|---|
| Higher half-lives (~21 days for IgG) provide extended protection against the infection | High cost: but improvement in production technology is expected to bring down the overall cost |
| Higher target specificity protects gut microbiome and mitigates adverse effects | A combination of MAbs may be required to target different components of a pathogen |
| Safe and time-tested molecules for treating diseases | Should be of human origin to prevent immunogenicity and suitable animal models should be available for testing |
| Can be less susceptible to development of resistance due to controlled use in only life-threatening conditions | The clinical trials may be expensive |
5. MABs APPROVED FOR THE TREATMENT OF BACTERIAL INFECTIONS
Three MAbs have been approved for the treatment of diseases caused by bacteria [Table 2]. Bezlotoxumab is a human MAb (IgG1), which was discovered using transgenic mice technology and developed by Merck (Trade name Zinplava), and was approved by FDA in 2016 to reduce infection risk caused by Clostridium difficile [18, 76]. This MAb specifically recognizes the Toxin B of C. difficile and neutralizes its effect. C. difficile is a nosocomial Gram-positive bacterium that causes diarrhea in hospitalized patients. On infection, it secretes exotoxins such as Toxin A and B in the gut, which interact with the surface receptors on the mucosa leading to the degradation of actin filaments. This, further, results in necrosis in the lumen of the colon and an inflammatory response in the gut [48]. Bezlotoxumab has been reported to reduce the recurrence of C. difficile infection when administered along with antibiotic therapy [49].
Table 2: Monoclonal antibodies approved for the treatment of bacterial diseases.
| Name | Target/Action | Technology used for discovery | Year of Approval | Reference |
|---|---|---|---|---|
| Bezlotoxumab | Neutralizes enterotoxin B of Clostridium difficile | Transgenic mice | 2016 | [76] |
| Obiltoxaximab | Neutralizes protective antigen of Bacillus anthracis | Hybridoma | 2016 | [77] |
| Raxibacumab | Neutralizes protective antigen of Bacillus anthracis | Phage display | 2012 | [50] |
Raxibacumab is a human MAb discovered using phage display technology and developed by GlaxoSmithKline/Human Genome Sciences, Inc. (Trade name Abthrax), and was approved by FDA in 2012 for the treatment of inhalational anthrax caused by Bacillus anthracis [50,51]. This MAb specifically recognizes the protective antigen (PA) of B. anthracis and neutralizes its toxic effect. The bacteria produce PA, edema factor (EF) and lethal factor (LF), and PA facilitates entry of EF and LF in mammalian cells [51]. Raxibacumab specifically recognizes PA and neutralizes its effect.
Similarly, Obiltoxaximab is a chimeric MAb (IgG1) discovered using traditional hybridoma technology and developed by Elusys Therapeutics Inc. (Trade name Anthim) and was approved by FDA in 2016 for the treatment of inhalational anthrax caused by B. anthracis. Like Raxibacumab, Obiltoxaximab also recognizes the PA of B. anthracis and neutralizes its toxic effect [52].
6. ANTI-BACTERIAL MABs AT VARIOUS STAGES OF THE DRUG DEVELOPMENT PIPELINE
As per the WHO data, in 2021, about five MAb leads targeted against S. aureus, Campylobacter jejuni, and against the biofilms formed by several Gram-negative and Gram-positive bacteria are under clinical trials as non-traditional antibiotics [53]. The molecules are listed in Table 3.
