1. INTRODUCTION
The ongoing trend of globalization and industrialization in agriculture continues to evoke global concerns, particularly regarding biodiversity loss and health risks. These issues stem from the prevalence of monoculture practices that diminish the genetic diversity of farmlands. In addition, the uneven distribution of technological advancements favors a select few resource-intensive plant species, notably monocotyledonous species [1,2]. Consequently, global food security largely depends on a limited range of crops, such as corn, rice, and wheat, which provide the majority of the world’s caloric requirements. Despite their importance in many diets, these grains lack essential micronutrients (essential vitamins and minerals required in small amounts for health). Consequently, an estimated 2 billion people worldwide suffer from micronutrient deficiencies, worsening the problems of malnutrition and public health (FAOSTAT, 2020).
Heavy reliance on some cereal crops raises concerns about risks to global food security. The agricultural sector, often considered a nation’s backbone, faces a significant threat due to such dependency on limited crop selection. At present, the agro-industry is dealing with significant challenges as it works to ensure an adequate food supply while maintaining high standards for productivity and quality in world with over 8.16 billion people. Moreover, projections suggest that the global population will exceed 10.9 billion by 2050, making the task of addressing the impending food crisis exceedingly demanding [3]. Addressing this challenge requires a comprehensive approach that transcends the traditional boundaries of disciplinary approaches. It involves crucial steps to expand the range of food options and enhance access to nutritious choices. Strategies, such as biofortification, nutritional supplements, and enrichment programs, are essential to ensure that individuals have access to a varied and nourishing diet, thereby promoting health and sustainability. Safeguarding the availability of nutritious foods is crucial for overall food security [4]. Moreover, it is important to acknowledge that adverse environmental effects, such as biodiversity loss, soil degradation, and erosion, along with apprehensions regarding crop variety extinction, have prompted government organizations, academic institutions, and researchers worldwide to take action. They are actively involved in studying and sharing information about the cultivation and use of underutilized, unexplored, and novel plant species, often termed as “alternative crops” [5].
Despite their nutritional richness and historical significance as dietary staples in diverse cultures, ancient grains, which are rich in micronutrients and phytonutrients, are currently undervalued. The cultivation of these crops is confined to only a few niches in the global food system. Globally, numerous individuals depend on a wide array of plant species to support their livelihood, health, and cultural heritage [6]. These plants provide a wealth of resources that are crucial for human survival, including fuel, fiber, food, and therapeutic compounds. From staple crops such as rice, wheat, and maize to medicinal herbs such as quinine and artemisinin, they have played a significant role in human history and continue to do so in the contemporary era. Most of these plant species or crops are of local or regional importance and are referred to by various colloquial terms, such as small, neglected, underutilized, or underexplored crops [7,8].
In this context, it is crucial to reassess the cultivation and use of underutilized crops that possess favorable nutritional profiles and are suitable for the demands of the 21st century [9]. Furthermore, certain underutilized crops, particularly amaranth and quinoa, have garnered global recognition in the nutraceutical industry due to their significantly superior nutritional profiles compared with traditional cereals. While amaranth is known for its high protein and lipid content, with a greater proportion stored in the embryo, quinoa is known for its complete amino acid profile, and buckwheat is notable for its rich flavonoid content, particularly rutin, which contributes to its antioxidant properties. Amaranth’s higher lipid content may be advantageous in formulations requiring fatter, while quinoa’s amino acid profile makes it a superior plant-based protein source. This heightened nutritional value has generated increased research interest in these crops and their potential use in the development of functional foods and nutraceutical products [10]. Amaranth (Amaranthus spp.), a member of the family Amaranthaceae, has a distinct anatomical contrast from staple cereals. Underutilized grains, such as amaranths, generally possess a higher quantity of embryos, which accumulate proteins and lipids, and a reduced amount of endosperm, which stores starch [11]. It is important to note that while higher calorie content might be seen as a disadvantage in the food systems of developed nations, where a calorie surplus is a common issue, it can actually be advantageous in less developed regions where problems such as undernutrition and food scarcity are major challenges [12].
Amaranths are a nutrient-dense powerhouse rich in bioactive compounds such as carotenoids, polyphenols, and flavonoids. These compounds are associated with a range of health benefits, including antioxidant, anti-inflammatory, and anticancer properties. The presence of these bioactive compounds enhances amaranth’s potential to promote overall health, reduce oxidative stress, and prevent disease. Additional discussion involving multiple pseudocereals including quinoa and buckwheat will display beneficial aspects along with practical uses for these underused crops. Amaranth is recognized for its high protein content and lipids but quinoa delivers all essential amino acids while buckwheat stands out with its flavonoids such as rutin that act as antioxidants. Their special nutritional properties make them attractive choices instead of conventional cereal products. Although this amaranth has anti-nutritional factors, oxalates, phytase, and saponins, which can reduce nutrient absorption and increase amaranth digestibility. To counter these results, different processing methods, including soaking, fermentation, and cooking, are used to increase bioavailability of nutrients and reduce the amounts of antinutrients. For maximum health benefits of amaranths and its use in functional foods, these aspects are critical.
Moreover, it is advantageous for these underutilized grains to have high protein contents and well-balanced amino acid compositions. This trait can significantly augment their nutritional value and suitability as a source of essential nutrients, particularly in regions where protein deficiency is widespread [13]. The observations indicate that the protein content in amaranth grains closely aligns with the protein intake guidelines established by the Food and Agriculture Organization/World Health Organization (FAO/WHO). This alignment was attributed to the well-balanced amino acid sequences. According to the FAO/WHO recommendations, adults are advised to consume approximately 0.75 of protein per kilogram of body weight daily [14,15]. Encouraging the inclusion of this underutilized vegetable in the diet is essential. Recent research has shed light on its potential, exploring both grains and leaves as functional foods or additives to enhance various dishes, such as cereals, bread, cakes, pasta, and cookies. However, there is still considerable untapped potential. Therefore, conducting clinical studies to gain deeper insights into amaranth’s bioactive components is crucial. This comprehensive investigation will enable its effective use across various industries, including pharmaceuticals, packaged foods, natural health supplements, and cosmetics. In this context, this review emphasizes the latest developments in the use of amaranth and quinoa as nutraceutical and functional foods, encompassing their bioactive compounds, anti-nutritional factors, processing methods, health benefits, and potential applications.
2. THE DIVERSITY OF THE AMARANTH
Amaranth, which originated in Latin America, is renowned as one of the world’s ancient crops and boasts, a history that traces back to approximately 6700 BC [16]. The genus Amaranth comprises approximately 60 distinct species, many of which are cultivated for a variety of purposes. These include the production of grains, leafy vegetables, and ornamental plants, at times, they may grow as weeds. Amaranth grains have historically played a significant role in the dietary traditions of numerous countries across Africa, India, China, Southeast Asia, Mexico, South America, and North America. Today, in numerous tropical and temperate regions, amaranths are extensively cultivated as highly nutritious leafy vegetables [17].
Among the various species within the genus Amaranthus, Amaranthus cruentus, Amaranthus caudatus, and Amaranthus hypochondriacus are frequently used for their grains and are recognized as dual-purpose varieties [17,18]. In addition, several popular leafy species, such as A. hypochondriacus, Amaranthus tricolor, Amaranthus hybridus, and Amaranthus blitum, are cultivated for their edible greens, as indicated in Table 1 [17,19-21]. It is worth noting that amaranths belong to a group of plants within the same genus that are typically classified as annual or short-lived perennials. Furthermore, amaranths exhibit notable drought resistance and high tolerance for arid environments when compared with soybeans. However, extended dry periods can trigger flowering and reduce leaf yield [22,23].
Table 1: Origin types and uses of amaranth species worldwide.
| S. No. | Amaranth species | Origin | Use | References |
|---|---|---|---|---|
| 1. | Amaranthus cruentus | America/India | Food, medicine, ornamental | [76] |
| 2. | Amaranthus thunbergii | Africa | Medicine and fodder for livestock. | [13] |
| 3. | Amaranthus spinosus | America | Medicine, food | [15,16] |
| 4. | Amaranthus graecizans | Africa | Food | [15] |
| 5. | Amaranthus hypochondriacus | America | Ornamental, medicine | [15,77] |
| 6. | Amaranthus dubius | America | Medicine | [16] |
| 7. | Amaranthus blitum | Africa | Medicine | [16] |
| 8. | Amaranthus caudatus | America | Medicine, ornamental | [76] |
| 9. | Amaranthus lividus | Nigeria | Food | [24] |
| 10. | Amaranthus hybridus | Africa | Medicine, food | [24] |
| 11. | Amaranthus deflexus | Nigeria | Medicine and fodder for livestock. | [25] |
| 12. | Amaranthus retroflexus | Africa | Food | [24] |
| 13. | Amaranthus tricolor | Africa, India | Food | [24] |
| 14. | Amaranthus viridis | Chennai, Tamil Nadu, India. | Medicine, food | [72] |
| 15. | Amaranthus polygonoides | India, Erode, Tamil Nadu | Medicine | [78] |
| 16. | Amaranthus Hypochondriacus | Mexico | Antioxidant capacities and higher secondary metabolite levels | [79] |
The amaranth species harvested in South Africa are in fallow lands and fields as young leaves, whole seedlings, and growth tips. Harvested amaranth is consumed as cooked meal, primarily as a porridge blend. The boost in the cultivation of this indigenized, traditional plant species offers greater potential to improve nutritional food security through crop breeding. The genus Amaranthus is an underutilized and neglected plant species in Southern Africa [24]. Most amaranth species originated in America and were introduced to Nigeria. Some commonly cultivated amaranth species are as follows:
A. blitum commonly found in Nigeria is also known as wild amaranth or purple amaranth. This variety has black seeds, and its leaves are sweet, its color may vary from green to red. Amaranthus lividus is cultivated in Nigerian ruderals for its leaf usage in various applications. This species is not native to Nigeria but has been introduced from Arabia and the Mediterranean. This species grows annually and can grow up to 6–90 cm. Leaves of this variety are ovate, with tapering bases and terminal spikes. A. caudatus also known as the red and pendant amaranths is native to southern Nigeria [15,16]. A. cruentus, commonly known as African spinach, is consumed as a grain. A. cruentus is native to the United States America and is valued in Nigeria for ornamental properties [24].
