Food is a necessary ingredient for existence of each living forms. Each and every living being consumes food for survival, from micro-organisms consuming macro- and micro-molecules, namely, fats, sugars, proteins, nitrogenous components, vitamins and minerals to human consuming fruits, vegetables, meat, and dairy products to name a few. Some major problems arise from the mismanagement of food in any stage of its life cycle result in serious outcomes at socioeconomic and environmental level. Food and Agricultural Organization (FAO) has predicted that 1.3 billion tons globally annual waste has been generated by edible part of food [1]. Food waste may be assumed as edible and inedible portion of food. These wastes are generated through all stages of food’s life cycle starting from agricultural practices followed by postharvest operations to their processing and manufacturing in industries and finally distribution of the packaged food products. Most of the food wastes are generated in household activities, food manufacturing plants, food service sectors, and remainder of the food waste is contributed during storage and transport [2]. Landfill or incineration of surplus food products results in production of greenhouse gases such as methane, carbon dioxide, and carbon monoxide and they directly affect climate change, air quality, and well-being of living organisms [3]. To maintain environmental stability and socioeconomic balance, valorization of food waste with an emphasis on extraction of value-added compounds is an approach gaining global attention.
Bioactive compounds have been consumed by humans from the very inception of their existence. Various evidences have been found throughout the years of usage of medicinal plants of specific foods for curing specific types of disease. Across South-East Asia, there are several ancient architecture that date backover a 1000 years, describing the usage of different foods for curing ailments [4]. It is proven that bioactive compounds from animal and plant-based food products have several beneficial effects on human [5]. There are varieties of bioactive components present including long chain polyunsaturated fatty acids, vitamins, carotenoids, peptides, tannins, flavonoids, phenolic acids, and minerals [6]. Food products from animal origin, such as meat and other meat originated products contains sizeable amount of bioactive compounds present in them [7].
Nutraceuticals has shifted to be the leading norm in the allied area of food and nutrition. The term nutraceutical was first coined by Stephen L. De Felice first in the year 1989 [8]. Nutrient factors combined with biologically active substances are thought to play an important role in overall human health. As presented by its inventers, the description of nutraceuticals can be elaborated as an object that may be food itself or part of food, providing medical and/or health advantages which may include the forestallment and treatment of some kind of illness or disease [9]. It could be comprised components such as isolated nutrients, dietary supplement, and processed food [8]. Nutraceuticals are therapeutic resources but cannot supersede chemically synthesized medicines. Metabolic syndrome, heart disease, and diabetes can be staved off with the aid of nutraceuticals or they can be used for those patients who do not qualify for standard pharmacological treatments [10,11]. The possibilities that nutraceuticals provide to support or as an alternative to traditional pharmacological treatments, may be a possible path to cure pathological, chronic, and long-term diseases which previously cannot be cured by traditional treatments. Simultaneously, new sources of nutraceuticals from edible source and their wastes are being explored which might provide novel nutraceuticals and/or their new sources that can be used in some specific health conditions [12].
Carotenoids are from the tetraterpenes family, a colorful liposoluble natural pigment, giving fruits and vegetables their bright red, orange, and yellow color. An array of things features carotenoids in them including plants, microbes such as bacteria, fungi, and algae, and in many fruits, vegetables, and aquatic flora and fauna [13]. Various extraction method of carotenoids are Soxhlet solvent extraction (SE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), super critical fluid extraction, enzyme aided extraction, high-shear dispersant extraction, and pulsed electric field (PEF)-assisted extraction. The trend to utilize carotenoids as bioactive agents or valued nutraceutical component has skyrocketed over past decade. Being a nutraceutical, carotenoids can be characterized according to its source, similar to prebiotics, probiotics, and polyphenols [8]. In some research, work conducted in the past by several research groups showed various nutraceutical applications of carotenoid in human diseases such as reducing obesity-related pathophysiology, type two diabetes and cardiovascular disease, as well as anti-carcinogenic and antioxidant properties. Suggestions were made on the antioxidant, anti-apoptotic, and anti-inflammatory effects of carotenoid helps in suppressing various effects of diabetes. a-carotene, b-carotene, g-carotene, and lycopene have antioxidant activities. Beta-carotene is also precursor of Vitamin A [14]. Astaxanthin, a xanthophyll carotenoid, is suggested for use as therapeutic agent for cardiovascular diseases [15]. Lutein and zeaxanthin have pharmacological effects on visual disorders and cognition disease [16].
Although carotenoids are present in lot of foods such as fruits and vegetables, the wastage part of those food components provides an enormous opportunity to collect the important bioactive compound from food waste. This property will add value to the waste utilization of surplus postharvest loss of such food products. Advances in current scientific and technological capabilities allow us to extract, characterize, and use nutraceuticals and bioactive components from foods as well as their wastage parts. Fruits and vegetables of bright red and yellow colors such as mango [17], passion fruit [18], peach [19], banana, persimmon [20], tomato [21,22], and carrot [23] have high quantity of carotenoids present in their peels which usually get thrown out and wasted. The present review discusses the various sources and extraction methods of carotenoids from food wastes that can be utilized as potential nutraceutical components.
2. CHEMICAL NATURE OF CAROTENOIDS
Carotenoids are tetraterpenoids which belong to the groups of lipids named terpenoids. It displays orange, red, yellow, and purple color. They are lipophilic compounds, which tend to be insoluble in water due to their extreme hydrophobia. Carotenoids are wide-ranging in nature, from photosynthetic bacteria to fungi, algae, archaea, animals, and plants [24]. Natures composition contains over 650 types of carotenoids. Among them almost 100 of them are present in the human food diet [25].
Most of the carotenoids are made up of eight isoprene units along with a 40-carbon skeleton. Overall, structure of carotenoids is made up of a polyene chain with nine conjugated double bonds and an end group at both ends of the polyene chain [24]. The structures of various types of carotenoids are shown in Figure 1 below. Carotenoids can be classified into provitamin A, and non-provitamin A compounds. However, on the basis of their functional groups, they can be divided as: carotenes, which does not have any functional group except the parent hydrocarbon chain (such as a-carotene, b-carotene, and lycopene) and xanthophylls, that contain oxygen as a functional group (such as lutein and zeaxanthin). In xanthophyll molecules, oxygen atoms are present as hydroxyl, carboxyl, carbonyl, aldehyde, epoxide, and furanoxide groups [13,24]. Carotenoids can have different isomeric configurations, trans and cis isomers, from their carbon-carbon bond. The isomers of carotenoids differ in their solubility, melting points, stability, and ultraviolet characteristics [26,27]. In Figure 1, different chemical structures of various carotenoids compounds are shown.
 | Figure 1: Structures of different carotenoids. [Click here to view] |
Besides the general classifications, carotenoids may have sub-classes based on their number of carbon atoms and double bonds. Normal carotenoids are formed from 40 carbon atoms and eight C5 isoprenoid unit. Despite the most abundance of C40 carotenoids in nature, some of them might have shorter (C30) or longer (C45, C50) carbon chain. As the carbon chain gets shorter or longer, the isoprenoid units also decrease or increase, respectively. For example, C30 carotenoids contain six C5 isoprenoid units whereas C45 and C50 are made up of 9 and 10 units of isoprenoids, respectively [Figure 1]. Decaprenoxanthin is an example of C50 carotenoids. Another subclass is secocarotenoids. In this subclass, a bond between the adjacent carbons is broken, except the first and sixth carbon in ring [28].
3. SOURCES OF CAROTENOIDS
An abundance of carotenoids can be found in the natural world, which can be seen in various parts of plants (leaves, flowers, vegetables, fruits, seeds, and roots). Further, they are located in fatty matter like yolks of eggs or other internal fats. In fatty fishes like tuna, salmon carotenoids are found in fat tissues or bound with flesh. A variety of plant and animal material, as well as microbial life, produces carotenoids.
3.1. Plants
Carotenoids, considered as secondary metabolites, are necessary for many biological activity and growth of plants. They give the bright red, yellow, and orange color to plants, leaves, flours, and fruits. Many flowers contain chromoplasts that contain carotenoids giving them their bright colors. Similar thing occurs in case of fruits. Phytoene, phytofluene, lycopene, and β-carotene are found in red-, yellow-, and orange-colored fruits and xanthophylls are found in green vegetables. Dark green leaves such as spinach and kale have higher concentration of carotenoids than light green vegetables such as lettuce and cabbages. Bright colored fruits such as tomato, watermelon, apricot peaches, mango, guava, and orange have high concentrations of carotenoids. Other vegetables such as beet roots, carrot, sweet potatoes, and bell papers also contain carotenoids [27,29].
3.2. Micro-organisms
Microalgae are a great source of carotenoids. They can be classified according to their photosynthetic pigments such as red algae (rhodophyta), green algae (chlorophyta), and brown algae (phaeophyta). Bioactive compounds such as lutein, β-carotene, and lycopene are widely available in microscopic algae. They are some significant carotenoids with high economic merit [26]. Many bacteria are able to produce carotenoids by chromatophores. They act as secondary metabolites to play key roles in cell adaptability. Bacteria, namely, Flavobacterium spp., Agrobacterium spp., Chromobacterium spp., Arthrobacter spp., Pseudomonas aeruginosa, and Rheinheimera spp. are some prominent producer of carotenoid pigments [27,30]. In comparison with other micro-organisms, fungi have the ability for production and accumulation of appreciably greater levels and concentrations of carotenoids intracellularly. Several classes of fungi including zygomycetes, ascomycetes, basidiomycetes, and the deuteromycetes are capable of producing greater level of carotenoids. Some example include Basidiomycete yeast, Blakeslea trispora, Neurospora crassa, and Xanthophyllomyces dendrorhous [27,31].
3.3. Animals
In case of bright colored birds, their red, orange, yellow, pink, and various color are the result of carotenoids colorization. These colors come from the various carotenoid rich diets of the birds, such as fruits and seeds high in a and b-carotene. Some examples of these birds are song birds and flamingo [32]. In case of fishes, most of the red fish and fishes with more fat concentration have high carotenoids stored in them, such as salmon, trout, and tuna. These carotenoids mainly come from their diet of microalgae, plankton, small insect, and shrimps. Red shrimps and lobsters also contain high level of dietary carotenoids such as astaxanthin which got released while cooking [33]. In case of eggs, the yellow-orange color comes from carotenoids also. β-carotene can also be detected in cow milk.