Table 3: Antibody-based products in the clinical development pipeline for the treatment of bacterial diseases [53].
| Name | Target/Action | Phase of development |
|---|---|---|
| AR-301 (tosatoxumab) | AR-301 is an anti-S. aureus IgG1 monoclonal antibody that targets virulence factor α-toxin | Phase-3 |
| AR-320 (suvratoxumab) | AR-302 is an anti-S. aureus IgG monoclonal antibody that targets the surface-localized clumping factor A and virulence factor a-toxin | Phase-3 |
| LMN-101 | LMN-101 is a variable heavy chain-derived protein that binds and inhibits a flagellin filament protein FlaA of C. jejuni | Phase-2 |
| 9MW1411 | 9MW1411 is a MAb that recognizes the α-toxin monomer of S. aureus and reduces its toxicity | Phase-2 |
| TRL1068 | TRL1068 is a MAb that binds to homologues of DNABII made by Gram-negative and Gram- positive bacteria and disrupts the formation of biofilms | Phase-1 |
AR-301 (tosatoxumab) is a fully human MAb (IgG1) being developed by Aridis Pharmaceuticals, which targets the alpha-toxin of S. aureus [54]. This MAb was isolated by screening B-cells from a patient suffering from pneumonia caused by S. aureus. The alpha-toxin has hemolytic and cytotoxic effects and binds to immune cells to modulate their activity. AR-301 MAb neutralizes the alpha toxin and is effective as an adjunctive treatment strategy for hospital-acquired bacterial pneumonia caused by S. aureus [54]. It is currently in Phase-3 of clinical trials [55].
AR-320 (suvratoxumab) is a fully human MAb (IgG1) licensed by Aridis Pharmaceuticals from AstraZeneca, which targets the alpha-toxin of S. aureus [56]. This MAb also neutralizes the effects of alpha-toxin and is being developed for reducing the risk of pneumonia in patients on mechanical ventilation. It is currently in Phase-3 of clinical trials [57].
LMN-101 is a variable antibody heavy chain-derived protein-based product (VHH) being developed by Lumen Biosciences that bind and inhibit a flagellin filament protein FlaA of C. jejuni [58]. It is currently in Phase-2 of clinical trials [58].
TRL1068 is a fully human MAb (IgG1) that binds to homologues of the DNABII family (integration host factor and histone-like proteins) made by Gram-negative and Gram-positive bacteria and disrupts the formation of biofilms [59]. It is being developed by Trellis Bioscience LLC and was isolated by B cell screening [60]. It is currently in Phase-1 of clinical trials for the treatment of prosthetic joint infections [61].
9MW1411 is a human MAb being developed by Mabwell (Shanghai) Bioscience Co., Ltd. that targets alpha-toxin of S. aureus for the treatment of acute bacterial skin and skin structure infections. It is currently in Phase-2 of clinical trials [62].
Further, several antibody-based molecules are currently under the preclinical stage of development [47,53]. AR401 is a fully human MAb being developed by Aridis Pharmaceuticals [63]. It was isolated from B-cells of patients infected with A. baumannii and targets novel putative targets of this bacterial species. VXD-003 also targets A. baumannii [47]. Cd-ISTAb is a cocktail of two antibodies engineered for the treatment of C. difficile and targets Toxin A [64]. Two antibodies, namely, ASN-4 and ASN-5 have been developed against E. coli ST131 and K. pneumoniae, respectively. Both antibodies were developed by Arsanis, Inc. and have been now licensed to Bravos biosciences (known as BB100 and BB200) [47].