Amaranthus deflexus also called pigweed is widely cultivated in central and southern Nigeria. Amaranthus dubius commonly known as the spleen amaranth is not native to Nigeria but grows there. It is an annual cultivated crop that can grow up to 30–150 cm with broad leaves. This variety is cultivated well on diverse soils and favorable climatic conditions; therefore, it is widely cultivated in the northern and southern parts of Nigeria [25].
Amaranthus retroflexus, also known as tumbleweed, is one of the most commonly grown species in Africa. It is also cultivated in irrigated farmlands in Nigeria. The leaves of this species can be consumed as salads or used in soups. Amaranthus thunbergii, also known as wild spinach, is widely cultivated in northern Nigeria and is used to flavor food. The leaves of this species are also edible. These can grow up to 55 cm. The stem of this variety is hairless and the flowers are green in color, valued for ornamental importance. The fruits of this variety are ovoid and native to America [25,26].
A. tricolor, also known as Chinese spinach and Joseph’s coat. As its name suggests, it is multicolored species. Its colors may range from yellow to green to red. Due to its wide color range, it has ornamental importance in America and Africa. This variety can grow up to 125 cm, and it requires moist soil for cultivation. This variety shows resistance to harsh and hot climatic conditions. Amaranthus viridis, commonly known as local tote is branched, has edible leaves and stems, and can grow up to 60–80 cm. This is a weedy variety of amaranth species. This species is native to East Asia [27].
3. NUTRITIONAL CONSTITUENT OF AMARANTH
Amaranths are versatile and nutrient-dense grains that have been consumed for thousands of years by various cultures worldwide. Amaranth is a multipurpose crop that supplies a high level of nutrition to the human diet [28]. Amaranth’s importance concerning the chemical composition, morphological characteristics, and rheological properties when used as flour is well known. Studies related to properties, processing aspects, and health effects are on the rise. Amaranths are a good source of protein, fiber, vitamins, and minerals [27]. According to the USDA National Nutrient Database, a 100-g serving of cooked amaranth contains calories (kcal): 103, protein: 3.8 g of, fat: 1.6 g of, carbohydrates: 19 g of, fiber: 2.1 g of, calcium: 16% of daily value (DV), iron: 14% of the DV, magnesium: 36% of the DV, phosphorus: 20% of the DV, potassium: 4% of the DV, zinc: 8% of the DV, copper: 18% of the DV, and manganese: 105% of the DV (USDA, 2005) [Table 2].
Table 2: Nutritional composition of amaranths.
| Nutrients (%) | Composition (%) |
|---|---|
| Moisture content | 6.23–11.29 |
| Crude protein | 13.5–20.5 |
| Crude fat | 6.2–7.2 |
| Ash | 2.1–4.2 |
| Carbohydrate | 60.9–65.25 |
| Crude fiber | 3.4–6.7 |
| Mineral (mg/100g) | |
| Iron | 7.61 |
| Calcium | 159 |
| Magnesium | 248 |
| Manganese | 3.3 |
| Potassium | 508 |
| Zinc | 287 |
| Sodium | 31 |
| Copper | 1.2 |
Amaranth contains high-quality protein; moreover, it contains more protein than most other grains, such as wheat, rice, and corn. Amaranth proteins are considered to be of higher quality because they contain all the essential amino acids in adequate amounts. The amino acid profile is similar to that of animal protein, making it a suitable plant-based protein source for vegetarians and vegans. Research has demonstrated that the protein present in amaranths exhibits excellent digestibility and high bioavailability [29].
The proteins present in amaranths can be easily absorbed by the body. Furthermore, it has also been reported that amaranth proteins have antioxidant and anti-inflammatory properties. Furthermore, amaranths are a notable source of dietary fiber, which plays a crucial role in promoting digestive health, regulating blood sugar levels, and lowering the risk of chronic conditions, such as heart disease and diabetes [30]. Therefore, amaranth stands out as a commendable dietary choice for individuals seeking blood sugar management [28]. Amaranths offer a rich supply of vitamins and minerals, with a notable emphasis on calcium, iron, magnesium, and manganese, as previously mentioned. These minerals play essential roles in maintaining bone health, bolstering the immune system and blood pressure. Amaranth is also a good source of Vitamin B6, folate, and Vitamin E, which are important for maintaining a healthy skin, producing red blood cells, and protecting cells from oxidative damage [31].
3.1. Carbohydrate
Amaranth seeds primarily consist of polysaccharides, notably starch; typically contain 65–75% starch along with 5% dietary fiber. The sucrose content in the amaranth seed is 2–3 times higher than that obtained from wheat. Amaranth seed samples in addition to sucrose contain raffinose as the major sugar followed by inositol, maltose, and stachyose in minor amounts. A. cruentus and A. caudatus contain low-molecular-weight sucrose, and the sucrose content in both varieties ranges from 0.58% to 0.75%. The rheological and physicochemical properties of starch play important roles in the bioavailability and processing of amaranth seeds [32]. These properties undergo change when amaranth seeds are exposed to thermal and chemical treatments. The size of starches varies in different species, which affects their physiochemical and functional properties. The granule size of starch typically ranges from 10 μm to 25 μm but in amaranth, the granule size of starch ranges from 1 μm to 3 μm. A. hypochondriacus starch after cooking, their storage, as well as the potential to be used in the development of starchy products increased [28]. However, a decrease in carbohydrate levels of was noticeable during the treatment with amaranth under the stimulation of He-Ne laser and magnetic fields. Amaranth grain genotypes “Mittlerer Typ” and “Neuer Typ” from the A. hypochondriacus variety contain a carbohydrate content of about 72.7%. To obtain amaranth starch and fractions various processes and treatments were employed. The endosperm of amaranth contains 79% of the high starch fraction [33].
3.2. Fiber
Amaranths are considered a rich source of dietary fiber. Dietary fiber, in general, is represented as total, soluble and insoluble dietary fiber. Depending on the variety selected for consumption, the studies on A. caudatus and A. hypochondriacus showed that a higher percentage of crude fiber was present. Different varieties of A. caudatus understudies show a higher percentage of fiber in “Centenario” (16.4%) than in other available species of A. caudatus which is “Oscar Blanco” in which dietary fiber sums up to 13.8%. When dietary fiber is combined with nitrogen and phosphorus, the potassium content of dietary fiber is reduced on a residual basis. The water-insoluble dietary fiber in amaranth is more than 25%, which is higher than that found in several bowls of cereal. The resistant starch content of amaranths is approximately 0.65% [34]. When amaranths undergo processing such as extrusion cooking or fluidized heating, the resistant starch content in amaranths tends to increase. Resistant starch is a type of starch that resists digestion in the small intestine, acting like fiber to promote gut health and assist with blood glucose control. Some processing techniques such as popping and longer cooking duration decrease the resistant starch content. The soluble dietary fiber content in amaranth protein concentrate extracted from A. cruentus is around 12.9% at the pH of 11 extracted from A. cruentus. Amaranth seed flour has a relatively lower amount of soluble dietary fiber, which is 4.29% [35].