3.4. Human Diet
Human body can acquire carotenoids from various fruits and vegetables, animal foods, and other nutritional supplements. Microalgae are widely used to produce nutritional supplements containing carotenoids. Some of the examples are brown sea-weed (Undaria pinnatifida) and red algae (Chondrus crispus) [34]. When it comes to foods derived from animals, the yolk of eggs is particularly high in carotenoids. Milk, flesh, liver, and fat tissues of poultry and cattle also contain significant level of carotenoids. Red fishes, such as salmon and trout, have carotenoids in their tissue [35,36]. In case of plant and plant-based food products, carotenoids are available in bright red, yellow, and orange colored fruits (mango, orange, guava, papaya, watermelon, apricot, and peach) and vegetables (tomato, bell paper, carrot, beet roots, spinach, and kale), herbs (basil and coriander), legumes [37], cereals (wheat, maize, rice, and corn) [38], and seeds and seed oil (palm oil). In Table 1, various carotenoids found in a human’s daily dietary intake are shown.
Table 1: The different sources of carotenoids that can be found in human dietary intake.
| Carotenoids | Sources | References |
|---|---|---|
| α-Carotene | Tomato, Carrot, Broccoli, Spinach, and Corn | [141] |
| β-Carotene | Carrot, Mango, Tomato, Apricot, Pumpkin, Spinach, and Kale | [141,142] |
| Lutein | Egg yolk, Corn, Tomato, Banana, Kale, and Spinach | [143,144] |
| Zeaxanthin | Paprika, Red paper, Maize, and Green vegetables | [143,144] |
| Astaxanthin | Crab, Trout, Green Algae, and Salmon | [145] |
| Lycopene | Watermelon, Tomato, Guava, and Peach | [146,147] |
| Canthaxanthin | Mushroom, Salmon, and Crustacea | [148] |
| Fucoxanthin | Brown sea weed | [149] |
| β-Cryptoxanthin | Orange, Papaya, Mango, Peas, and Peach | [141,150] |
3.5. Food Wastes
Although carotenoids are present in several human dietary sources, a huge amount of them get wasted as a form of food waste such as, some of fruits peels contain more carotenoids than the pulp. Such fruits and vegetables of which the peels and pulps (after extracting juice out of it) get wasted as most of the time those things were used in landfill or animal feed or in biofertilizer/biogas production. Food waste such as mango peel, peach peels and pulp, apricot waste, tomato peel and pulp, carrot peels, and passion fruit peels are high sources of carotenoids. Some other food waste except fruits and vegetables include coffee grounds, having rich number of carotenoids present in them. Sea food waste like shrimp shells also contain collectable amount of carotenoid.
4. HEALTH BENEFITS OF CAROTENOIDS
Apart from using as a natural dye in food to make it more appealing to customers, carotenoids have various health benefits.
4.1. Lutein-Zeaxanthin
Zeaxanthin is the stereoisomer of lutein. Both of them have similar function in human health, first as an antioxidant [12]. They act as a screen to safeguard one’s skin from ultraviolet (UV)-light; in addition, lutein has been suggested as having the potential to diminish cardiovascular system risks. Zeaxanthin and lutein are found in the macular pigment of human retina. Supplementation of lutein and zeaxanthin can increase macular pigment optical density (MPOD) values and reduce the risk of age-related macular degeneration in individuals with low MPOD (0.2 or lower) [39,40]. They also protects retina from photochemical injury and UV-induced peroxidation [41]. Several studies showed lutein having anti-cancerous properties against certain types of cancer. It improves the immune response also, both humoral and cell mediated [42].
4.2. Astaxanthin
It has similar antioxidant properties such as lutein as well as anti-inflammatory properties. Human clinical trials on eye health showed positive effects of dietary astaxanthin on diabetic retinopathy, eye strain and fatigue, and macular degeneration [27]. It increases the phagocytic and fungicide capacity of neutrophils. It increases lymphocyte proliferation and the cytotoxic activity of natural killer cells [43]. Increased consumption of astaxanthin decreases the risks of certain types of cancers [44]. Moreover, it has been shown to reduce cardiovascular health disease since it reduces low-density lipoprotein peroxidation and improves the blood flow capacity and blood lipid profiles [45]. It is one of the strongest antioxidant of carotenoids family, exhibiting Vitamin E and β-carotene. Application of astaxanthin with aspirin shows sun-proofing and anti-aging properties as well as anti-inflammatory activities [26].
4.3. Canthaxanthin
Canthaxanthin shows positive effect on neurological disorders, as a neuroprotective agent due to its antioxidant and anti-inflammation properties [13]. It enhances the communication between cells through gap junctions either directly or by forming 4-oxo-retinic acid, stimulating the retinoic acid stimulator. These mechanisms provide it with antitumor activities. Canthaxanthin can prevent some blood disorder disease; however, consumption of large quantity is required which might be unsafe [27,46].
4.4. Lycopene
The carotenoid is reported to have anticancer properties especially prostate cancer. A study conducted by Zu et al. [47] on 49,898 male health professionals showed lower risk of fatal prostate cancer for the candidates having dietary intake of lycopene as compared to those who consumed less lycopene. The sources of lycopene in diet were pink grapefruit, tomato, tomato sauce, tomato juice, watermelon, and pizza [47]. Lycopene enhances the integrity of vascular barrier, inhibits the barrier permeability, cells adhesion molecule, and prevents leucocyte adhesion and trans-endothelial migration. Doing so lycopene suppresses pro-inflammatory response. It also shows antioxidant activities and has potential roles in cardiovascular health [48].
4.5. β-carotene
Beta-carotene shows provitamin A functions in human body. It is used as a supplement for Vitamin A deficiency. Regular intake of β-carotene can affect eye health and reduce macular diseases. It also shows anti-cancerous and antioxidant activities. In various studies, β-carotene has shown protection against sunburns [49].
5. EXTRACTION METHODS OF CAROTENOIDS FROM VARIOUS SOURCES
The stability and yield of carotenoids depend on their source and extraction methods or conditions [Table 2]. Usage of organic solvent is a traditional method for extraction but it is getting replaced by green extraction techniques for being challenging and time consuming. One of the more traditional methods is Soxhlet SE method, but new methods, such as MAE, UAE, and supercritical fluid extraction (SCF) methods, are replacing it. New studies are investigating and comparing them for better quality and yield [50]. In Figure 2, various processes for the extraction of carotenoids are shown.
Table 2: The methods of extraction of carotenoids.
| S. No. | Source | Method of extraction | Extraction yield | References |
|---|---|---|---|---|
| 1. | Carrot waste | Ultrasound-assisted extraction | 83.32% | [85] |
| 2. | Lemon waste | Microwave-assisted extraction | 3.2−6.2% w/w | [151] |
| 3. | Tomato pulp waste | Soxhlet solvent extraction | 94.7% | [152] |
| 4. | Pumpkin rind | Super critical fluid extraction | 20.50 mg/100 g | [153] |
| 5. | Blueberry byproducts | Pulsed electric field-assisted extraction | 55% | [154] |
| 6. | Tomato peel | Enzyme-aided extraction | 55.15 mg/100 g | [69] |
| 7. | Bael fruit pulp | High-shear dispersant extraction | 4.14 μg/100 g | [155] |
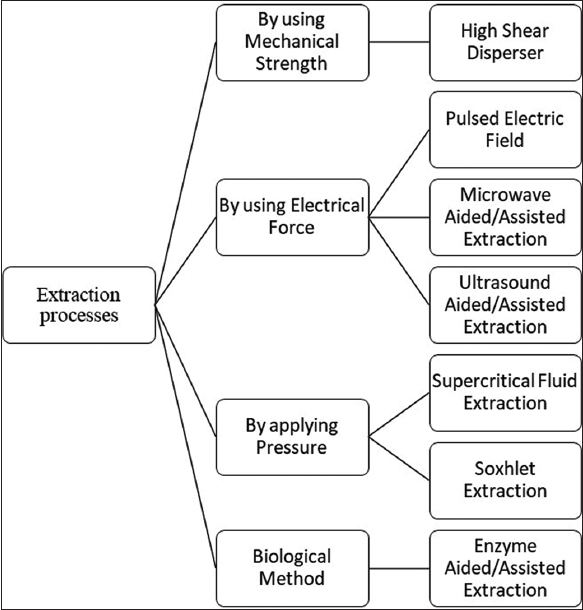 | Figure 2: Various extraction processes of carotenoids. [Click here to view] |
5.1. Soxhlet SE
SE is one of the traditional methods. It utilizes a solid-liquid extraction method that employs continuous reflux of natural solvents to enable the transfer of non-harmful biological molecules in large quantities. Some of the solvents used for carotenoids extraction include hexane, ethanol, acetone and chloroform. Although this technique extracts the largest amount of carotenoids from a given sample; nowadays, it is not preferred due to more time consumption, lengthy processing time, requirement of high amount of organic solvents, and increased extraction cost [51,52].
5.2. MAE
This method employs microwave radiation to heat up the solvent that further enhances transfer of solutes present in the matrix to the solvent. Microwave radiation is capable of rupture plant cell walls, resulting in an easy release of bioactive compounds present in cells into solvents. Both polar and non-polar solvent are used in microwave extraction such as aglycones, flavonoid glycosides, acetone, chloroform methanol, and ethanol with different concentration. This method is more appreciated presently, for being simple, time convenient and cost effective. Although this method requires less time and less amount of solvent, the yield of this technique is very low as compared to Soxhlet extraction method. Increase of extraction time can cause thermal degradation of bioactive compound, so microwave time and power optimization is critical issue in this technique [51,53].