Several antibody-based molecules against different bacterial targets are also in the preliminary discovery stage of development. Aguilar et al. have shown that passive administration of MAbs against Staphylococcal enterotoxin K can protect mice from lethal shock and sepsis induced by a community-acquired MRSA USA300 strain [65]. Further, since antibodies are highly specific to their target, efforts are also underway to discover molecules against bacterial targets that can have broad-spectrum effects. For example, polysaccharide poly-β-1,6-N-acetylglucosamine (PNAG) has been identified on the surface of many AMR pathogenic bacteria and has been reported to be upregulated during drug resistance [66,67]. A fully human MAb, F598 IgG, has been found to be specific to PNAG and exhibit protective action against several bacterial species [68]. Similarly, Szijarto et al. developed a high affinity humanized mouse MAb – A1102 against the LPS O-antigen, D-galactan-III of K. pneumoniae carbapenemase producing K. pneumonia isolates associated with ST258 clades, and MAb A1102 showed protection against the ST258 whole bacteria in mice and rabbits [69]. The same group also developed another humanized MAb against LPS O-antigen, O25b of multi-drug resistant H30 subclone of extraintestinal pathogenic E. coli ST131, which is responsible for 10–25% of extraintestinal E. coli infection [70]. Since O25b is a conserved antigen, the MAb is expected to be effective against 10–25% of extraintestinal pathogenic E. coli and more than 50% of the MDR isolates. Diago-Navaro et al. reported two broadly reactive MAbs 17H12 and 8F12, which specifically recognize epitopes of the capsular polysaccharide of clade 2 ST258 carbapenem-resistant K. pneumoniae. These MAbs caused agglutination of all clade-2 strains and promoted bacterial killing by biofilm inhibition, complement deposition, opsonophagocytosis, and deployment of neutrophil extracellular traps [71]. Similarly, efforts should be made to identify conserved targets for developing broad-spectrum antibody-based therapy against other pathogenic bacteria. Furthermore, in some cases, it may be necessary to neutralize more than one antigen/toxin secreted by pathogenic bacteria. For example, multiple toxins drive the pathogenesis of S. aureus, which are a major pathogenic bacterium. Rouha et al. developed two neutralizing human MAbs ASN-1 and ASN-2, which were found to simultaneously neutralize alpha-hemolysin and 5 leukocidins of S. aureus [72]. The molecules entered clinical trials, but the trials were eventually terminated [73].
7. KEY CONSIDERATIONS AND CHALLENGES ASSOCIATED WITH MABs AS NON-TRADITIONAL ANTIBIOTICS
After the recent success of broadly neutralizing MAbs as effective antivirals against SARS-CoV-2 and its emerging variants, they have emerged as a viable option for the treatment of AMR bacteria [74]. The identification of bacterial antigens that should be initially targeted for developing narrow or broad-spectrum antibody-based therapy is important. The target bacteria should be the pathogens from the WHO priority list, which have been identified as the leading cause of antibiotic-resistant infections caused globally in hospitals [6]. The target antigens could be lipopolysaccharides, capsular polysaccharides, conserved exopolysaccharides, pilus formation proteins, extracellular vesicle components, efflux pumps, antibiotic degrading enzymes, etc. [75]. It is also important to develop sensitive and rapid diagnostic tools for the identification of disease-causing bacteria to determine the proper course of the therapy [9]. Further, the knowledge about the effector functions of the MAbs with different isotypes/subclasses should be exploited to obtain desired binding and effector functions for effective bacterial killing. It is also imperative that proper animal models should be available for evaluating the antibody molecules during the preclinical stages of development. At the same time, knowledge about disease etiology and progression is very essential to determine the time window most suitable for the administration of MAb therapy [75]. The cost of MAbs can be a challenge for their viability as a therapeutic alternative to antibiotics. However, with continuous technological advancements in the field, it is expected that MAb-based treatments will become increasingly affordable in the future. Addressing such issues can make antibody-based antibacterial therapy successful and can revolutionize the course of treatment of bacterial infections.
8. CONCLUSION
MAbs are an important class of protein-based biologics that are used to treat multiple disease conditions [18]. AMR is emerging as a significant threat globally, and as per the WHO, lack of action against AMR can make it a leading cause of fatalities. Due to the paucity of small molecule-based antibiotics in the development pipeline against the drug-resistant pathogens of the ESKAPEE category, other molecules are continuously being explored as non-traditional antibiotics. Due to their specificity and proven safety in human subjects, MAbs have a high potential to serve as non-traditional antibiotics for AMR bacteria. Several technologies exist for the development of human MAbs and the recent success with antibodies as antivirals for the treatment of COVID-19 gives hope that the same strategies can be explored for rapidly developing antibody-based antibiotics. Such molecules can be used as standalone or adjunct therapies with existing antibiotics to mitigate AMR. MAbs can be used as a mixture to address various aspects of curing bacterial infections including neutralization of toxins, killing, and clearance of bacterial cells. In the future, careful selection of bacterial targets (toxins, surface proteins, etc.), the discovery of broadly neutralizing antibodies, and the optimization of antibody production costs will allow the availability of MAb-based treatments for treating AMR bacteria. Overall, with three antibody molecules already approved and many more in various stages of the drug development pipeline, it is envisaged that antibodies will emerge as key players on the clinical landscape of therapeutic interventions for AMR bacteria.
9. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FUNDING
This study was supported by Start-up Research Grant (SRG/2022/000486) provided to VV by Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This study does not involve experiments on animal and human subjects.
13. DATA AVAILABILITY
The author confirmed that all the relevant data are included in the article.
14. PUBLISHER’s NOTE:
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization. The Top 10 Causes of Death;2020. Available from:https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Last accessed on 2022 Jul 05].
2. Ventola CL. The antibiotic resistance crisis:Part 1:Causes and threats. P T 2015;40:277-83.
3. Das B, Chaudhuri S, Srivastava R, Nair GB, Ramamurthy T. Fostering research into antimicrobial resistance in India. BMJ 2017;358:j3535. [CrossRef]
4. Kakkar M, Walia K, Vong S, Chatterjee P, Sharma A. Antibiotic resistance and its containment in India. BMJ 2017;358:j2687. [CrossRef]
5. World Health Organization. Essential Medicines List Antibiotic Book. 2021;Version 1.1. Available from:https://www.who.int/publications/m/item/the-who-essential-medicines-list-antibiotic-book-improving-antibiotic-awareness [Last accessed on 2022 Jun 01].
6. World Health Organization. WHO Publishes List of Bacteria for which New Antibiotics are Urgently Needed;2017. Available from:https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed [Last accessed on 2022 Jul 05].
7. Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A,
8. Butler MS, Gigante V, Sati H, Paulin S, Al-Sulaiman L, Rex JH,
9. Tse BN, Adalja AA, Houchens C, Larsen J, Inglesby TV, Hatchett R. Challenges and opportunities of nontraditional approaches to treating bacterial infections. Clin Infect Dis 2017;65:495-500. [CrossRef]
10. Thomas D, Wessel C. The State of Innovation in Antibacterial Therapeutics. Bio Industry Analysis;2022. Available from:https://www.bio.org/sites/default/files/2022-02/The-State-of-Innovation-in-Antibacterial-Therapeutics.pdf [Last accessed on 2022 Jun 01].
11. Tizard IR. Passive immunization. Vaccines for Veterinarians. Amsterdam, Netherlands:Elsevier;2021. 141-52.e1. [CrossRef]
12. Klassen SA, Senefeld JW, Senese KA, Johnson PW, Wiggins CC, Baker SE,
13. Gray A, Bradbury AR, Knappik A, Plückthun A, Borrebaeck CA, Dübel S. Animal-free alternatives and the antibody iceberg. Nat Biotechnol 2020;38:1234-9. [CrossRef]
14. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495-7. [CrossRef]
15. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs 2015;7:9-14. [CrossRef]
16. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 2010;9:325-38. [CrossRef]
17. Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012;4:413-5. [CrossRef]
18. Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ,
19. Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect Dis 2022;22:e311-26. [CrossRef]
20. Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov 2021;20:491-5. [CrossRef]
21. Suzuki M, Kato C, Kato A. Therapeutic antibodies:Their mechanisms of action and the pathological findings they induce in toxicity studies. J Toxicol Pathol 2015;28:133-9. [CrossRef]
22. Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci 2018;11:540-52. [CrossRef]