3.3. Protein
The protein content present in various species of amaranth, namely, A. hybridus, A. caudatus, A. hypochondriacus, and A. cruentus, ranges from 12% to 16% [36]. Besides the presence of albumin and globulin, the protein in the amaranth also comprises alkali-soluble glutelin up to 20.8% and alcohol-soluble prolamin up to 12%. Amaranthus dolorosa variety of amaranths was compared to 12 cereal species, and studies proved that the amount of protein present in amaranths was about 15.4% with the highest concentration of Met, Arg, and Lys. Based on this study, it is suggested that the use of amaranths in the improvement of poor Met and Lys content ratios presents in common wheat for consumption [37]. Consumption of a diet rich in gluten derived from rye, barley, and wheat triggers an immune response in celiac disease patients. In such cases, a gluten-free diet is advised. The quality of baked products is closely linked to gluten and structural characteristics of proteins. Amaranths contain easily digestible and highly nutritious components, particularly albumin and globulin. In a separate study, A. dubius, when processed into flour, was found to contain approximately 73.42% albumin, 6.47% prolamin and 6.41% glutelin. Another study involving 11 species (A. viridis, Amaranthus powellii, Amaranthus muricatus, Amaranthus deflexus, Amaranthus blitoides, Amaranthus graecizans, A. retroflexus, Amaranthus albus, A. blitum, A. cruentus, and A. hypochondriacus) from wild populations utilized gel filtration chromatography and denaturing electrophoresis to analyze protein profiles and amino acid composition. These findings revealed that the molecular weights and concentrations of primary seed proteins exhibited only slight variations across all these species [38-40].
Amaranth seeds are considered a good source of superior-quality protein and function as a relevant raw material for developing amaranth protein concentrate. Amaranth flour yields about 26–28% protein, and the starch yield in amaranth is estimated to be about 52% extracted by the enzymatic method, which results in the preparation of glucoamylase and α amylase [41]. Emulsion stability was increased in the obtained amaranth protein isolate due to the presence of steric repulsion effect. The standard procedure in use for protein extraction requires prior acid washing and heat treatment during alkaline extraction at 50°C for the preparation of protein concentrate. This conventional method, when compared with the isoelectric precipitation and dialysis methods of protein concentrate extraction, shows that the method used for the extraction adversely affects the product properties. The conventional method exhibits a positive impact on the characteristics of amaranth protein concentrates in terms of their improved composition, color, thermal stability, and protein solubility. The thermal process has a positive impact on the color of the obtained protein concentrate, but it has a negative impact on the solubility of the protein [42].
3.4. Crude Lipid
The crude lipid content in amaranths varies according to species and cultivation mode. The amount of crude lipids in amaranth depends on the extraction method, as well as the solvent used for lipid extraction. The processing conditions applied to amaranth seeds also influence the rate of extractable lipids. Extraction of the lipophilic fraction from the seed of amaranth was performed with non-polar organic solvents, commonly used for the extraction process, including hexane and petroleum ether. Oil in amaranth seeds can be recovered using the pressing technique, but the recovery rate is low. The lipid content in amaranth is between 4.8% and 8.1% [40]. Lipids are an important component of amaranth seeds loaded with tocopherol, phospholipid, and triacylglycerol (TAG). Some minor fractions of amaranths are phytosterols, terpene alcohols, and waxes. Any heat treatment applied to amaranths decreases the extractable lipid content. TAG is present in the range of 80.3–82.3% in the lipid fraction of amaranth species, namely, A. caudatus and A. cruentus, when petroleum ether is used as the solvent for lipid extraction. Diacylglycerol content is approximately 6.5%, whereas the presence of phospholipid is 9.1–10.2% approximately, depending on the variety used for lipid extraction. Monoacylglycerol is present at a range from 3.0% to 3.5% [39].
3.5. Fatty Acids
The composition of fatty acids is vital for the determination of the nutritional and stability properties of edible oils. Among the various fatty acids present in amaranth, the main ones are palmitic, oleic, and linoleic acids. Their percentages vary depending on the species and cultivar used for the production of a particular species. Stearic acid is present in trace amounts 4.62% and other minor fatty acids are present in lesser amounts. Some major fatty acids in the amaranth species oil are oleic acid found between 19.0% and 39.0%, linoleic acid ranging from 36.7% to 55.9%, and palmitic acid present from 19.0% to 23.0% [43]. There was a significant increase in the content of fatty acids in amaranths under electromagnetic stimulation with the help of laser lights or in the presence of a magnetic field around the seeds of amaranths. The fatty acid profile of amaranth was compared with those of other crops, such as corn, buckwheat, oat, barley, and lupin. We concluded that the amaranth profile was similar to the fatty acid profiles of buckwheat and corn oil. The A. curentus fatty acid profile was similar to that of the fatty acids obtained from cotton and sesame seed oils [44].
3.6. Other Microconstituents
Amaranth seeds without processing are flavorless. The volatile compounds in amaranth seeds are 4-methyl heptane, 2,4-dimethyl heptane, and the C12H24 isomer. The folate content in amaranth ranged from 52.8 to 73.0 μg-1. Folate concentrate in the bran fraction was higher than that in the flour fraction of amaranth grain. The natural defense component of amaranths is lignans, and most of the available lignans are metabolized in the gut by microflora. The total concentration of lignans in amaranth grain varies depending on the extraction method used. The phenolic compounds of amaranths are vanillic acid, gallic acid, syringic acid, protocatechuic acid, caffeic acid, sinapic acid, rutin, quercetin, isoquercitrin, and nictiflorin [45].
3.7. Squalene
It is a triterpene in the cholesterol biosynthesis pathway. Squalene can be used in cosmetic dermatology. It is the major component of polyunsaturated lipids and acts as an antioxidant, emollient, antitumor, and hydrating agent (Huang et al. 2009). Shark liver oil is the richest source of available squalene, but it is present in relatively higher concentrations in olive oil, palm oil, wheat germ, rice bran oil, and amaranth oil. Amaranths are considered a major source of squalene. Studies on the extraction of squalene from different amaranth species were conducted. Extraction from A. tricolour is proven to be highest ranging from around 6.0–6.1%, while other species of amaranth were also utilized for the extraction process, namely, A. cruentus and A. hypochondriacus, from which 5.67% and 3.6% of squalene are extracted, respectively [46]. Studies related to the squalene extraction process stated that even if the extraction occurs from the same species, its concentration differs. The concentration of squalene extracted from A. caudatus exhibits different concentrations when extracted from the seeds of Oscar Blanco and Victor red seed variants. To increase the squalene fraction, there is a need to decrease the solvent pressure in the separator. Amaranth seed processing affects the rate of extraction of squalene. The amount of squalene presents in amaranth grains after processing was distributed between coarse fractions. Contrariety of amaranth seeds increased the squalene content in A. caudatus and A. cruentus by up to 26.5% and 14.5%, respectively. Puffing and roasting of amaranth seeds up to 290°C and 150°C for 15 to 20 min causes no loss in squalene content [47].
3.8. Tocopherol and Tocotrienols
Tocopherols, as, as well as tocotrienol, are organic substances of plant origin that are found in nature. They are primarily obtained from nuts, oilseeds, and vegetable oils in varying proportions. In the diet, α- as well as β-tocopherol are the major forms present due to their high content in several food items. However, α-tocopherol is present in major quantities in the context of Vitamin E content. Both tocopherols and tocotrienol are considered more metabolically active in their α-forms [47]. The amount of tocopherol present varies according to the species. α-tocopherol and β-tocopherol are commonly present in A. hypochondriacus and A. cruentus. A. cruentus also contains a significant amount of δ tocopherol. Vitamin E profile comparison of amaranth with other crops, such as corn, oat, wheat oils, buckwheat, and barley, the value of tocopherol in amaranth grain is higher than in any other crop. In the extraction of tocopherol, the highest yield was obtained at a pressure of 55 MPa with the addition of 5% ethanol as a solvent [48].
4. BIOACTIVE COMPOUNDS IN THE AMARANTH INDIVIDUAL
A food ingredient known as a bioactive chemical is one that when consumed alters the body’s cellular or physiological functions and may offer positive health effects in addition to simple nourishment. In the Indian subcontinent, Mexico, and South Africa, where public health concerns about malnourishment and food security are pressing, local vegetable intake and cooking practices are being taken into account as important aspects of a healthy lifestyle. Growing scientific data suggests that eating vegetables like Amaranthus regularly may help reduce the risk of lifestyle diseases such as diabetes mellitus, hypertension, and cardiovascular disease. This is because the plant’s numerous bioactives work in concert to target different cell signaling pathways at the same time, which regulates organ pathologies [49]. The wide range of bioactive chemicals present in different Amaranthus species presents a substantial opportunity for creating innovative functional foods and nutraceutical supplements. According to studies conducted in laboratories, Amaranthus’s ability to promote health is due to the synergistic interactions or combination of its various phyto-constituents, which are found throughout the plant. Regarding nutraceuticals, Amaranthus offers more affordable option than the expensive artificial supplements that many health conscious consumers choose [45]. Furthermore, encouraging the use of Amaranthus leaves as a vegetable and its grains as flour may have the practical effect of gaining popular acceptance and improving the plant’s availability and use in regular meals [50] [Table 3].