5.3. UAE
This technique utilizes the mechanical energy produced by ultrasound waves. The sonication generates small bubbles or gaps in the liquid, resulting in the falling apart of the internals, due to reaching high temperature (4500°C) and pressure (50 MPa) [54]. In this method, ultrasonic probe is submerged in the solution and generates ultrasonic power at least 100 times greater than that of the bath, with a typical sonication time of 5 min or less. In the study of Goula et al., [55] a Ti-Al-V sonoprobe (13 mm) equipped 130 W maximum nominal output power, 20 kHz frequency VCX-130 Sonics and Materials (Danbury, CT, USA) sonicator was employed for pulsed mode ultrasound-assisted extraction for extracting carotenoids from pomegranate waste. The sample was combined with 200 mL solvent to obtain different peels/solvent ratios. The sample container was kept in a thermostat-controlled water bath during the extraction procedure. The extracts were collected at 10, 20, 30, 40, 50, and 60 min and filtered through glass microfiber paper to eliminate particle residues before to carotenoids analysis. As this technique is performed in low temperature, thermal damages can be avoided [55].
5.4. Super Critical Fluid Extraction of Carotenoids
Supercritical fluids are either liquid or gas used above critical pressure and temperature where gas and liquid can coexist. For this nature, gas and liquid phase become indissociable form each other. In this method, mostly pressurized fluid is used as solvent and carbon dioxide gas is being used. It becomes advantageous for its greater range of extraction ability [56]. Total carotenoids were extracted by super fluid extraction (SFE) utilizing S-CO2 at 15 g/min and optimized processing settings were 30 min at 59°C, 350 bar, and 15.5% (v/v) ethanol [57].
5.5. Enzyme-Aided Extraction
Enzymes act as catalysts in the process of extracting bioactive compounds. The raw material is treated with enzymes, allows it to hydrolyze the cell wall and the phytochemicals will come out along with carbohydrate chains and lipid molecules. Drawback of this process is factors affecting the extractions such as type, dosage, and condition of enzyme particle size of sample materials and its water content among other factors. Some of its advantages compared to previous methods are high yield and low-energy consumption. Some of the enzymes used in this method are proteases, tannases, pectinases, and cellulose [50].
5.6. High-Shear Dispersant Extraction
In this technique, cell wall and membrane of source is disrupted by mechanical pressure, using high-shear dispersant, releasing the compound present within the cells. To effectively and selectively extract water-soluble proteins, carbohydrates, and C-phycocyanin, the use of a mechanical pre-treatment like high-shear homogenization (HSH - 20,000 rpm, 96 kJ/kgSUSP) in combination with (PEFs - 20 kV/cm, 100 kJ/kgSUSP) has been advocated [58]. This process is less time consuming than many other traditional techniques. Vegetable oils are used as an organic solvent in this process, which reduces the energy consumption as well as environment friendly. It also avails the possibility to obtain uncontaminated carotenoids, directly usable without any need of purification. A disadvantage of using vegetable oil is its viscosity, elevated viscosity reduces solvent diffusivity [55,59].
5.7. PEF-Assisted Extraction
PEF-assisted techniques are based on the electroporation, or electropermeabilization, phenomenon, which depicts the development of pores in cell membranes as a result of electric pulses with short durations (from several nanoseconds to several milliseconds) and high electric field strengths (from 100–300 V/cm up to 300 kV/cm) [60]. Using electric fields for extraction processes can decrease the necessary temperature and increase the overall yield. This is extremely important for thermolabile compounds like carotenoids. The cellular components must be exposed to a strong electric field (external) in range of 1–50 kV/cm for a brief period during the extraction process. The cell membrane barrier allows for the electrical potential to divide molecules depending on its charge. The formation of pores is a result of this electro-permeabilization technique, which may or may not be reversible, in the cell membrane or wall, allowing the components of the cells to come out. Low-energy consumption and processing cost along with high efficiency and low temperature are some of the advantages of this process. The absence of any petrochemical solvent makes this technique environment friendly. Without damaging the matrix, the technique enhances the selective separation of biologically active components. However, the technique must be adjusted differently for different types of samples, as the parameters for a process depend on the texture and electrical conductivity of the sample [61-64].
6. EXTRACTION OF CAROTENOIDS FROM VARIOUS SOURCES
Industrialization and progressive globalization has increased the demand for production of more food. Along with the lack of infrastructure and management for this huge quantity of food contributed to a huge loss and wastage of food and its by-products. As reported by food loss index of 2016, in between harvest and distribution, about 13.8% of food is being wasted worldwide. In 2020, this decreases very little making it 13.3% (FAO, 2023). Most of these loses are in fruits and vegetables due to their short shelf life and high expenses of storage. In addition, processing the gathered fruits and vegetables generates a sizable number of by-products in the form of pieces that are not edible, including peels, cores and pomace as well as damaged, rotten, and unripe ones. However, these waste products are rich in bioactive compounds and nutraceuticals as plants hold them in both their edible and nonedible segments of veggies and fruits. In case of carotenoids, they are stored more in non-consumable sections of edible fruits and veggies, like skin, as they give them their bright red/orange colors. Some examples include tomato, carrot, oranges, and grape, and these parts are often discarded producing a huge amount of food waste, for example, juice production facilities produce 5.5 million metric tons of by-products [65]. The following article presents extraction process of carotenoids from various types of food wastes.
6.1. Extraction from Tomato Waste
Tomato (Lycopersicon esculentum) one of the most important components in Mediterranean diet making it the second most important vegetable worldwide. Approximately 80 million tons of waste is generated from over 130 million tons of production worldwide. After industrial processing, the remaining tomato pomace including peels and seeds are mostly used as animal feed. However, these wastes contain a plethora of bioactive compounds, including phenolic components, vitamins, and carotenoids. Of all the pigments, lycopene [Table 1] makes up about 80–90%. Consumption of lycopene shows various health benefits including cardiovascular and anticancer activities, supported by various epidemiological studies [66,67].
The rudimentary carotenoids present in tomato are lycopene and β-carotene, where lycopene is present mostly in the peels and the seeds contain β-carotene. For being beneficial to human health, the demand of low cost available and high purity carotenoids is increasing. The extraction of lycopene from tomatoes is commonly done using traditional methods that involve the application of a range of organic solvents and their combinations in established protocols. One of the most efficient ways to obtain lycopene is by utilizing the peels of tomato generated by juice production industries.
Using enzymes to aid in extraction is often viewed as a pre-treatment step rather than a standalone method of extraction. This technique breaks down the polysaccharide chains of the tomato cell wall in which the majority of pigments are located. This helps the solvents to penetrate and improve the release of carotenoids and reduce extraction time and temperature [68]. Using cellulolytic and hemicellulolytic enzymes as a pre-treatment method for tomato peels is an effective method for recovering carotenoids [69]. In an experiment of Catalkaya and Kahveci, the study demonstrated that the polar nature of the solvent used is a significant factor in the process of extraction (e.g., 1:2 acetone-chloroform, 50:25:25 hexane-acetone-ethanol [70].
It is one of the safest techniques where carbon dioxide is used as super critical solvent among many, for being environmentally safe, non-toxic, and easily available. In an experiment performed by Kehili et al., the extraction yield for lycopene and β-carotene was up from 32.02% to 60.85%, and 28.38% to 58.8%, respectively. The raw material used here was Tunisian industrial tomato peels [71]. Latest research has shown that usage of additional cosolvent can increase the carotenoids solubility. de Andrade Lima and the team used ethanol as a cosolvent in SFE method, that increases the polarity of CO2 which ultimately increases the recovery rate of more than 90% of carotenoid. Lycopene recovery was also efficient (almost 95%) due to the high content of moisture found in peels of tomato [57].
The electrical and/or heat treatment’s induction of cell disintegration at the cuticular level enhances the solvent’s entry into the cytoplasm, amplifying the carotenoids’ ability to be extracted. Degradation is made possible by PEF extraction technology, which also cuts down on time, energy costs, and solvent usage. An investigation was conducted to determine the relationship between the breakdown index of cells present in tomato peel tissues and different electric field strengths as well as temperatures used in steam blanching (SB). The result of steam blanch treatment 60°C applied with PEF treatment with an intensity more than 0.75 kV/cm shows a much higher yield of extraction of total carotenoids (PEF-188% and steam blanching-189%) and power of antioxidant (PEF-372% and steam blanching- 305%) when compared to untreated peels of tomato (Pataro et al., 2018). In case of solid liquid extraction, pretreatment with PEF (5 kV/cm, 5 kJ/kg) increases the extraction yield of lycopene to 12-18% and total extraction rate to 27–38%, in acetone or ethyl lactate solvent [72].
Application of UAE method on tomato pomace for lycopene extraction using ethyl lactate and ethyl acetate mixture as solvent along with optimized extraction conditions (30% v/v ethyl acetate in solvent mixture, 63.4°C, 100 mL/g solvent to sample ratio, 20 min) yielded higher (1334.8 ± 83.9 µg/g) at shorter time and lower temperature. Compared to this, the yield without UAE was 9% lower [73]. Combined treatment of UAE and freeze drying increases the yield by 4.12 folds from industrial tomato waste of lycopene extraction [74]. Hydrophobic eutectic mixtures of DL-methanol (hydrogen-bond acceptor) and lactic acid (hydrogen-bond donor) in the UAE yielded as high as 1.45 g of lycopene/Kg dry weight of tomato pomace. The yield is reportedly higher than the traditional organic solvent mixture of ethyl acetate and ethyl lactate for the same raw material [75,76].
6.2. Extraction from Carrot Waste
Carrots are a kind of tuber or root vegetables, present in various colors, including, purple, red, orange, yellow, and white [77]. Carrots are considered the most abundant source of β-carotene [Table 1] [78]. Carrot waste is abundant in carotenoids, particularly β-carotene. Researchers have shown that the peel of carrots contains a higher concentration of β-carotene (204.5 mg/g) as compared to carrot pomace waste (19.81 mg/g) and carrot pulp waste (39.2 mg/g) [79,80]. β-carotene is the rudimentary carotenoid present in carrot. Selective SE techniques are used to extract both polar and non-polar carotenoids using various mixtures of organic solvents [81]. Other green extraction processes such as MAE, ultrasound extraction, and super critical CO2 assisted extraction methods are also can be used. Water-induced hydrocolloidal complexation method was used to co-extract β-carotene and pectin from carrot peels where complexation of these two bioactive components under different operating stirring conditions yielded in 1.17 mg of β-carotene in 100 g wet sample [82].