23. Pedrioli A, Oxenius A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol 2021;42:1143-58. [CrossRef]
24. Zambrano N, Froechlich G, Lazarevic D, Passariello M, Nicosia A, De Lorenzo C,
25. Chen WC, Murawsky CM. Strategies for generating diverse antibody repertoires using transgenic animals expressing human antibodies. Front Immunol 2018;9:460. [CrossRef]
26. Norman RA, Ambrosetti F, Bonvin AM, Colwell LJ, Kelm S, Kumar S,
27. Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM,
28. Pan Y, Du J, Liu J, Wu H, Gui F, Zhang N,
29. Ferrara F, Erasmus MF, D'Angelo S, Leal-Lopes C, Teixeira AA, Choudhary A,
30. Chan SK, Rahumatullah A, Lai JY, Lim TS. Naïve human antibody libraries for infectious diseases. In:Lim TS, editor. Recombinant Antibodies for Infectious Diseases. Cham:Springer International Publishing;2017. 35-59. [CrossRef]
31. Burkovitz A, Ofran Y. Understanding differences between synthetic and natural antibodies can help improve antibody engineering. MAbs 2016;8:278-87. [CrossRef]
32. Cobaugh CW, Almagro JC, Pogson M, Iverson B, Georgiou G. Synthetic antibody libraries focused towards peptide ligands. J Mol Biol 2008;378:622-33. [CrossRef]
33. Prassler J, Thiel S, Pracht C, Polzer A, Peters S, Bauer M,
34. Jian JW, Chen HS, Chiu YK, Peng HP, Tung CP, Chen IC,
35. Yuan TZ, Garg P, Wang L, Willis JR, Kwan E, Hernandez AG,
36. Tung CP, Chen IC, Yu CM, Peng HP, Jian JW, Ma SH,
37. Chen G, Sidhu SS, Nilvebrant J. Synthetic antibodies in infectious disease. In:Lim TS, editor. Recombinant Antibodies for Infectious Diseases. Cham:Springer International Publishing;2017. 79-98. [CrossRef]
38. Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol 2007;25:1134-43. [CrossRef]
39. Wang C, Li W, Drabek D, Okba NM, van Haperen R, Osterhaus AD,
40. Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E,
41. Ouisse LH, Gautreau-Rolland L, Devilder MC, Osborn M, Moyon M, Visentin J,
42. Prashar P, Swain S, Adhikari N, Aryan P, Singh A, Kwatra M,
43. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O,
44. Cavaco M, Castanho MA, Neves V. The use of antibody-antibiotic conjugates to fight bacterial infections. Front Microbiol 2022;13:835677. [CrossRef]
45. Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance:The most critical pathogens. Pathog Basel Switz 2021;10:1310. [CrossRef]
46. Wenzel EV, Bosnak M, Tierney R, Schubert M, Brown J, Dübel S,
47. Zurawski DV, McLendon MK. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics (Basel) 2020;9:E155. [CrossRef]
48. Riddle DJ, Dubberke ER. Trends in
49. Lee Y, Lim WI, Bloom CI, Moore S, Chung E, Marzella N. Bezlotoxumab (Zinplava) for
50. Tsai CW, Morris S. Approval of raxibacumab for the treatment of inhalation anthrax under the US food and drug administration “Animal Rule.“Front Microbiol 2015;6:01320. [CrossRef]
51. Mazumdar S. Raxibacumab. MAbs 2009;1:531-8. [CrossRef]
52. Yamamoto BJ, Shadiack AM, Carpenter S, Sanford D, Henning LN, Gonzales N,
53. World Health Organization. Antibacterial Products in Clinical Development for Priority Pathogens;2022. Available from:https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/antibacterial-products-in-clinical-development-for-priority-pathogens [Last accessed on 2022 Jul 01].