Table 3: Bioactive compounds in amaranths.
| Bioactive compounds | Subcategory | Structure | Health benefit | References |
|---|---|---|---|---|
| Phenolic acids | Vanillic acid | 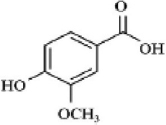 | Amaranths are recognized for their potential anti-cancer, anti-obesity, antidiabetic, antibacterial, anti-inflammatory, and antioxidant properties. | [80] |
| Gallic acid | 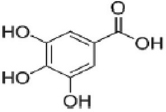 | Amaranth is associated with anti-inflammatory, anti-oxidative, anti-tumor, anti-bacterial, anti-diabetes, anti-obesity, anti-microbial, and antimyocardial ischemia properties. | [81] | |
| Sallicylic acid | 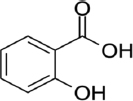 | Amaranths are associated with free-radical-scavenging activity | [81] | |
| Flavonoids | Quercetin | 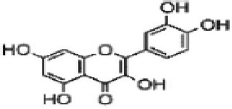 | Amaranth has antioxidant, antifungal, anti-carcinogenic, anti-inflammatory, hepatoprotective, and cytotoxic activity. | [82] |
| Rutin | 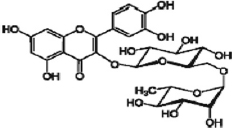 | Has anticancer, antioxidant, anti-diabetic, anti-inflammatory, antibacterial, antifungal, neuroprotective, cardioprotective, hepatoprotective, nephroprotective, and hematoprotective properties. | [83] | |
| Apigenin | 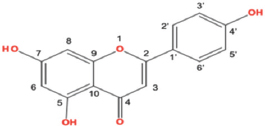 | All taxa roots contained apigenin with antioxidant activities. | [72] | |
| Amaranthine | 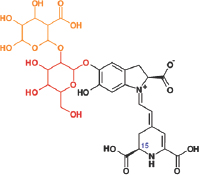 | Amaranths have antioxidant, antimicrobial, and cytotoxic properties. | [84] | |
| Carotenoids | Lutein | 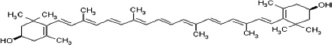 | Amaranth has antioxidant, antidiabetic, anti-inflammatory, and anticancer properties. | [85] |
| Zeaxanthin | 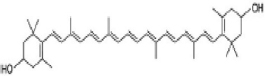 | Amaranth has antioxidant, anthelmintic, anti-osteoporotic, anti-inflammatory, and anticancer properties. | [86] | |
| Betanine | 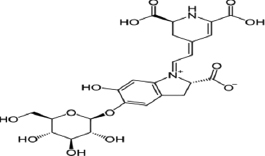 | Amaranth has antioxidant, antimicrobial, antidiabetic, anti-inflammatory, and anticancer properties. | [86] | |
| Sterol | Kaempferol | 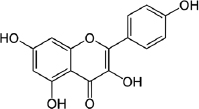 | Antibacterial against pathogens associated with urinary tract infections. | [86] |
| Fatty acids | Campesterol | 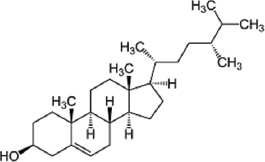 | Campesterols possess unique nutraceutical properties. | [86] |
4.1. Phenolic Acids
Amaranths are known to contain bioactive phytochemicals within their outer layers, which serve as natural defenses against insects and microorganisms [51]. These bioactive components can be either “lipophilic” or “hydrophilic” in nature. Amaranth seeds are rich in phenolic compounds, particularly phenolic acids, which are primarily found in their seed coats [52]. Amaranth, a nutritionally valuable pseudocereal, has been found to contain a variety of bioactive substances in its sprouts and seeds, including p-hydroxybenzoic acid, rutin, vanillic acid, and gallic acid [51]. These bioactive compounds in amaranths contribute to their nutritional and potential health-promoting properties, making them valuable supplements for a balanced diet [50].
4.2. Betalains
Betalain, a nitrogen-containing pigment, is notably abundant in pseudocereals. They are derived from tyrosine and subsequently transformed into L-3,4-dihydroxyphenylalanine. Betalains can be categorized into two main groups: betacyanins, which exhibit red-violet hues, and betaxanthin, which display yellow-orange colors [53]. The amaranth contains betacyanins, such as amaranthine and isoamaranthine [51]. These betalains contribute not only to the visual appeal of pseudocereals and their potential health benefits and antioxidant properties [50].
4.3. Carotenoids
Carotenoids are crucial compounds present in numerous fruits and vegetables and renowned for their antioxidant properties and potential to be converted into Vitamin A in the human body. These compounds offer a spectrum of benefits, including the amplification the activity of specific enzymes, enhancement of cell-to-cell communication, regulation of gene expression, fortification of the immune system, and assistance in plant photosynthesis as photosensitizers [51]. Amaranth is a high source of carotenoids, especially lutein and zeaxanthin which are powerful antioxidants. Amaranth is prized for its vibrant color, which comes from these carotenoids, and they provide health benefits such as helping maintain eye health and preventing age related macular degeneration. Eating amaranths as part of the diet provides natural sources of these essential nutrients.
5. ANTI-NUTRITIONAL FACTORS
The word “anti-nutritional factor” refers to substances found most abundantly in plant foods that can be associated with many nutrients present in different foodstuffs by digestion or utilization and can be dangerous to human health if ingested in higher quantities proportion [54]. Despite their medicinal benefits, particularly in the prevention and treatment of oxidative stress-related infections, phenolic compounds have been shown to influence protein digestion due to their ability to bind to stomach-related enzymes and protein substrates. According to several researchers, dietary polyphenols can interact with gluten proteins through protein sequestration and reduce their adverse effects on patients with celiac disease [55] [Table 4].
Table 4: Anti-nutritional components present in amaranths.
| S. No. | Antinutritional factor | Structure | Health impact | References |
|---|---|---|---|---|
| 1. | Phytic Acid | 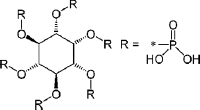 | The bioavailabilities of calcium, iron, copper, and zinc are significantly affected by | [87,88] |
| 2. | Tannins | 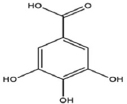 | A protein tannin complex is formed, which retards protein digestibility by inactivating digestive enzymes. | [87,88] |
| 3. | Oxalates | 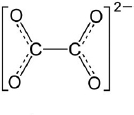 | Decreases mineral bioavailability in humans. | [87,88] |
| 4. | Trypsin inhibitor |  | Growth is hindered by unavailable proteins. | [58,87,88] |
| 5. | Saponins |  | Protein digestibility is affected by digestive enzyme inactivation. Examples such as glucosidase, trypsin, chymotrypsin, and lipase | [89] |
| 6. | Nitrates | 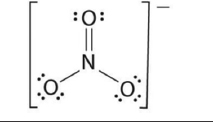 | The pulse rate is significantly affected, and weakness is also caused. | [90,91] |
Amaranth seeds contain 2.90–7.90 g/kg of phytic acid [56]. According to Kumar et al. [57], pseudocereals are rich sources of minerals and phytic acid. By chelating their divalent cations, phytic acids have been shown to be reduced the bioavailability of various minerals, including iron, zinc, magnesium, and calcium. This binding affects the limits of body ability to absorb these crucial elements, which can be harmful in populations in which these nutrients are deficient. In various amaranth cultivars, tannin content ranged from 0.40 to 5.20 mg/g. The tannin content isolated from raw, cooked, and roasted seeds of amaranth has been shown to have anti-diabetic characteristics, reducing α-amylase activity by 50% and α-glucosidase by 78% [47]. However, tannin poses a nutritional challenge by forming complexes with proteins, which reduces protein digestibility and digestive enzyme inactivity. The presence of antinutritional components, such as phytic acid, saponins, oxalates, and nitrates, may limit the health benefits of amaranths. The main storage form of phosphorus is phytic acid, which is present in pseudocereals. Similarly, the presence of tannins and other factors emphasizes the need for appropriate processing methods to reduce the concentrations of tannins and their adverse effects on human health [58]. Several processing methods have been investigated to reduce these anti-nutritional factors so as to enhance nutrient bio-digestibility. Fermentation has been found to inhibit the phytic acid effect up to 60% levels, leading to enhance absorption of zinc and iron. It can be immersed in water for 12–24 h, which reduces oxalate and increases the calcium solubility. Enzyme treatment particularly phytate enzymes have been found highly effective in the degrading of phytic acid, so far, the mineral bioavailability has increased by 30–50%. Heating techniques such as roasting and cooking also aids in decreasing the amount of tannin present which increases the digestibility of proteins. A comparative evaluation of these techniques indicates that fermentation and enzymatic methods are the best in the reduction of anti-nutrients with minimized effects on bioactive compounds.
6. HEALTH BENEFITS OF AMARANTH
The need of the current world is for functional food or food enriched in different nutrients that can promote the health of consumers, including reducing heart disease, diabetes, hypocholesterolemia, and chronic diseases. The grains obtained from small amaranth seeds can be used as an alternative or a substitute for grains such as rice and wheat [44]. Amaranths are a good source of various amino acids. Amino acids, such as arginine, serine, glutamic acid, methionine, lysine, and aspartic acid, were found in abundant amounts in amaranth seed grains. Compared with maize, amaranth has higher-quality protein content (high lysine content which cannot be found in cereals such as maize, wheat, and rice). It was also observed that the protein content of amaranths was almost as per the FAO/WHO-recommended standards [45]. Amaranth grain is also rich in oil (6–10%) as compared to other cereals and has about 77% of polyunsaturated oil rich in linoleic acid (50%). The Amaranth seed contained palmitic acid (19%), stearic acid (3.4%), oleic acid (34%), and linoleic acid (33%). The lipid ratio present in amaranth is rare as compared to other cereals due to the presence of various biologically active compounds such as 8% squalene, 2% tocopherol, 10% phospholipids, and 2% phytosterols. The high nutritional content of amaranth provides various health benefits to consumers, which include [59].