Carrot juice processing waste was frozen at –40°C and then freeze dried to reduce moisture content to <1%. A definite amount of waste powder was mixed in flaxseed oil, used as solvent. The mixture was placed in an extraction vessel and it was put in a microwave extraction device. The temperature and time can be set in different variation to obtain maximum yield but should not exceed 110°C. A closed microwave system is used to carry out the process. After extraction process, the flaxseed oil, enriched with carotenoids, was stored at –20°C in a dark or black glass bottle. The highest recovery yield was achieved at 170 W microwave power, 9.46 min of extraction time with oil to waste ratio as 8:1. The recovery percentage was 77.91 ± 0.33 [83]. The quantification of extracted β- carotene was done by high-performance liquid chromatography (HPLC) using methanol/acetonitrile (9:1) with 9 µM triethylamine mixture as mobile phase at 0.9 mL/min flowrate, 450 nm wavelength [83]. Changes in carrot tissue caused by sonication impacted total carotenoid content and color change [84]. Carrot pomace was used as a raw material, while the UAE was carried out for 16 min of sonication at 29°C under 59% v/v concentration of ethanol yielded 14.37 μg/g of β-carotene, 5.35 μg/g of lutein, and 2.5 μg/g of lycopene [23].
The aim of this process was to assess and improve the method for extracting carotenoids from peels of carrots using supercritical CO2 (SCO2) in combination with a cosolvent like ethanol. The factors that were assessed included temperature and pressure and the concentration of a cosolvent [23]. Carrot peels were used as sample, which were dried and mixed 5 g of it with 95 g inert glass beads for homogenization. The mixture was put in an extraction vessel and CO2 flow was applied at a rate of 15 g/min with ethanol as a cosolvent. The extraction time was 80 min. After every run, the collected carotenoids, dissolved into ethanol, were evaporated using a rotavapor to remove the ethanol and the collection was weighed and redissolved in methanol for total carotenoid concentration analysis [85]. According to a validated model, the best conditions for achieving the highest mass yield (5.31%, d.b.) were determined to be 58.5°C, 306 bar, and 14.3% ethanol. In addition, the most efficient conditions for carotenoid recovery (86.1%) were identified as 59.0°C, 349 bar, and 15.5% ethanol [85].
The results of this study indicate that using PEF technology can effectively enhance the carotenoid resistance of natural food ingredients, such as carrot pomace. The use of electroporation in PEF treatment has the potential to enhance the ability to extract carotenoids from carrot pomace. Roohinejad et al. [79] have optimized β-carotene extraction through microemulsion (made of medium chain triglycerides, phosphate buffer, and tween 80) using Box–Behnken experimental design. The optimal conditions at 49.4 min of extraction time under temperature of 52.2°C having carrot to microemulsion ratio as 1:70 (w/w) yielded 19.6 µg/g of β-carotene in the microemulsion. Based on the given conditions, it was predicted that the β-carotene content would have a response value of 19.6 µg/g [79,86].
6.3. Extraction from Mango Waste
Mango (Mangifera indica L.) is cultivated in sub-tropical and tropical regions, which belongs to Anacardiaceae family. It is produced in more than ninety countries [87-89]. Mangoes contain a diverse array of bioactive compounds, such as phenolic compounds and carotenoids [Table 1], that can be found in ripe mangoes [90,91]. Depending on cultivars and weight of pulp can vary from 33 to 85%, skin 7-24%, and seeds 9–40% (w/w). Therefore, the processing of mangoes generates 35-60% (w/w) agro-industrial waste [92,93]. Peeling and recovery of skins and seeds, respectively, as rich source of bioactive compounds can not only aid in managing waste but also yield substances that can be utilized in various industries, such as food and beverages, pharmaceutical, and cosmetic products [94]. Peel and seed are the main by-product of mango fruit. Ripen mango skin contains various kind of carotenoids including α and β-carotene. Green extraction process such as MAE and supercritical CO2 (SrCO2) extraction is used to extract the bioactive compounds present in the mango waste. Mango peels were freeze dried at –35°C for 48 h under 4.0 Pa pressure or air dried (50°C, 24 h), size reduced and extracted using SrCO2 (operated at 40°C temperature, 30 MPa pressure, and 1.1 L/min CO2 flow rate). The process yielded total carotenoid of 5.6 ± 0.51 mg β-carotene/g dry basis of mango peel [95,96]. High pressurized ethanol can be used as a co-solvent [97] or the residue of SrCO2 extraction can be treated with it under the same conditions [96]. The output of extracted carotenoid is affected by the duration of extraction, the temperature, the pressure, and the concentration of the cosolvent. The amount of carotenoids can be determined through methods such as UV-visible spectrophotometry and ultra-performance liquid chromatography having diode array detector [97]. Application of polar solvent such as ethanol in SrCO2 extraction increases the extraction yield due to the presence of polar components in sample [98]. Segatto et al. [99] extracted secondary metabolites, namely, hyperoside (flavonol) and mangiferin (xanthone) under MAE system and matrix solid-phase dispersion (MSPD) technique from mango processing waste (MPW). They revealed higher extraction yield of hyperoside (398.52 mg/Kg of dried MPW) and mangiferin (352.90 mg/Kg of dried MPW) using MSPD technique as compared to MAE system [100].
6.4. Extraction from Orange Waste
Orange has various biochemical compounds loaded with secondary metabolites including amino acids, proteins, carbohydrates, phenolic compounds, flavonoids, and carotenoids [101]. Carotenoid is one of the main natural pigment presents in orange give it its orange color. A variety of carotenoids are present in orange including mutatoxanthin, β-cryptoxanthin, violaxanthin, zeaxanthin, and antheraxanthin. The main compound in orange juice is α- and β-carotene measuring almost 1.5 µg/mL [102]. Of the oranges grown worldwide, four out of ten are used to manufacture concentrated juice. While extracting the juice, it produces by-products including peels core and frit. The pulp is also produced as by-product during filtration of extracted juice. These by-products contain a variety of carotenoids which can be extracted using green extraction methods [101]. After extraction of juice, the by-products can be treated for carotenoid extraction. Orange peel is the most favorite sample for these extraction process probably due to less moisture content in it. Traditional green SE techniques can be used to extract carotenoids from orange as well as green extraction techniques such as ultrasound-assisted extraction can be used.
Carotenoids are extracted from homogenized orange peels by SE technique. In this process, various organic solvents, such as acetone, hexane, petroleum ether, and KOH, is used to extract the carotenoids from the peels. Final separation of carotenoids from solvent is done using separating funnel and diethyl ether and distilled water for washing, where the upper lipophilic phase contains carotenoids. Rota vapor and nitrogen was used to concentrate the final extract [103]. Orange peels were dried to reduce moisture content and then grounded using electric mill. An ultrasound-assisted extraction method was utilized by combining powdered orange peel with various solvents, including olive oil and different types of ionic liquids including olive oil [104] and different types of ionic liquid such as 1-n-butyl-3-methylimidazolium tetrafluoroborate, 1-hexyl-3-methylimidazolium chloride, and 1-butyl-3- methylimidazolium chloride [105] and 1-butyl-3- methylimidazolium hexafluorophosphate [106]. In case of ionic liquids, ethanol is used as a cosolvent. The utilization of frequency and power of ultrasonication can vary depending on the specific experiment, as well as factors such as time required for extraction, temperature, and the ratio of liquid to solid. Utilizing a 4000 rpm speed on the centrifuge, the plant material was divided through spinning for a quarter of an hour [104,105]. The analysis of extracted carotenoid can be done using HPLC with DAD detector [104,107].
6.5. Extraction of from Shrimp Waste
Shrimp, a highly sought-after seafood item, is prepared for consumption by removing the shell, head, and tail, which are typically considered as unusable parts. These were part measures almost 60% of shrimp’s body weight [108,109]. The waste parts are good source of proteins, lipids, chitins, and carotenoids, having potential industrial uses [110,111]. In terms of carotenoid representation within raw shrimp, astaxanthin and β-carotene dominated with astaxanthin contributing 75.18–88.86% of the total carotenoid content [112]. Astaxanthin having high antioxidant activity and anti-diabetic, neuroprotective, and anti-inflammatory activity [113-116] and the current pollution issues faced by the sea food industries for dumping the waste generate a demand for utilization of shrimps wastes to produce astaxanthin [117]. The main carotenoid content of shrimp waste is astaxanthin and there is very little information available on its extraction process. Powdered sample of shrimp waste is mixed with different solvents (acetone and methanol) with different ratio and put in a high-pressure container and different pressure (210 MPa) and holding time (10 min) is applied. The research team then used a centrifuge to separate the sample and collected the liquid layer on top. Mixture of distilled water and hexene (1:1 v/v) was added to it and the resulting upper layer was collected and dried using oxygen free nitrogen [118].
Total carotenoid content was determined using spectrophotometer and the yield of astaxanthin was measured using HPLC [118,119]. Utilizing commercial, crude and unconstructed enzymes for the purpose of enzymatic hydrolysis are a technique that has been investigated for the extraction of astaxanthin from shrimp. A combination of proteolytic enzymes (barbel and bovine trypsin) and ethylenediamine tetraacetic acid EDTA (0.5 M) is used on Dried Shrimp shell powder to obtain the dry matter “carotenoprotein.” This carotenoprotein underwent treatment with lipase and protease for hydrolysis, resulting in the production of 900 mg/g of soluble protein and 66 mg/g of total carotenoids through the use of 10 lipolytic and 15 proteolytic units [120,121].
The enzyme alcalase can also be used for recovering carotenoids although suitable range of pH is needed for proper activity of this enzyme. The range of recovery was 57.5–64.6% measuring 4.7–5.7 mg/100 g of dry waste [113]. Cabanillas-Bojórquez et al. [122] used super critical CO2 and ethanol to extract astaxanthin from dried exoskeleton of shrimp. After extraction, the supernatant was collected form the extract through centrifugation, dried, and resuspended into ethyl acetate for astaxanthin concentration analysis using HPLC.