54. François B, Mercier E, Gonzalez C, Asehnoune K, Nseir S, Fiancette M,
55. Aridis Pharmaceuticals, Inc. Randomized Double-blind Placebo-controlled Multicenter Phase 3 Study of Efficacy and Safety of AR-301 as Adjunct Therapy to Antibiotics in the Treatment of Ventilator-Associated Pneumonia (VAP) Caused by
56. François B, Jafri HS, Chastre J, Sánchez-García M, Eggimann P, Dequin PF,
57. Aridis Pharmaceuticals, Inc. Phase 3, Randomized, Double-blind, Placebo-controlled, Single-dose Study to Evaluate the Efficacy and Safety of Suvratoxumab in Mechanically Ventilated Adults and Adolescents for the Prevention of Nosocomial Pneumonia;2022. Report No.:NCT05331885. Available from:https://www.clinicaltrials.gov/ct2/show/NCT05331885
58. Lumen Bioscience, Inc. Phase 2 Randomized, Double-Blind, Placebo-Controlled, Single Dose Regimen Study of LMN-101 in Healthy Volunteers Challenged With Campylobacter Jejuni;2022. Report No.:NCT04182490. Available from:https://www.clinicaltrials.gov/ct2/show/NCT04182490
59. Estellés A, Woischnig AK, Liu K, Stephenson R, Lomongsod E, Nguyen D,
60. Xiong YQ, Estellés A, Li L, Abdelhady W, Gonzales R, Bayer AS,
61. Trellis Bioscience LLC. Phase 1, Blinded, Single Ascending Dose Study to Evaluate Safety, Pharmacokinetics, and Activity of TRL1068 in Subjects With Prosthetic Joint Infection of the Knee or Hip, Undergoing Primary Two Stage Exchange Arthroplasty;2022. Report No.:NCT04763759. Available from:https://www.clinicaltrials.gov/ct2/show/NCT04763759 [Last accessed on 2022 Jul 01].
62. Mabwell (Shanghai) Bioscience Co., Ltd. Multicenter, Randomized, Double-blind, Placebo-controlled Phase II Clinical Study of the Efficacy and Safety of 9MW1411 Injection Combined With Antibiotics in Patients With Acute
63. Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant
64. Shiferaw H. Our Science. In:Integrated Biotherapeutics. Available from:https://www.integratedbiotherapeutics.com/our-science/[Last accessed on 2020 Jul 31].
65. Aguilar JL, Varshney AK, Pechuan X, Dutta K, Nosanchuk JD, Fries BC. Monoclonal antibodies protect from Staphylococcal Enterotoxin K (SEK) induced toxic shock and sepsis by USA300
66. Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH,
67. Skurnik D, Davis MR Jr., Benedetti D, Moravec KL, Cywes-Bentley C, Roux D,
68. Soliman C, Walduck AK, Yuriev E, Richards JS, Cywes-Bentley C, Pier GB,
69. SzijártóV, Guachalla LM, Hartl K, Varga C, Badarau A, Mirkina I,
70. Guachalla LM, Hartl K, Varga C, Stulik L, Mirkina I, Malafa S,
71. Diago-Navarro E, Motley MP, Ruiz-Peréz G, Yu W, Austin J, Seco BM,
72. Rouha H, Weber S, Janesch P, Maierhofer B, Gross K, Dolezilkova I,
73. Koulenti D, Xu E, Song A, Sum Mok IY, Karageorgopoulos DE, Armaganidis A,
74. Planchais C, Fernández I, Bruel T, de Melo GD, Prot M, Beretta M,
75. Motley MP, Fries BC. A new take on an old remedy:Generating antibodies against multidrug-resistant gram-negative bacteria in a postantibiotic world. mSphere 2017;2:e00397-17. [CrossRef]
76. Markham A. Bezlotoxumab:First global approval. Drugs 2016;76:1793-8. [CrossRef]
77. Greig SL. Obiltoxaximab:First global approval. Drugs 2016;76:823-30. https://doi.org/10.1007/s40265-016-0647-3 https://doi.org/10.1007/s40265-015-0533-4 https://doi.org/10.1007/s40265-016-0577-0 https://doi.org/10.1007/s40265-015-0522-7 https://doi.org/10.1007/s40265-016-0634-8