6.1. Hypocholesterolemia Effect
The presence of unsaturated fatty acids, total and soluble fiber, and the amino acid profile of its protein may be responsible for cholesterol level modulation. Tocotrienols, phytosterols, and tocopherol phytochemicals are also responsible for the hypocholesterolemic effect of amaranth. In the effect of supplementing the diet with amaranth for 6 weeks, it was observed that total and low-density lipoprotein cholesterol values in the blood of chicken decreased significantly, whereas the high-density lipoprotein-cholesterol density remained the same [45]. An enzyme such as cholesterol 7 alpha-hydroxylase was increased from 10% to 18% from the original value in the experimental group; however, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity in the experimental lot’s liver is lower (9%). The reduction in HMG-CoA reductase activity is the result of the presence of various important inhibitors that cause the synthesis of cholesterol [60]. Another study in which amaranth is combined with oat bran and fed to male buffalo rats showed that there was a significant decrease in the cholesterol present in total blood and liver, and the main decrease is the result of oat bran incorporation, whereas amaranth is responsible for the decrease in the TAG level in the liver [61].
6.2. Antitumor Effect
Chronic disease such as cancer is caused by the consumption of unhealthy diets. Diet is considered the main factor in the inhibition, as well as the promotion, of any disease. Elevation in the concentration of the Thomsen-Friedenreich antigen (TF-antigen, Gal-beta-3GalNAc alpha) serves as a primary indicator of epithelial malignant tumor and premalignancy [62]. The proliferative impact of diets containing two lectins capable of preventing TF-antigen formation – jaclin (from Artocarpus integrifolia) and amaranth lectin (from A. caudatus) – was investigated. Diets containing TF-binding lectins could serve as makers for detecting malignant gastrointestinal epithelial cells, thereby playing a significant role in the diagnosis of intestinal cancer [63].
6.3. Antioxidant Activity
Antioxidant activity of the two varieties was determined using lipid peroxidation. Antidiabetic activity of amaranths was due to the presence of methanolic extracts (50.5%) from A. caudatus. In this study, we examined the influence of amaranth grains on oxidative stress in the plasma, heart, kidney, and pancreas of rats. Oxidative stress was assessed by measuring fructose levels, and changes in malondialdehyde levels and antioxidant enzyme capacity in plasma and selected tissues were used as indicators [50]. This study demonstrated that after consuming amaranth grain at two different doses (310 and 150 g/kg of diet), there was a considerable increase in enzyme levels. In addition, a significant decrease in oxidative stress was noted, evidenced by a reduction in malondialdehyde levels, an elevation in ferric ion reduction capacity in plasma (FRAP), and increased activity of antioxidant enzymes, such as erythrocyte superoxide dismutase, catalase, and glutathione peroxidase 1 [64,65] [Figure 1].
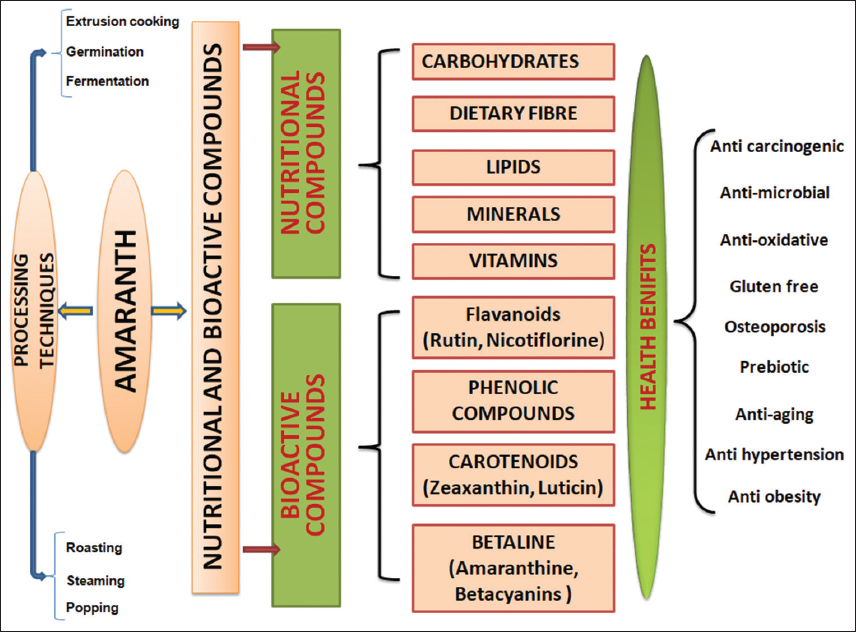 | Figure 1: Graphical representation of nutritional and bioactive compounds, processing techniques, and health benefits of amaranth. [Click here to view] |
7. AMARANTH PROCESSINGS AND ITS APPLICATIONS IN FOOD INDUSTRY
7.1. Processing Techniques
The processing parameters used can influence the levels of antinutrients, mineral content, antioxidant activity, and in vitro protein digestibility of grains and flours derived from various cereals and pseudocereals. Amaranth grains undergo various processing methods to produce products tailored to the preferences and usage habits of consumers [45] [Figure 2].
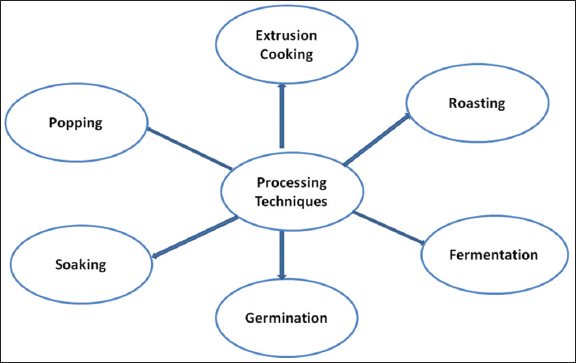 | Figure 2: Various processing methods for amaranth grains. [Click here to view] |
Amaranth grains are renowned for their nutritional value and culinary applications. To maximize their potential and cater to diverse consumer preferences, they employ various processing techniques. One popular method is popping, in which the grains are rapidly heated until they burst open, similar to popcorn, resulting in a crunchy, light texture. This process enhances the natural sweetness of grain while preserving its nutritional value, making popped amaranth an excellent addition to healthy snacks or cereals [66]. Another prevalent method is milling, where grains are finely processed into flour for various culinary uses, including thickening soups and sauces and baking bread and cakes. The resulting flour provides a gluten-free option for individuals with dietary restrictions while retaining the grain’s nutritional benefits. Cooking amaranth grains is a straightforward boiling or steaming process until they reach a soft consistency, making them suitable for incorporation into a variety of recipes, such as salads, stir-fries, side dishes, and soups. Cooking also enhances delicate texture and subtle flavors [67]. Moreover, enzymatic activity is stimulated and antinutrient levels, such as phytic acid and tannins, are reduced during germination, which is achieved by soaking grains in water for a specified duration. This process activates enzymes such as amylase and protease, which break down complex proteins and carbohydrates, respectively. This enhances the nutritional profile and digestibility of grains. Due to its ability to enhance the bioavailability of essential nutrients, germinated amaranths are excellent choices for health-conscious consumers. Extrusion, on the other hand, is a high-pressure and high-temperature processing technique that induces mechanical and chemical changes in amaranth grains. During this process, heat and shear forces denature proteins, gelatinize starch, reduce anti-nutritional factors, and significantly enhance the texture, digestibility, and shelf life of amaranth grains by shaping them into ready-to-eat snacks, breakfast cereals, or pasta varieties [66]. Traditional fermentation of amaranth grains with beneficial bacteria yields products with enhanced flavor, digestibility, and nutritional bioavailability, such as sourdough bread and fermented beverages. Roasting involves dry heating of grains in an oven or in a skillet to achieve a crispy texture and to impart a rich, nutty flavor. This makes them ideal as salad, yogurt, or dessert toppings to enhance the flavor and nutritional content of various foods. All these processing techniques improve the nutritional value and sensory qualities of amaranth grains while meeting the diverse dietary requirements and culinary demands of customers around the world [68].