6.6. Extraction from Pumpkin Waste
Pumpkin is a good source of bioactive compound which includes more than 800 varieties of its species [123]. Pumpkin pulp is considered a good source of carotenoids. Major carotenoids present in pumpkin is β-carotene, as well as little amount of lutein, lycopene, and α-carotenes are present also [124]. In spite of having the potential to use pumpkin waste as an alternative for carotenoid production, most of the waste of pumpkin is used as landfill material or converted into biofertilizer. It is also used for animal feeding [125]. To extract bioactive compounds from vegetables, green extraction technologies using vegetable oil as a solvent or cosolvent were studied before. These techniques are environment friendly and do not require any use of harmful chemicals. To harvest carotenoids from pumpkin waste, it is suggested to use freeze dried sample. Using methods such as ultrasound-aided extraction and microwave-aided extraction can be implemented to implement green extraction techniques.
In their experiment, M. Sharma and Bhat extracted carotenoids from pumpkin peels and pulp, using corn oil as a green solvent and ultrasonication technique. The final extract was separated from the oil through centrifugation. The total carotenoid content was determined by spectrophotometer at 470 nm [126]. In the same experiment, they used MAE technique to extract carotenoids from pumpkin waste using same solvent and MAE extractor (130W, 30 min, 45°C). After extraction process, the cooled extract were separated from the oil through centrifugation [126]. The total carotenoid content was determined by and the antioxidant capacity of extracted carotenoid was estimated by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay. Ghosh, Biswas [127] used pumpkin peels and pumpkin industrial waste in their experiment to extract carotenoids, such as lycopene, using enzymes such as cellulase and pectinase. The hand blended pumpkin wastes are treated with these two enzymes separately to extract carotenoids and the extracts are filtrated and undergone SE using acetone and petroleum ether. Effects of pectinase were shown better than cellulase in case where the raw material was fruit pulp waste. Same conclusion can be drawn in case of whole carotenoid extraction and lycopene extraction from pumpkin pulp. However, pectinase showed better yield in case where industrial pumpkin waste, such as peels, were used as raw material [127].
6.7. Extraction from Passion Fruit Waste
Passion fruit waste refers to the parts of the passion fruit that is not typically consumed, such as the peel and seeds. The waste created by juice processing industries weigh almost 60% of the whole fruit gets discarded [128]. These waste parts may contain valuable nutrients such as carotenoids, vitamins, and minerals. Passion fruit waste parts contain carotenoids such as beta-carotene and lutein. These carotenoids have antioxidant properties and may have health benefits such as promoting eye health and reducing the risk of certain diseases [12]. Passion fruit waste can be used for various purposes such as animal feed, composting, and extraction of bioactive compounds. The extraction of biologically active compounds from these types of waste materials has the potential to provide significant industrial benefits.
Conventional extraction process using petrochemical solvents is useful for extraction of carotenoid from plant based materials but they comes with issues such as generates harmful chemical compounds, having low extraction rates and long extraction time [129]. Therefore, methods of green extraction such as ultrasound-assisted and microwave-assisted extraction using bio-based solvents like vegetable oils are gaining popularity due to its low to zero chances of environmental harming by products production, low extraction time, and high efficiency and low-energy costs [130,131].
In their experiment, Chutia and Mahanta used UAE technique and MAE technique to extract carotenoids from freeze dried passion fruit peels. In both techniques, olive oil and sunflower oil were taken as solvent and in case of UAE method; the mixture of solvent and passion fruit peels were ultrasonicated using a probe and in case of MAE method microwave system was used for extraction process. Further, filtration was done using glass microfiber paper [18]. The total antioxidant activity of extracted carotenoid was measured using DPPH free radical scavenging activity.
Passion fruit peel powder was mixed with a solvent containing mixture of n-hexane, ethanol, ethyl acetate, and acetone (2:1:1:1) in volumetric ratio. The mixture was put into extraction thimble and extraction was done inside Soxhlet apparatus. After the end of extraction process, the solvents were evaporated using vacuum evaporator to separate the extracted carotenoids [83]. To quantify the amount of carotenoids and βcarotene, a dilution of carotenoids extract in n-hexane was performed for the investigation [18].
6.8. Extraction from Guava Waste
Guava fruit contains several carotenoids, including lycopene, beta-carotene, and lutein. These compounds are responsible for the red or yellow color of the fruit and have been found to have antioxidant properties [132]. Guava is used in food industry for production of juice, syrup, jelly, and flavored food. It is a popular tropical fruit. However, during various stages of industrial productions, it produces wastes containing seeds, peel, and leftover pulp, making almost 30% of the fruit weight [133]. This waste can create environmental problems. However, the amount of carotenoids left in the waste parts of guava holds industrial value if extracted properly [132]. The extraction of carotenoids from guava waste products can be done through maceration but is not accepted well due to its labor intense process, high extraction time, and use of excessive solvents. Hence, alternative non-conventional green extraction techniques, such as UAE, are now being interested due to their high extraction yield, low extraction time, and processing labor and most importantly by production of less to none toxic chemicals [134,135]. da Silva Lima et al. [136] used different UAE techniques to extract carotenoids, mostly lycopene and β-carotene from dried guava pulp, peel, and left-over waste. Three different methods, maceration extraction, bath type and probe type UAE, were used for comparison purpose to understand the best possible method for extraction while ethyl acetate was being used as a solvent. Lycopene was found to be the major carotenoid present in the extract. Ultrasonic bath type extraction method was found to be most effective with high yield, where probe type extraction method showed the most degradation of carotenoids. Maceration extraction process was also found to be efficient for extraction but did not get entertained due to its high usage of organic solvent making it impractical for large scale commercial use [136].
6.9. Extraction from Pomegranate Waste
Pomegranate fruit contains various carotenoids, including lycopene, β-carotene, and lutein. Research has demonstrated that certain carotenoids possess antioxidant properties and may offer potential health advantages, such as a decreased likelihood of heart disease, cancer, and diabetes. In addition, pomegranate fruit also contain high amounts of punicalagins and punicic acid, which are unique antioxidants found in pomegranate and are responsible for many of its health benefits. As the yield of extracted juice from pomegranate is less than half of its weight, it produces a lot of waste by-products. Pomegranate waste, such as the peels and seeds, also contain various carotenoids, including lycopene, beta-carotene, and lutein. Although previously pomegranate peels are mostly applied as animal feed with recent advance in studies, the industrial value of it came to known for carotenoid production [137,138]. There has not much studies been published regarding the extraction of carotenoids from pomegranate. Instead of using traditional method, green SEs such as use of vegetable oils as solvents are now being considered as alternatives.
Goula et al. [55] used vegetable oil as an alternative solvent to extract carotenoids from pomegranate peels by applying ultrasound-assisted techniques. They used both bath type and probe type ultrasonication device and sunflower and soy oil for extraction and the extracts were filtered using glass microfiber. In this process, they managed to extract almost 85.7 and 93.8% of total carotenoid present in the peel waste. Moreover, with increase in temperature (20–40°C), the extraction yield was also increased [55].
7. FUTURE SCOPE AND PROSPECT
Carotenoids are a class of naturally occurring pigments that are widely distributed in the plant kingdom, and are responsible for the bright red, orange, and yellow colors of fruits and vegetables. These pigments are not only important for the esthetic appeal of fruits and vegetables but they also have important health benefits, such as acting as antioxidants and protecting against cancer and heart disease. To create carotene radicals with various characteristics, carotenoids can also squelch other free radicals. These antioxidant effects include reducing the oxidation of lipids during digestion in the gastrointestinal lumen, blocking the oxidation of lipids in brain cells, preventing the oxidation of polyunsaturated lipids in the retina, and preventing the oxidation of low-density lipoprotein particles. One of the most promising areas for the extraction of carotenoids from food waste materials is in the field of food processing. Food processing industries generate large amounts of waste, such as peels, cores, and pulp from fruits and vegetables, which can be a rich source of carotenoids. For example, orange peels are known to contain high levels of beta-carotene, a precursor of Vitamin A, which is essential for eye health. Similarly, tomato skins and pulp are rich in lycopene, which is known to protect against cancer.
Another area where carotenoid extraction from food waste materials has great potential is in the field of biotechnology. There is a growing interest in developing micro-organisms that can produce carotenoids as a mean of producing natural food colorants. Micro-organisms such as yeasts, fungi, and algae are known to produce carotenoids and research is currently underway to optimize the conditions for carotenoid production by these organisms.
In addition to food processing and biotechnology, carotenoid extraction from food waste materials also has great potential in the field of agriculture. Many farmers use chemical pesticides to protect their crops from pests and diseases, but these pesticides can have negative effects on human health and the environment. Carotenoids have been shown to have natural insecticidal and fungicidal properties, and thus, their extraction from food waste materials can be used as a natural alternative to chemical pesticides [139]. Furthermore, carotenoids extraction from food waste materials can also be used to produce animal feed. Many species of fish and poultry require carotenoids in their diet to maintain good health, and carotenoids are also used as natural colorants to enhance the appearance of fish and poultry products [140].
8. CONCLUSIONS
The extraction of carotenoids from food waste materials using green extraction processes is a promising field with many potential benefits. Green extraction methods, such as using solvents that are less toxic and more environmentally friendly, can help to minimize the environmental impact of carotenoid extraction. In addition, the use of food waste materials as a source of carotenoids can help to reduce waste and minimize the environmental impact of food production. The extraction of carotenoids from food waste materials not only has the potential to provide a source of natural colorants and health-promoting compounds but it also has the potential to reduce end product of food waste and minimize the environmental impact of food production. However, all the work in this area are still in research stages and to utilize the proper value of these waste by-products the systems need to be industrialized at a larger scale. That will increase the production and decrease the cost of the end product, making it available and affordable to byers. With further research and development, the extraction of carotenoids from food waste materials using green extraction processes may become a viable and sustainable industry in the future. Moreover, the use of food waste in the production of carotenoids can also help to promote sustainable agriculture by reducing the need for chemical pesticides, and providing a natural alternative for animal feed. This way, the green extraction of carotenoids from food waste can help to create a more sustainable and environmentally friendly food production system. The green extraction of carotenoids from food waste materials is an area that has a lot of potential for future development. Using environmentally friendly extraction methods and utilizing food waste as a source of carotenoids, we can create a more sustainable and environmentally friendly food production system.
9. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FUNDING
There is no funding to report.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. Use of artificial intelligence (AI)-assisted technology
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sharma P, Gaur VK, Kim SH, Pandey A. Microbial strategies for bio-transforming food waste into resources. Bioresour Technol 2020;299:122580. [CrossRef]
2. Kumar K, Yadav AN, Kumar V, Vyas P, Dhaliwal HS. Food waste:A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour Bioprocess 2017;4:1-14. [CrossRef]
3. Barnhill A, Civita N. Food waste:Ethical imperatives &complexities. Physiol Behav 2020;223:112927. [CrossRef]
4. Segura Campos MR. Bioactive Compounds:Health Benefits and Potential Applications. Sawston:Woodhead Publishing;2018.
5. Santos DI, Saraiva JM, Vicente AA, Moldão-Martins M. Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds and Nutrients, Innovative Thermal and non-thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds. Netherlands:Elsevier Inc.;2019. 23-54. [CrossRef]
6. Karasawa MM, Mohan C. Fruits as prospective reserves of bioactive compounds:A review. Nat Prod Bioprospect 2018;8:335-46. [CrossRef]
7. Pogorzelska-Nowicka E, Atanasov AG, Horbanczuk J, Wierzbicka A. Bioactive compounds in functional meat products. Molecules 2018;23:307. [CrossRef]
8. Bera S. Carotenoids:Updates on legal statutory and competence for nutraceutical properties. Curr Res Nutr Food Sci 2019;7:300-19. [CrossRef]
9. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols:A concise overview on the chemistry, occurrence, and human health. Phytother Res 2019;33:2221-43. [CrossRef]
10. Santini A, Novellino E. Nutraceuticals - shedding light on the grey area between pharmaceuticals and food. Expert Rev Clin Pharmacol 2018;11:545-7. [CrossRef]
11. Daliu P, Santini A, Novellino E. From pharmaceuticals to nutraceuticals:Bridging disease prevention and management. Expert Rev Clin Pharmacol 2019;12:1-7. [CrossRef]
12. Salehi B, Venditti A, Sharifi-Rad M, Kregiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of Apigenin. Int J Mol Sci 2019;20:1305. [CrossRef]
13. Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids:Biochemistry, pharmacology and treatment. Br J Pharmacol 2017;174:1290-324. [CrossRef]
14. Lakey-Beitia J, Doens D, Jagadeesh Kumar D, Murillo E, Fernandez PL, Rao K, et al. Anti-amyloid aggregation activity of novel carotenoids:Implications for Alzheimer's drug discovery. Clin Interv Aging 2017;12:815-22. [CrossRef]
15. Visioli F, Artaria C. Astaxanthin in cardiovascular health and disease:Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct 2017;8:39-63. [CrossRef]
16. Jia YP, Sun L, Yu HS, Liang LP, Li W, Ding H, et al. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 2017;22:610. [CrossRef]
17. Ranganath KG, Shivashankara KS, Roy TK, Dinesh MR, Geetha GA, Pavithra KC, et al. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J Food Sci Technol 2018;55:4566-77. [CrossRef]
18. Chutia H, Mahanta CL. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent:Optimization, comparison, kinetics, and thermodynamic studies. Innov Food Sci Emerg Technol 2021;67:102547. [CrossRef]
19. Vargas EF, Jablonski A, Flôres SH, Rios AO. Waste from peach (Prunus persica) processing used for optimisation of carotenoids ethanolic extraction. Int J Food Sci Technol 2017;52:757-62. [CrossRef]
20. Zhou C, Zhao D, Sheng Y, Tao J, Yang Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011;16:624-36. [CrossRef]
21. Saini RK, Moon SH, Keum YS. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res Int 2018;108:516-29. [CrossRef]
22. Trombino S, Cassano R, Procopio D, Di Gioia ML, Barone E. Valorization of tomato waste as a source of carotenoids. Molecules 2021;26:5062. [CrossRef]
23. Umair M, Jabbar S, Nasiru MM, Lu Z, Zhang J, Abid M, et al. Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules 2021;26:6763. [CrossRef]
24. Maoka T. Carotenoids as natural functional pigments. J Nat Med 2020;74:1-16. [CrossRef]
25. Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophy 2018;652:18-26. [CrossRef]
26. Gong M, Bassi A. Carotenoids from microalgae:A review of recent developments. Biotechnol Adv 2016;34:1396-412. [CrossRef]
27. Langi P, Kiokias S, Varzakas T, Proestos C. Carotenoids:From plants to food and feed industries. Microbial carotenoids. Methods Mol Biol 2018;1852:57-71. [CrossRef]
28. Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, et al. A global perspective on carotenoids:Metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res 2018;70:62-93. [CrossRef]
29. Chacón-Ordóñez T, Carle R, Schweiggert R. Bioaccessibility of carotenoids from plant and animal foods. J Sci Food Agric 2019;99:3220-39. [CrossRef]
30. Ram S, Mitra M, Shah F, Tirkey SR, Mishra S. Bacteria as an alternate biofactory for carotenoid production:A review of its applications, opportunities and challenges. J Funct Foods 2020;67:103867. [CrossRef]
31. Liu C, Hu B, Cheng Y, Guo Y, Yao W, Qian H. Carotenoids from fungi and microalgae:A review on their recent production, extraction, and developments. Bioresour Technol 2021;337:125398. [CrossRef]
32. Zakynthinos G, Varzakas T. Carotenoids:From plants to food industry. Curr Res Nutr Food Sci 2016;4:38-51. [CrossRef]
33. Vincent U, Serano F, von Holst C. Development and validation of a multi-analyte method for the regulatory control of carotenoids used as feed additives in fish and poultry feed. Food Addit Contam A 2017;34:1285-97. [CrossRef]
34. Zarekarizi A, Hoffmann L, Burritt D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J Appl Phycol 2019;31:281-99. [CrossRef]
35. Álvarez R, Meléndez-Martínez A, Vicario I, Alcalde M. Carotenoid and vitamin A contents in biological fluids and tissues of animals as an effect of the diet:A review. Food Rev Int 2015;31:319-40. [CrossRef]
36. Meléndez-Martínez AJ. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol Nutr Food Res 2019;63:1801045. [CrossRef]
37. Kan L, Nie S, Hu J, Wang S, Bai Z, Wang J, et al. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem 2018;260:317-26. [CrossRef]
38. D'hooghe P, Picot D, Brunel-Muguet S, Kopriva S, Avice JC, Trouverie J. Germinative and post-germinative behaviours of Brassica napus seeds are impacted by the severity of s limitation applied to the parent plants. Plants (Basel) 2019;8:12. [CrossRef]
39. Loughman J, Nolan JM, Howard AN, Connolly E, Meagher K, Beatty S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Investig Ophthalmol Vis Sci 2012;53:7871-80. [CrossRef]
40. Yao Y, Qiu QH, Wu XW, Cai ZY, Xu S, Liang XQ. Lutein supplementation improves visual performance in Chinese drivers:1-year randomized, double-blind, placebo-controlled study. Nutrition 2013;29:958-64. [CrossRef]
41. Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, et al. Lutein, zeaxanthin, and meso-zeaxanthin:The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 2016;50:34-66. [CrossRef]
42. Woodside JV, Mcgrath AJ, Lyner N, Mckinley MC. Carotenoids and health in older people. Maturitas 2015;80:63-8. [CrossRef]
43. Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. [CrossRef]
44. Dore J. Astaxanthin and cancer chemoprevention. In:Bagchi D, Preuss HG, editors. Phytopharmaceuticals in Cancer Chemoprevention. Boca Raton FL:CRC Press;2005. 555-70.
45. Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr 2011;66:363-9. [CrossRef]
46. Zhang W, Wang J, Wang J, Liu T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour Technol 2014;158:329-35.
47. Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, et al. Dietary lycopene, angiogenesis, and prostate cancer:A prospective study in the prostate-specific antigen era. J Nat Cancer Inst 2014;106:djt430. [CrossRef]
48. Müller L, Caris-Veyrat C, Lowe G, Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases-a critical review. Crit Rev Food Sci Nutr 2016;56:1868-79. [CrossRef]
49. Heinrich U, Ga¨rtner C, Wiebusch M, Eichler O, Sies H, Tronnier H, et al. Supplementation with b-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr 2003;133:98-101. [CrossRef]
50. Kultys E, Kurek MA. Green extraction of carotenoids from fruit and vegetable byproducts:A review. Molecules 2022;27:518. [CrossRef]
51. Saini RK, Keum YS. Progress in microbial carotenoids production. Indian J Microbiol 2017;57:129-30. [CrossRef]
52. Pocha CK, Chia WY, Chew KW, Munawaroh HS, Show PL. Current advances in recovery and biorefinery of fucoxanthin from Phaeodactylum tricornutum. Algal Res 2022;65:102735. [CrossRef]
53. Seçilmis S, Yanik DK, Fadiloglu S, Gögüs F. A novel bleaching approach:Microwave assisted sunflower oil bleaching and optimization. Grasasy Aceites 2021;72:437-7.