7.2. In the Beverage Industry
Kunnu is said to be a fermented nonalcoholic drink prepared by various cereal grains targeting generally children, adults, and infants, where kannu is consumed as a weaning food in Nigeria. It has a distinct sweet and sour taste with a rather creamy appearance and low density. It can be prepared throughout the year and often consumed after the main meal as a carbonated drink due to its thirst-quenching properties, generally in summer. To enhance the flavor of this drink, different ingredients were also added to it, which include ginger, black pepper, or red pepper that not only improve the aroma quality but also act as purgatives and antidotes for flatulence and function to enhance lactation in nursing mothers. Due to the easy availability of its raw material, sunnis are cheap and nutritious compared with other carbonated drinks. The availability of cheap, highly nutritious, and easily accessible amaranth grains has enabled the production of various functional foods for the treatment of malnutrition, poverty, and food insecurity. Amaranth kannu is a functional food that can promote the health of consumers [69]. Kunnu production involves various processing operations. The amaranth grains were thoroughly cleaned to remove foreign particles such as dirt and sand. The weighed amaranth grains were then steeped in double the amount of water for 12, 24, and 36 h. Spices like ginger were then added to the sample, followed by wet milling and sieving of the wet sample. The sample was then allowed to ferment for 12–36 h followed by decanting. The fermented sample was then cooked for 5 min at 45°C. Addition of sugar was carried out, followed by packaging or pasteurization (65°C, 15 min) and then cooled at 29°C followed by storage if required [50].
7.3. Amaranth-containing Composite Flours in the Leavened Product
FAO recommended the formulation of fortified food with the help of “The Composite Flour Program” where various bread-making, baking goods, and pasta products have been prepared using the mixture of wheat flour and non-wheat high protein components such as legumes, tubers and roots, and other raw materials. The main objective of the program is to identify components or mixtures that are highly nutritious and have appropriate processing characteristics [50]. Due to its crop agricultural benefits and high nutritional value, amaranth has been considered a potential ingredient for non -wheat materials in composite flours. The only limitation of using amaranth in baking products is its lack of gluten, due to which the proper texture cannot be provided by the amaranth to the baked products, along with its distinct pungent flavor and aroma that causes the bitter aftertaste, further affecting the sensory appeal of the final product [70]. According to a study conducted by [71], composite flours containing amaranth have different pasting and rheological properties, which depend on the type of cereals used. It was observed that there was an increase in the initial gelatinization temperature and viscosity due to an increase in the replacement ratio of refined wheat flour with amaranth flour from 0% to 30%. In the readings obtained from the extremograms, it was recorded that there was an increase in the strength of the dough containing amaranth due to the gradual decrease in extensibility, which could further hinder the desirable texture and handling properties of the dough [64].
7.4. Amaranths as Feed
After the prohibition of meat-based farm feed and the occurrence of bovine spongiform and encephalopathy, the use of amaranth grains for animal feed has increased as they are considered high-quality raw materials. Amaranth can be utilized as feed for polygastric species due to its high protein content, rapid growth rate, and better results from studies carried out on its bypass protein or rumen undegraded intake protein [72]. Countries like China are cultivating amaranths in their fields solely for cattle feed. From the study carried out to check the forage quality parameters of amaranth (neutral detergent fiber, acid detergent fiber acid detergent lignin, in vitro dry matter digestibility, crude protein, and undegraded protein intake), it was observed that at a certain stage of growth, amaranth has good-quality forage [73]. High nitrate concentrations of amaranth grains were the main concern in this study, which led to the conclusion that amaranths cannot be fed to animals within 84 days of the plantation. The forage can be ensiled to reduce the nitrate concentration and improve the amaranth digestibility. It was also concluded from this study that A. lividus and Amaranthus mantegazzianum are more suitable for the ensilage and should be used with maize and sorghum (in the ratio of 1:1 and 1:5:1). It was also observed that amaranth harvesting should be conducted during the fruiting period [19].
7.5. Amaranth Oil-incorporated Infant Formulas
Enzymatic modification of amaranth oil can be used to achieve properties similar to breast milk fat analogs. A structural lipid was created by combining amaranth oil and milk fat, matching the levels of docosahexaenoic acid (DHA) with palmitic acid esterified at the sn-2 position of the TAG backbone. To produce amaranth oil-incorporated infant formula, customized amaranth oil was prepared through the of amaranth oil and ethyl palmitate using a lipase biocatalyst. DHA esterification was then carried out using lysozyme RM IM and sn-1, 3 specific lipases as biocatalysts [73].
In contrast, the commonly available infant formulas on the market typically use a blend of vegetable oils for preparation. This blend consists of palm oil (62%), soybean oil (25%), coconut oil (8%), and high oleic sunflower oil (5%). The coconut and palm oils were melted at 30°C before mixing with the soybean and high-oleic-sugar oils. These ingredients were thoroughly blended at temperatures of 50–60°C using a high-speed benchtop homogenizer. After double homogenization, the formula was collected in opaque screw-type containers and rapidly cooled [72]. These formulas were stored at 4°C for 24 h to ensure stability [74]. From this study, it was concluded that the developed formula consisted of 3.6% fat, 3% protein, and 4.5% carbohydrate. Most of the reduction in fat globule size occurs at the first homogenization stage (150–200 bar). The following homogenization, the average size of the fat globules was approximately 4 μm (with a maximum of 2 μm). In addition, a substantial size difference between the milk fat globule and other fat globules was observed, potentially contributing to the enhanced accessibility of TAGs from milk fat globules compared with other fat globules. This difference may lead to improved absorption and metabolism [75].
7.6. Commercialization Challenges
However, challenges act as barriers toward commercial use of amaranth-based composite flours in the food industry. Market penetration is limited due to the distinct pungent flavor and bitter aftertaste of amaranth, which lowers the sensory properties of baked products. Consumer acceptance is also hindered by limited knowledge on amaranth products and consumers’ traditions of taking wheat-based products. Furthermore, the cost of handling and maintaining product quality could be slightly more expensive than normal wheat flour especially for bulk production. There are also regulatory factors; labeling, claims, and fortification standards differ from country to country which poses a challenge to the company when expanding into new markets. Overcoming these barriers by adopting better processing methods, marketing strategies and policies could increase the market appeal of bakery products containing amaranth.
8. CONCLUSIONS
Amaranth (Amaranthus) is a promising pseudocereal with exceptional nutritional value, bioactive compounds, and health benefits. It is rich in essential components, such as proteins, dietary fiber, vitamins, and minerals, making it an ideal protein source for individuals with specific dietary requirements. In addition, amaranth contains bioactive compounds such phenolic compounds, flavonoids, and squalene, which exhibit antioxidant, anti-inflammatory, and anticancer properties. However, antinutritional factors, such as saponins, oxalates, and nitrates, may hinder their optimal use and pose risks if consumed excessively. These factors can be mitigated using proper processing methods that enhance the nutritional content of amaranth-based products. Regular consumption of amaranths has been associated with improved cardiovascular health, reduced chronic disease risk, and enhanced immune function. Moreover, the industrial potential of amaranths is expanding, with potential applications in gluten-free baking, as a source of unsaturated fatty acids and natural antioxidants, and in the development of functional foods, nutraceuticals, and pharmaceuticals. However, further research and development are required to enhance the efficacy, safety, sensory characteristics, techno-functional properties, and mechanisms underlying amaranth-based products.
9. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FUNDING
There is no funding to report.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This article does not contain any animal or human studies performed by any of the authors.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. Use of artificial intelligence (AI)-assisted technology
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Joshi DC, Sood S, Hosahatti R, Kant L, Pattanayak A, Kumar A, et al. From zero to hero:The past, present, and future of grain amaranth breeding. Theor Appl Genet 2018;131:1807-23.[CrossRef]
2. Hagos M, Chandravanshi BS, Redi-Abshiro M, Yaya EE. Determination of total phenolics, total flavonoid, ascorbic acid contents and antioxidant activity of pumpkin flesh, peel, and seeds. Bull Chem Soc Ethiopia 2023;37:1093-8.[CrossRef]
3. Bekkering CS, Tian L. Thinking outside the cereal box:Breeding underutilized (pseudo) cereals for improved human nutrition. Front Genet 2019;10:1289.[CrossRef]
4. Hoidal N, Jacobsen SE, Odone A, Alandia G. Defoliation timing for optimal leaf nutrition in dual-use amaranth production systems.J Sci Food Agric 2020;100:4745-55.[CrossRef]
5. Pirzadah TB, Malik B. Pseudocereals as super foods of 21st century:Recent technological interventions. J Agric Food Res 2020;2:100052.[CrossRef]
6. Mir NA, Riar CS, Singh S. Nutritional constituents of pseudocereals and their potential use in food systems:A review. Trends Food Sci Technol 2018;75:170-80.[CrossRef]
7. Morales D, Miguel M, Garcés-Rimón M. Pseudocereals:A novel source of biologically active peptides. Crit Rev Food Sci Nutr 2021;61:1537-44.[CrossRef]
8. Meena VS, Gora JS, Singh A, Ram C, Meena NK, Rouphael Y, et al. Underutilized fruit crops in arid and semi-arid regions of India:Importance, conservation conservation and use strategies. Horticulturae 2022;8:171.[CrossRef]
9. Peiretti P, Meineri G, Longato E, Tassone S. Chemical composition, in vitro digestibility, and fatty acid profiles of Amaranthus caudatus herbage during its growth cycle. Anim Nutr Feed Technol 2018;18:107-16.[CrossRef]
10. Aguilera JM. Food matrix:Implications in processing, nutrition and health. Crit Rev Food Sci Nutr 2019;59:3612-29.[CrossRef]
11. Gorinstein S, Vargas OJ, Jaramillo NO, Salas IA, Ayala AL, Arancibia-Avila P, et al. Total polyphenol content and antioxidant potential of selected cereals and pseudocereals. Eur Food Res Technol 2007;225:321-8.[CrossRef]
12. Orona-Tamayo D, Paredes-López O. Amaranth part 1-Sustainable crop for the 21st century:Properties of food and nutraceuticals for improving human health. In:Sustainable Protein Sources. Vol. 2. Netherlands:Elsevier;2017. 239-56.[CrossRef]
13. Manikandan S, Srimathi P. Studies on postharvest seed handling techniques for grain amaranth (Amaranthus hypochondriacus L.) . Suvarna. Curr Biotica 2014;8:13241.
14. Stetter MG, Vidal-Villarejo M, Schmid KJ. Parallel seed color adaptation during multiple domestication attempts of an ancient new world grain. Mol Biol Evol 2020;37:1407-19.[CrossRef]
15. Maurya NK, Arya P. Amaranthus grain nutritional benefits:A review.J Pharmacogn Phytochem 2018;7:2258-62.
16. Jimoh M, Afolayan A, Lewu F. Therapeutic uses of Amaranthus caudatus L. Trop Biomed 2019;36:1038-53.
17. Gangwal A, Parmar S, Sheth N. Phytochemical investigation and immunomodulatory activity of Lagenaria siceraria fruits.J Nat Remedies 2010;10:170-4.
18. Tatiya A, Surana S, Khope S, Gokhale S, Sutar M. Phytochemical investigation and Immunomodulator activity of Amaranthus spinosus Linn. J Agric Food Res 2007;41:337-41.
19. Manyelo TG, Sebola NA, van Rensburg EJ, Mabelebele M. Probable use of Genus Amaranthus as feed material for monogastric animals. Animals (Basel) 2020;10:1504.[CrossRef]
20. Apolot MG, Ssozi JJ, Namutebi A, Masanza M, Kizito EB, Rees DD, et al. Changes in the sensory and quality characteristics of S. (Shum) and A. lividus (Linn) leafy vegetables along the supply chain. Am J Food Technol 2018;6:161-6.
21. Van Volkenburg H, Guinel FC, Vasseur L. Effects of smooth pigweed (Amaranthus hybridus) on cover crops in Southern Ontario. J Agron 2020;10:529.[CrossRef]
22. Alemayehu FR, Bendevis M, Jacobsen SE. The potential for using the seed crop amaranth (Amaranthus spp.) East Africa as an alternative crop to support food security and climate change mitigation.J Agron Crop Sci 2015;201:321-9.[CrossRef]
23. Venskutonis PR, Kraujalis P. Nutritional components of amaranth seeds and vegetables:A review of composition, properties, and uses. Compr Rev Food Sci Food Saf 2013;12:381-412.[CrossRef]
24. Adegbola PI, Adetutu A, Olaniyi TD. Antioxidant activity of Amaranthus species from the Amaranthaceae family-A review. S Afr J Bot 2020;133:111-7.[CrossRef]
25. Akin-Idowu PE, Ademoyegun OT, Olagunju YO, Aduloju AO, Adebo UG. Phytochemical contents and antioxidant activities of five grain amaranth species. Am J Food Sci Technol 2017;5:249-55.
26. Thapa R, Blair MW. Morphological assessment of cultivated and wild amaranth species diversity. Agronomy 2018;8:272.[CrossRef]
27. Kachiguma NA, Mwase W, Maliro M, Damaliphetsa A. Chemical and mineral compositions of amaranth (Amaranthus L.) species collected from central Malawi. J Food Res 2015;4:92.[CrossRef]
28. Thakur P, Kumar K, Ahmed N, Chauhan D, Rizvi QE, Jan S, et al. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Curr Res Food Sci 2021;4:917-25.[CrossRef]
29. Coelho LM, Silva PM, Martins JT, Pinheiro AC, Vicente AA. Emerging opportunities for exploring the nutritional and functional value of amaranth. Food Funct 2018;9:5499-512.[CrossRef]
30. Rivero Meza SL, Hirsch Ramos A, Cañizares L, Raphaelli CO, Bueno Peres B, Gaioso CA, et al. A review of amaranth protein:Composition, digestibility, health benefits and food industry utilization. Int J Food Sci Technol 2023;58:1564-74.[CrossRef]
31. Bressani R. Amaranth composition and nutritional properties of amaranth. In:Amaranth Biology, Chemistry, and Technology. Florida:CRC Press;2018. 185-5.[CrossRef]
32. Wolosik K, Markowska A. Amaranthus cruentus taxonomy, botanical description, and review of its seed chemical composition. Nat Prod Commun 2019;14:1934578X19844141.[CrossRef]
33. López MG. Amaranth carbohydrates. In:Paredes-López O, editor. Amaranth Biology, Chemistry, and Technology. Boca Raton, FL:CRC Press Inc.;2018. 107-31.[CrossRef]
34. Kurek MA, Karp S, Wyrwisz J, Niu Y. Physicochemical properties of dietary fibers extracted from gluten-free sources:Quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and millet (Panicum miliaceum). Food Hydrocoll 2018;85:321-30.[CrossRef]
35. Zhu F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains:A review of their chemical structure, biological functions and food uses. Carbohydr Polym 2020;248:116819.[CrossRef]
36. Hejazi SN, Orsat V, Azadi B, Kubow S. Improvement of in vitro protein digestibility of amaranth grains through optimization of the malting process. J Cereal Sci 2016;68:59-65.[CrossRef]
37. Segura-Nieto M. Biochemistry of amaranth proteins. In:Amaranth Biology, Chemistry, and Technology. Vol. 1. United States:CRC Press;2018. 75-106.[CrossRef]
38. Bojórquez-Velázquez E, Barrera-Pacheco A, Espitia-Rangel E, Herrera-Estrella A, Barba de la Rosa AP. Protein analysis reveals differential accumulation of late embryogenesis abundant and storage proteins in seeds of wild and cultivated amaranth species. BMC Plant Biol 2019;19:59.[CrossRef]
39. Bojórquez-Velázquez E, Velarde-Salcedo AJ, De León-Rodríguez A, Jimnez-Islas H, Pérez-Torres JL, Herrera-Estrella A, et al. Characterization of morphological, proximal composition and bioactive compounds of wild and cultivated amaranth (Amaranthus spp.) species. J Cereal Sci 2018;83:222-8.[CrossRef]
40. Achilary DD, Khatri-Chhetri UU, Slaski J. Amaranth:An ancient and high-quality wholesome crop. In:Nutritional Value of Amaranth. London:IntechOpen;2020. 111-42.[CrossRef]
41. Constantino AB, Garcia-Rojas EE. Modification of the physiochemical and functional properties of amaranth protein isolates (Amaranthus cruentus BRS Alegria) treated with high-intensity ultrasound. J Cereal Sci 2020;95:103076.[CrossRef]
42. López DN, Galante M, Robson M, Boeris V, Spelzini D. Amaranth, quinoa, and chia protein isolates:Physicochemical and structural properties. Int J Biol Macromol 2018;109:152-9.[CrossRef]
43. Wirkowska-Wojdyla M, Ostrowska-Ligeza E, Górska A, BrysJ. Chromatographic and thermal methods to study fatty acid composition and positional distribution, oxidation kinetic parameters, and melting profile as important factors characterizing amaranth and quinoa oils. Appl Sci 2022;12:2166.[CrossRef]
44. Nasirpour-Tabrizi P, Azadmard-Damirchi S, Hesari J, Piravi-Vanak Z. Amaranth seed oil composition. In:Waisundara VY, editor. Amaranth Nutritional Value. Working Title. 1st ed. London:IntechOpen;2020. 91381.[CrossRef]
45. Aderibigbe OR, Ezekiel OO, Owolade SO, Korese JK, Sturm B, Hensel O. Exploring the potential of underutilized grain amaranths (Amaranthus spp.) the value chain for food and nutrition security:A review. Crit Rev Food Sci Nutr 2022;62:656-69.[CrossRef]
46. Srivastava S, Sreerama YN, Dharmaraj U. Effect of processing on the squalene content of grain amaranth fractions. J Cereal Sci 2021;100:103218.[CrossRef]
47. Garcia-Estepa RM, Guerra-Hernández E, Garcia-Villanova B. Phytic acid content of milled cereal products and breads. Food Res Int 1999;32:217-21.[CrossRef]
48. Tang Y, Li X, Chen PX, Zhang B, Liu R, Hernandez M, et al. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario, Canada and their overall contribution to nutritional quality. J Agric Food Chem 2016;64:1103-10.[CrossRef]
49. Jimoh MO, Afolayan AJ, Lewu FB. Nutrients and antinutrient constituents of A.. Cultivated on different soils. Saudi J Biol Sci 2020;27:3570-80.[CrossRef]
50. Jan N, Hussain SZ, Naseer B, Bhat TA. Amaranth and quinoa as potential nutraceuticals:A review of antinutritional factors, health benefits, and their applications in the food, medicinal and cosmetic sectors. Food Chem 2023;61:100687.[CrossRef]
51. Tang Y, Tsao R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory and potential health beneficial effects:A review. Mol Nutr Food Res 2017;61:1600767.[CrossRef]
52. Adetunji CO, Michael OS, Varma A, Oloke JK, Kadiri O, Akram M, et al. Recent advances in biotechnology applications for improving the production of secondary metabolites from quinoa. In:Biology and Biotechnology of Quinoa:Super Grain for Food Security. Vol. 1. Berlin:Springer;2021. 373-96.[CrossRef]
53. Esatbeyoglu T, Wagner AE, Schini-Kerth VB, Rimbach G. Betanin-a food colorant with biological activity. Mol Nutr Food Res 2015;59:36-47.[CrossRef]
54. De Oliveira Lopes C, Dessimoni GV, da Silva MC, Vieira G, Pinto D. Utilization, Nutritional and Antinutritional Composition of Quinoa Flour (Chenopodium quinoa). Alim Nutr 2010;20:669-75.