54. Weggler BA, Gruber B, Teehan P, Jaramillo R, Dorman FL. Inlets and sampling. In:Separation Science and Technology. New York:Elsevier Inc.;2020. 141-203. [CrossRef]
55. Goula AM, Ververi M, Adamopoulou A, Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason Sonochem 2017;34:821-30. [CrossRef]
56. Amran MA, Palaniveloo K, Fauzi R, Satar NM, Mohidin TB, Mohan G, et al. Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability 2021;13:11432. [CrossRef]
57. Andrade Lima M, Kestekoglou I, Charalampopoulos D, Chatzifragkou A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019;24:466. [CrossRef]
58. Carullo D, Donsi F, Ferrari G, Pataro G. Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal Res 2021;57:102341. [CrossRef]
59. Silva HR, Iwassa IJ, Marques J, Postaue N, Stevanato N, da Silva C. Enrichment of sunflower oil with b-carotene from carrots:Maximization and thermodynamic parameters of the b-carotene extraction and oil characterization. J Food Process Preserv 2020;44:14399. [CrossRef]
60. Parniakov O, Barba FJ, Grimi N, Marchal L, Jubeau S, Lebovka N, et al. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res 2015;8:128-34. [CrossRef]
61. Singh A, Ahmad S, Ahmad A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv 2015;5:62358-93. [CrossRef]
62. Redondo D, Venturini ME, Luengo E, Raso J, Arias E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov Food Sci Emerg Technol 2018;45:335-43. [CrossRef]
63. Soquetta MB, Terra LM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J Food 2018;16:400-12. [CrossRef]
64. López-Gámez G, Elez-Martínez P, Martín-Belloso O, Soliva-Fortuny R. Pulsed electric field treatment strategies to increase bioaccessibility of phenolic and carotenoid compounds in oil-added carrot purees. Food Chem 2021;364:130377. [CrossRef]
65. Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and vegetable waste:Bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf 2018;17:512-31. [CrossRef]
66. Kaur G, Uisan K, Ong KL, Lin CS. Recent trends in green and sustainable chemistry &waste valorisation:rethinking plastics in a circular economy. Curr Opin Green Sustain Chem 2018;9:30-9. [CrossRef]
67. Nayak A, Bhushan B. An overview of the recent trends on the waste valorization techniques for food wastes. J Environ Manage 2019;233:352-70. [CrossRef]
68. Lenucci MS, De Caroli M, Marrese PP, Iurlaro A, Rescio L, Böhm V, et al. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem 2015;170: 193-202. [CrossRef]
69. Prokopov T, Nikolova M, Dobrev G, Taneva D. Enzyme-assisted extraction of carotenoids from Bulgarian tomato peels. Acta Aliment 2017;46:84-91. [CrossRef]
70. Catalkaya G, Kahveci D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep Purif Technol 2019;219:55-63. [CrossRef]
71. Kehili M, Kammlott M, Choura S, Zammel A, Zetzl C, Smirnova I, et al. Supercritical CO2 extraction and antioxidant activity of lycopene and b-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) by-product of a Tunisian industry. Food Bioprod Process 2017;102:340-9. [CrossRef]
72. Pataro G, Carullo D, Falcone M, Ferrari G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Food Sci Emerg Technol 2020;63:102369. [CrossRef]
73. Silva YP, Ferreira TA, Celli GB, Brooks MS. Optimization of lycopene extraction from tomato processing waste using an eco-friendly ethyl lactate-ethyl acetate solvent:A green valorization approach. Waste Biomass Valori 2019;10:2851-61. [CrossRef]
74. Wei W. Improving extraction of lycopene from tomato waste by-products using ultrasonication and freeze drying. World J Adv Res Rev 2020;5:177-85. [CrossRef]
75. Gallo M, Ferrara L, Naviglio D. Application of ultrasound in food science and technology:A perspective. Foods 2018;7:164. [CrossRef]
76. Silva YP, Ferreira TA, Jiao G, Brooks MS. Sustainable approach for lycopene extraction from tomato processing by-product using hydrophobic eutectic solvents. J Food Sci Technol 2019;56:1649-54. [CrossRef]
77. Singh B, Koley T, Maurya A, Singh P, Singh B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. Schubl. &Martens). Physiol Mol Biol Plants 2018;24:899-907. [CrossRef]
78. Mirheli M, Taghian Dinani S. Extraction of b-carotene pigment from carrot processing waste using ultrasonic-shaking incubation method. J Food Meas Charact 2018;12:1818-28. [CrossRef]
79. Roohinejad S, Oey I, Everett D, Niven B. Evaluating the effectiveness of b-carotene extraction from pulsed electric field-treated carrot pomace using oil-in-water microemulsion. Food Bioprocess Technol 2014;7:3336-48. [CrossRef]
80. Hiranvarachat B, Devahastin S. Enhancement of microwave-assisted extraction via intermittent radiation:Extraction of carotenoids from carrot peels. J Food Eng 2014;126:17-26. [CrossRef]
81. Saini RK, Keum YS. Carotenoid extraction methods:A review of recent developments. Food Chem 2018;240:90-103. [CrossRef]
82. Jayesree N, Hang PK, Priyangaa A, Krishnamurthy NP, Ramanan RN, Turki MA, et al. Valorisation of carrot peel waste by water-induced hydrocolloidal complexation for extraction of carotene and pectin. Chemosphere 2021;272:129919. [CrossRef]
83. Elik A, Yanik DK, Gögüs F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT 2020;123:109100. [CrossRef]
84. Nowacka M, Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. App Acoust 2016;103:163-71. [CrossRef]
85. Andrade Lima M, Charalampopoulos D, Chatzifragkou A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J Supercrit Fluids 2018;133:94-102. [CrossRef]
86. Roohinejad S, Everett DW, Oey I. Effect of pulsed electric field processing on carotenoid extractability of carrot purée. Int J Food Sci Technol 2014;49:2120-7. [CrossRef]
87. Asif A, Farooq U, Akram K, Hayat Z, Shafi A, Sarfraz F, et al. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci Technol 2016;53:102-12. [CrossRef]
88. López-Cobo A, Verardo V, Diaz-de-Cerio E, Segura-Carretero A, Fernández-Gutiérrez A, Gómez-Caravaca AM. Use of HPLC-and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) its by-products. Food Res Int 2017;100:423-34. [CrossRef]
89. Coman V, Teleky BE, Mitrea L, Marteu GA, Szabo K, Calinoiu LF, et al. Bioactive potential of fruit and vegetable wastes. Adv Food Nutr Res 2020;91:157-225. [CrossRef]
90. Zhang Z, Zhu Q, Hu M, Gao Z, An F, Li M, et al. Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem 2017;219:76-84. [CrossRef]
91. Maldonado-Celis ME, Yahia EM, Bedoya R, Landázuri P, Loango N, Aguillón J, et al. Chemical composition of mango (Mangifera indica L.) :Nutritional and phytochemical compounds. Front Plant Sci 2019;10:1073. [CrossRef]
92. Aggarwal P, Kaur A, Bhise S. Value-added processing and utilization of mango by-products. In:Handbook of Mango Fruit. Production, Postharvest Science Process Technology and Nutrition. Berlin:Springer Nature;2017. 279-93. [CrossRef]
93. Wall-Medrano A, Olivas-Aguirre FJ, Ayala-Zavala JF, Domínguez-Avila JA, Gonzalez-Aguilar GA, Herrera-Cazares LA, et al. Health benefits of mango by-products. In:Food Wastes and by-Products:Nutraceutical Health Potential. New York:Wiley;2020. 159-91. [CrossRef]
94. García-Mahecha M, Soto-Valdez H, Carvajal-Millan E, Madera-Santana TJ, Lomelí-Ramírez MG, Colín-Chávez C. Bioactive compounds in extracts from the agro-industrial waste of mango. Molecules 2023;28:458. [CrossRef]
95. Garmus TT, Paviani LC, Queiroga CL, Magalhães PM, Cabral FA. Extraction of phenolic compounds from pitanga (Eugenia uniflora L.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J Supercrit Fluids 2014;86:4-14. [CrossRef]
96. Garcia-Mendoza MP, Paula JT, Paviani LC, Cabral FA, Martinez-Correa HA. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT Food Sci Technol 2015;62:131-7. [CrossRef]
97. Pilar Sanchez-Camargo A, Gutierrez LF, Vargas SM, Martinez-Correa HA, Parada-Alfonso F, Narvaez-Cuenca CE. Valorisation of mango peel:Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J Supercrit Fluids 2019;152:104574. [CrossRef]
98. Martinez-Correa HA, Magalhães PM, Queiroga CL, Peixoto CA, Oliveira AL, Cabral FA. Extracts from pitanga (Eugenia uniflora L.) leaves:Influence of extraction process on antioxidant properties and yield of phenolic compounds. J Supercrit Fluids 2011;55:998-1006. [CrossRef]
99. Segatto ML, Zanotti K, Zuin VG. Microwave-assisted extraction and matrix solid-phase dispersion as green analytical chemistry sample preparation techniques for the valorisation of mango processing waste. Curr Res Chem Biol 2021;1:100007. [CrossRef]
100. Vélez-Erazo EM, Pasquel-Reátegui JL, Dorronsoro-Guerrero OH, Martínez-Correa HA. Phenolics and carotenoids recovery from agroindustrial mango waste using microwave-assisted extraction:Extraction and modeling. J Food Process Eng 2021;44:13774. [CrossRef]
101. Okino Delgado CH, Fleuri LF. Orange and mango by-products:Agro-industrial waste as source of bioactive compounds and botanical versus commercial description-a review. Food Rev Int 2016;32:1-14. [CrossRef]
102. Hu X, Liu Y, Zeng G, Hu X, Wang Y, Zeng X. Effects of limonene stress on the growth of and microcystin release by the freshwater cyanobacterium Microcystis aeruginosa FACHB-905. Ecotoxicol Environ Saf 2014;105:121-7. [CrossRef]
103. Chedea VS, Kefalas P, Socaciu C. Patterns of carotenoid pigments extracted from two orange peel wastes (valencia and navel var.). J Food Biochem 2010;34:101-10. [CrossRef]
104. Savic Gajic IM, Savic IM, Gajic DG, Dosic A. Ultrasound-assisted extraction of carotenoids from orange peel using olive oil and its encapsulation in ca-alginate beads. Biomolecules 2021;11:225. [CrossRef]
105. Murador DC, Braga AR, Martins PL, Mercadante AZ, de Rosso VV. Ionic liquid associated with ultrasonic-assisted extraction:A new approach to obtain carotenoids from orange peel. Food Res Int 2019;126:108653. [CrossRef]
106. Martins PL, de Rosso VV. Thermal and light stabilities and antioxidant activity of carotenoids from tomatoes extracted using an ultrasound-assisted completely solvent-free method. Food Res Int 2016;82:156-64. [CrossRef]
107. Boukroufa M, Boutekedjiret C, Chemat F. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resour Technol 2017;3:252-62. [CrossRef]
108. Montero P, Calvo M, Gómez-Guillén M, Gómez-Estaca J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient:Characterization, stability, and bioaccessibility. LWT 2016;70:229-36. [CrossRef]
109. Roy VC, Getachew AT, Cho YJ, Park JS, Chun BS. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J Supercrit Fluids 2020;159:104773. [CrossRef]
110. Bahasan SH, Satheesh S, Ba-Akdah MA. Extraction of chitin from the shell wastes of two shrimp species Fenneropenaeus semisulcatusand Fenneropenaeus indicus using microorganisms. J Aquat Food Prod Technol 2017;26:390-405. [CrossRef]
111. Ximenes J, Hissa D, Ribeiro L, Rocha M, Oliveira E, Melo V. Sustainable recovery of protein-rich liquor from shrimp farming waste by lactic acid fermentation for application in tilapia feed. Braz J Microbiol 2019;50:195-203. [CrossRef]
112. Su F, Liu J. The carotenoid characteristics of the important wild shrimp Trachysalambria curvirostris (Stimpson, 1860) in China. J Oceanol Limnol 2019;37:706-12. [CrossRef]
113. Prameela K, Venkatesh K, Immandi SB, Kasturi AP, Krishna CR, Mohan CM. Next generation nutraceutical from shrimp waste:The convergence of applications with extraction methods. Food Chem 2017;237:121-32. [CrossRef]
114. Messina CM, Manuguerra S, Renda G, Santulli A. Biotechnological applications for the sustainable use of marine by-products:In vitro antioxidant and pro-apoptotic effects of astaxanthin extracted with supercritical CO2 from Parapeneus longirostris. Mar Biotechnol 2019;21:565-76. [CrossRef]
115. Chang MX, Xiong F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases:Recent advances and future directions. Molecules 2020;25:5342. [CrossRef]
116. Stachowiak B, Szulc P. Astaxanthin for the food industry. Molecules 2021;26:2666. [CrossRef]
117. Venugopal V, Sasidharan A. Seafood industry effluents:Environmental hazards, treatment and resource recovery. J Environ Chem Eng 2021;9:104758. [CrossRef]
118. Irna C, Jaswir I, Othman R, Jimat DN. Comparison between high-pressure processing and chemical extraction:Astaxanthin yield from six species of shrimp carapace. J Diet Suppl 2018;15:805-13. [CrossRef]