55. Ribeiro M, Sousa T, Poeta P, Bagulho AS, Igrejas G. Review of the features and binding capacity of polyphenols to gluten proteins and peptides in vitro:Relevance to celiac disease. Antioxidants (Basel) 2020;9:463.[CrossRef]
56. Koziol MJ. Evaluation of chemical composition and nutritional value of quinoa (Chenopodium quinoa Willd.). J Food Compos Anal 1992;5:35-68.[CrossRef]
57. Kumar V, Sinha AK, Makkar HP, Becker K. Dietary roles of phytate and phytase in human nutrition:A review. Food Chem 2010;120:945-59.[CrossRef]
58. Joye I. Protein digestibility of cereal products. Foods 2019;8:199.[CrossRef]
59. Namiya PS. Development of Snacks by the Incorporation of Amaranth Seeds. St. Teresa's College (Autonomous), Emakulamia, Doctoral Dissertation;2020. 89.
60. Shahwan M, Alhumaydhi F, Ashraf GM, Hasan PM, Shamsi A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance Int J Biol Macromol 2022;206:567-79.[CrossRef]
61. Chmelík Z, ŠnejdrlováM, Vrablík M. Amaranth as a potential dietary adjunct for lifestyle modification to improve cardiovascular risk profile. Nutr Res 2019;72:36-45.[CrossRef]
62. Madadi E, Mazloum-Ravasan S, Yu JS, Ha JW, Hamishehkar H, Kim KH. Therapeutic applications of betalains:Areview. Plant (Basel) 2020;9:1219.[CrossRef]
63. Quiroga AV, Barrio DA, Añón MC. Amaranth lectin presents potential antitumor properties. LWT 2015;60:478-85.[CrossRef]
64. Pulipati S, Babu PS, Naveena U, Parveen SR, Nausheen SS, Sai MT. Determination of total phenolic, tannin, flavonoid contents and evaluation of antioxidant property of Amaranthus tricolor (L). Int J Pharm Phytochem Res 2017;9:814-9.[CrossRef]
65. Sharma M, Usmani Z, Gupta VK, Bhat R. Valorization of fruit and vegetable waste and by-products to produce natural pigments. Crit Rev Biotechnol 2021;41:535-63.[CrossRef]
66. Malik M, Sindhu R, Dhull SB, Bou-Mitri C, Singh Y, Panwar S, et al. Nutritional composition, functionality, and processing technologies for Amaranth. J Food Process Preserv 2023;2023:1753029.[CrossRef]
67. Gelaye Y. A review of amaranth crop as a potential solution to Ethiopia's nutritional crisis. J Diet Supp 2023;15:101-10.[CrossRef]
68. Baraniak J, Kania-Dobrowolska M. The dual nature of amaranth-Functional food and potential medicine. Food J 2022;11:618.[CrossRef]
69. Isaac-Bamgboye FJ, Edema MO, Oshundahunsi O. Nutritional quality, physicochemical properties, and sensory evaluation of Amaranth-Kunu produced from fermented-grain amaranth (Amaranthus hybridus). Ann Food Sci Technol 2019;20:322-31.
70. Miranda-Ramos KC, Sanz-Ponce N, Haros CM. Evaluation of technological and nutritional quality of bread enriched with amaranth flour. Wheat 2019;114:108418.[CrossRef]
71. Chauhan A, Saxena D, Singh S. Physical, textural, and sensory characteristics of wheat and amaranth flour blend cookies. Convincing Food Agric 2016;2:1125773.[CrossRef]
72. Gandhi P, Samarth RM, Peter K. Bioactive compounds of amaranth (genus Amaranthus). In:Murthy HN, Paek KY, editors. Bioactive Compounds in Underutilized Vegetables and Legumes. Reference Series in Phytochemistry. Cham:Springer;2020. 1-39-74.[CrossRef]
73. Peiretti P. Amaranth in animal nutrition:A review. Livest Res Rural Dev 2018;30:1-20.
74. Fei P, Feng H, Wang Y, Kang H, Xing M, Chang Y, et al. Amaranthus tricolor crude extract inhibits Cronobacter sakazakiiisolated from powdered infant formula. JDS 2020;103:9969-79.[CrossRef]
75. Okoth JK, Ochola SA, Gikonyo NK, Makokha A. Development of a nutrient-dense complementary food using amaranth-sorghum grains. Food Sci Nutr 2017;5:86-93.[CrossRef]
76. Jimoh MO, Afolayan AJ, Lewu FB. Suitability of Amaranthus species to alleviate human dietary deficiencies. S Afr J Bot 2018;115:65-73.[CrossRef]
77. Soriano-García M, Ilnamiqui Arias-Olguín I, Carrillo Montes JP, Genaro Rosas Ramírez D, Mendoza Figueroa JS, FloresValverde E, et al. Functional nutritional value and therapeutic use of amaranth. J Anal Pharm Res 2018;7:596-600.[CrossRef]
78. Sharmila M, Rajeswari M, Vijayashalini P. Comparison of qualitative phytochemical analysis of Amaranthus polygonoides L and Amaranthus viridis L. Int J Appl Res 2017;3:280-3.
79. Reyes MF, Chávez-Servín JL, Gonzlez-Coria C, Mercado-Luna A, Carbot K, Aguilera-Barreyro A, et al. Comparative account of phenolics, antioxidant capacity, a-tocopherol and antinutritional factors in Amaranth (Amaranthus hypochondriacus) plants grown in greenhouses and open field. Int J Appl Res 2018;20:2428-36.
80. Kaur J, Gulati M, Singh SK, Kuppusamy G, Kapoor B, Mishra V, et al. Discovering the multifaceted role of vanillic acid beyond flavors:Nutraceutical and therapeutic potential. Trends Food Sci Technol 2022;122:187-200.[CrossRef]
81. Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, et al. Gallic acid:Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother 2021;133:110985.[CrossRef]
82. Bertazzo A, Comai S, Brunato I, Zancato M, Costa CV. The content of protein and nonprotein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem 2011;124:93-6.[CrossRef]
83. Prasad R, Prasad SB.A review of the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J Pharm Pharmacol 2019;5:1-20.[CrossRef]
84. Roriz CL, Xavier V, Heleno SA, Pinela J, Dias MI, Calhelha RC, et al. Chemical and bioactive properties of Amaranthus caudatus L flowers and optimized ultrasound-assisted extraction of betalains. Food J 2021;10:779.[CrossRef]
85. Srividya AR, Vishnuvarthan VJ. Physical, chemical, and biological properties of lutein:A review. World J Pharm Res 2014;3:105-18.
86. Bouyahya A, El Omari N, Hakkur M, El Hachlafi N, Charfi S, Balahbib A, et al. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci 2021;118:519-38.[CrossRef]
87. Samtiya M, Aluko RE, Dhewa T. Plant food antinutritional factors and their reduction strategies:An overview. FPPN 2020;2:6.[CrossRef]
88. Kaushik G, Singhal P, Chaturvedi S. Food processing to increase consumption:The case of legumes. In:Food Processing for Increased Quality and Consumption. Vol. 18. Cambridge, MA, USA:Academic Press;2018. 1-28.[CrossRef]
89. Nørgaard JV, Malla N, Dionisio G, Madsen CK, Pettersson D, Lærke HN, et al. Exogenous xylanase or protease activity in pigs fed barley cultivars with high or low enzyme inhibitors. AFST 2019;248:59-66.[CrossRef]
90. Nyonje WA. Nutrients, Anti-Nutrients and Phytochemical Evaluation of Ten Vegetable Amaranth (Amaranthus spp.) Varieties at Two Growth Stages. Doctoral Dissertation;2015.
91. Sinha K, Khare V. Review on antinutritional factors in vegetable crops. J Pharm Innov 2017;6:353-8.