119. Othman R. Biochemistry and Genetics of Carotenoid Composition in Potato Tubers. Colombo:Lincoln University;2009.
120. Sila A, Nasri M, Bougatef A. Isolation and characterisation of carotenoproteins from deep-water pink shrimp processing waste. Int J Biol Macromol 2012;51:953-9. [CrossRef]
121. Mao X, Guo N, Sun J, Xue C. Comprehensive utilization of shrimp waste based on biotechnological methods:A review. J Clean Prod 2017;143:814-23. [CrossRef]
122. Cabanillas-Bojórquez LA, Gutiérrez-Grijalva EP, González-Aguilar GA, López-Martinez LX, Castillo-López RI, Bastidas-Bastidas PJ, et al. Valorization of fermented shrimp waste with supercritical CO2 conditions:Extraction of astaxanthin and effect of simulated gastrointestinal digestion on its antioxidant capacity. Molecules 2021;26:4465. [CrossRef]
123. Perez Gutierrez RM. Review of Cucurbita pepo (pumpkin) its phytochemistry and pharmacology. Med Chem 2016;6:12-21. [CrossRef]
124. Seo JS, Burri BJ, Quan Z, Neidlinger TR. Extraction and chromatography of carotenoids from pumpkin. J Chromatogr A 2005;1073:371-5. [CrossRef]
125. Valdez-Arjona LP, Ramírez-Mella M. Pumpkin waste as livestock feed:impact on nutrition and animal health and on quality of meat, milk, and egg. Animals (Basel) 2019;9:769. [CrossRef]
126. Sharma M, Bhat R. Extraction of carotenoids from pumpkin peel and pulp:Comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil). Foods 2021;10:787. [CrossRef]
127. Ghosh D, Biswas PK. Enzyme-aided extraction of carotenoids from pumpkin tissues. Indian Chem Eng 2016;58:1-11. [CrossRef]
128. Dos Reis LC, Facco EM, Salvador M, Flôres SH, de Oliveira Rios A. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J Food Sci Technol 2018;55:2679-91. [CrossRef]
129. Song J, Yang Q, Huang W, Xiao Y, Li D, Liu C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod Process 2018;107:104-12. [CrossRef]
130. Chemat F, Abert-Vian M, Fabiano-Tixier AS, Strube J, Uhlenbrock L, Gunjevic V, et al. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal Chem 2019;118:248-63. [CrossRef]
131. Chemat F, Vian MA, Fabiano-Tixier AS, Nutrizio M, Jambrak AR, Munekata PE, et al. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem 2020;22:2325-53. [CrossRef]
132. Kumar K, Ahmed N, Jan S, Thakur P, Chauhan D, Kaur J. Guava wastes and by-products:Chemistry, processing, and utilization. In:Handbook of Fruit Wastes and by-Products. United States:CRC Press;2022. 99-112. [CrossRef]
133. Silva Lima R, Ferreira SR, Vitali L, Block JM. May the superfruit red guava and its processing waste be a potential ingredient in functional foods?Food Res Int 2019;115:451-9. [CrossRef]
134. Cárcel J, García-Pérez JV, Benedito J, Mulet A. Food process innovation through new technologies:Use of ultrasound. J Food Eng 2012;110:200-7. [CrossRef]
135. Ladole MR, Nair RR, Bhutada YD, Amritkar VD, Pandit AB. Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason Sonochem 2018;48:453-62. [CrossRef]
136. Silva Lima R, Nunes IL, Block JM. Ultrasound-assisted extraction for the recovery of carotenoids from guava's pulp and waste powders. Plant Foods Hum Nutr 2020;75:63-9. [CrossRef]
137. Siano F, Straccia MC, Paolucci M, Fasulo G, Boscaino F, Volpe MG. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J Sci Food Agric 2016;96:1730-5. [CrossRef]
138. El Moujahed S, Dinica RM, Cudalbeanu M, Avramescu SM, Msegued Ayam I, Ouazzani Chahdi F, et al. Characterizations of six pomegranate (Punica granatum L.) of global commercial interest in morocco:Pomological, organoleptic, chemical and biochemical studies. Molecules 2022;27:3847. [CrossRef]
139. Paparella A, Shaltiel-Harpaza L, Ibdah M. -Ionone:Its occurrence and biological function and metabolic engineering. Plants (Basel) 2021;10:754. [CrossRef]
140. Nabi F, Arain MA, Rajput N, Alagawany M, Soomro J, Umer M, et al. Health benefits of carotenoids and potential application in poultry industry:A review. J Anim Physiol Anim Nutr 2020;104:1809-18. [CrossRef]
141. Beltrán-de-Miguel B, Estévez-Santiago R, Olmedilla-Alonso B. Assessment of dietary vitamin A intake (retinol, a-carotene, b-carotene, b-cryptoxanthin) and its sources in the national survey of dietary intake in spain (2009–2010). Int J Food Sci Nutr 2015;66:706-12. [CrossRef]
142. Maiani G, Periago Castón MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, et al. Carotenoids:Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 2009;53:194-218. [CrossRef]
143. Abdel-Aal ES, Akhtar H, Zaheer K, Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013;5:1169-85. [CrossRef]
144. Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and zeaxanthin-food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients 2017;9:120. [CrossRef]
145. Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin:Sources, extraction, stability, biological activities and its commercial applications-a review. Mar Drugs 2014;12:128-52. [CrossRef]
146. Khan UM, Sevindik M, Zarrabi A, Nami M, Ozdemir B, Kaplan DN, et al. Lycopene:Food sources, biological activities, and human health benefits. Oxid Med Cell Longev 2021;2021:2713511. [CrossRef]
147. Cooperstone JL. Lycopene:Food sources, properties, and effects on human health. In:Handbook of Nutraceuticals and Functional Foods. United States:CRC Press;2019. 37-53.
148. Esatbeyoglu T, Rimbach G. Canthaxanthin:From molecule to function. Mol Nutr Food Res 2017;61:1-49. [CrossRef]
149. Din NA, Mohd Alayudin AS, Sofian-Seng NS, Rahman HA, Mohd Razali NS, Lim SJ, et al. Brown algae as functional food source of fucoxanthin:A review. Foods 2022;11:1-35. [CrossRef]
150. Burri BJ, La Frano MR, Zhu C. Absorption, metabolism, and functions of b-cryptoxanthin. Nutr Rev 2016;74:69-82. [CrossRef]
151. Martínez-Abad A, Ramos M, Hamzaoui M, Kohnen S, Jiménez A, Garrigós MC. Optimisation of sequential microwave-assisted extraction of essential oil and pigment from lemon peels waste. Foods 2020;9:1493. [CrossRef]
152. Poojary MM, Passamonti P. Optimization of extraction of high purity all-trans-lycopene from tomato pulp waste. Food Chem 2015;188: 84-91 [CrossRef].
153. Wang X, Wang C, Zha X, Mei Y, Xia J, Jiao Z. Supercritical carbon dioxide extraction of b-carotene and a-tocopherol from pumpkin:A box-behnken design for extraction variables. Anal Methods 2017;9:294-303. [CrossRef]
154. Pataro G, Bobinaite R, Bobinas C, Šatkauskas S, Raudonis R, Visockis M, et al. Improving the extraction of juice and anthocyanins from blueberry fruits and their by-products by application of pulsed electric fields. Food Bioprocess Technol 2017;10:1595-605. [CrossRef]
155. Kamble MG, Singh A, Kumar Prabhakar P, Meghwal M, Veer Singh S, Chinchkar AV. Effect of high shear homogenization on quality characteristics of bael fruit pulp. Food Qual Saf 2022;6:fyac011. [CrossRef]