1. INTRODUCTION
The Gerbera, or Gerbera jamesonii L., is one of the five most popular cut flowers in the world and is renowned for its eye-catching look and vivid hues [1]. The rapid wilting of Gerberas, however, poses a serious problem for the cut flower business since it influences consumer choices and the profitability of their manufacturing. Neck bending in the stems is a primary factor contributing to this issue, leading to reduced longevity [2,3]. Research has shown that neck bending results from a combination of low turgor pressure and insufficient mechanical support in the stems. The imbalance between low water absorption and high transpiration rates, along with bacterial blockages in the vascular system, creates negative pressure that diminishes turgor in stem cells [4]. In recent years, the quest to enhance the vase life of cut flowers has led to the widespread use of synthetic antimicrobial agents and growth regulators [5,6]. While studies such as those examining dithiothreitol and thioglycolic acid have demonstrated effectiveness in prolonging vase life, they often carry significant drawbacks, including potential cytotoxic effects that can compromise plant longevity. Notably, dithiothreitol has been shown to extend the vase life of cut gerbera flowers by approximately 3 days compared to the control; however, the reliance on synthetic chemicals raises concerns regarding their long-term safety in post-harvest treatments [7].
On the other hand, our study concentrates on phenylalanine (Phe), a naturally occurring lignin pre-cursor, as a viable substitute that improves the structural integrity of stems without the negative side effects connected to synthetic agents. A crucial pre-cursor in the production of lignin, Phe is essential to the phenylpropanoid pathway. Through the action of phenylalanine ammonia-lyase (PAL), Phe is converted into trans-cinnamic acid, which is subsequently transformed into lignin monomers such as p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol [8]. These monomers are then polymerized by enzymes, such as peroxidases and laccases to form the complex lignin structure in cell walls [9]. Phenylalanine provides a more comprehensive approach to flower preservation problems by addressing the root causes of neck bending and low turgor pressure through the promotion of lignin synthesis [10]. Furthermore, present research frequently overlooks the fundamental flaws that lead to stem failure in favor of band-aid fixes that only postpone senescence. This disparity emphasizes the need for our strategy, which seeks to produce long-lasting gains in stem strength and blossom longevity. Our study adds to the increasing amount of studies supporting natural solutions in post-harvest floriculture by highlighting the shortcomings of existing techniques and offering phenylalanine as a safer, more efficient substitute.
This makes our study, which aims for better flower quality and environmental sustainability, a significant breakthrough in the field. By investigating this strategy, the study hopes to offer valuable insights that help improve post-harvest management procedures, which would ultimately benefit both growers and consumers.
2. MATERIALS AND METHODS
2.1. Plant Material
The “Jent” Gerbera variety at 3 months old was grown at the Center for Business Incubation of Agricultural High Technology in Ho Chi Minh City, Vietnam.
2.2. Experimental Treatments
The two-factor experiment was conducted using a completely randomized design, consisting of 15 treatments, each replicated 3 times, with 20 plants per replication. Foliar application of phenylalanine was conducted when the plants reached 90 days old, utilizing varying concentrations (the first factor) and application intervals (the second factor). The phenylalanine (Phe) concentrations tested were 0 mg/L, 50 mg/L, 100 mg/L, 150 mg/L, and 200 mg/L. The application intervals were every 5 days, once every 10 days, and once every 15 days. After 35 days, flower stems measuring 40 cm in length were harvested and placed in test tubes containing water, stored under laboratory conditions at 30°C ± 5°C and 70 ± 5% relative humidity. The parameters assessed included flower diameter and flower branch circumference. Morphological changes were monitored, and the vase life of the flowers was recorded.
2.3. Cellulose, Hemicellulose, and Lignin Content
To extract cellulose, hemicellulose, and lignin, 1 g of the sample was finely chopped and placed in a 200 mL reaction flask containing 150 mL of 2 M NaOH solution. The mixture was heated to 90°C for duration of 2 h. Following this, the mixture was filtered, and the residue was washed with 200 mL of 0.1 M NaOH solution, subsequently followed by washing with 200 mL of 0.1 M HCl solution. The filtrate was collected, while the residue was further washed with 200 mL of distilled water and then dried at 50°C for 24 h to yield cellulose. The filtrate was concentrated to approximately half its original volume and the pH was adjusted to 5.5 using HCl solution. Subsequently, 95% ethanol was added (with a volume ratio of ethanol to filtrate of 3:1) and allowed to stand for approximately 6 h. The resulting hemicellulose precipitate was filtered and washed with 100 mL of 70% ethanol. After distilling the filtrate to recover the ethanol, the pH was adjusted to 1.5 using an HCl solution. The precipitate was then filtered and washed with HCl solution (pH 2) before being dried to obtain lignin [11].
2.4. PAL Activity
The enzyme PAL activity is measured by the change in absorbance at 290 nm [12]. The experiment was conducted in triplicate, including a control sample alongside the experimental samples. The control, adjusted to zero at 290 nm, comprised 0.7 mL of 0.1 M borate buffer at pH 8.7, 1 mL of 1.0 M L-Phe solution, and 0.15 mL of distilled water. For each measurement, the reaction mixture contained 0.5 mL of 0.1 M borate buffer at pH 8.7, 1 mL of 0.1 M L-Phe solution, 0.15 mL of distilled water, and 0.2 mL of enzyme solution. The reaction was carried out at 37°C for 40 min, and was subsequently halted by adding 0.2 mL of 5.0 N HCl.
2.5. Lignin Peroxidase Activity
The activity of lignin peroxidase was determined using 2,4-dichlorophenol (2,4-DCP) [13]. The final volume of the reaction mixture was 1 mL and contained 100 mM sodium succinate buffer (pH 5.5), 82 mM 4-aminoantipyrine, 1.0 mM 2,4-DCP, 4.0 mM H2O2, and 100 μL of enzyme solution. The reaction was initiated by the addition of H2O2, and the increase in absorbance was monitored at a wavelength of 510 nm for 1 min at 37°C.
2.6. Laccase Activity
The activity of laccase was determined spectrophotometrically by using ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) as the substrate [14]. The laccase reaction mixture, with a total volume of 1 mL, contained 0.3 mL of diluted enzyme in acetate buffer (100 mM, pH 5.5) and 0.7 mL of 0.02 M ABTS. The reaction was monitored by measuring the change in optical density at a wavelength of 415 nm over a period of 2 min.
2.7. Statistical Analysis
A total of 900 plants were evaluated (15 treatments × 3 replications × 20 plants). Data were processed and visualized using Microsoft Excel 2010. The experimental data were analyzed using analysis of variance and Duncan’s multiple range test at significance levels of α = 0.05, utilizing SAS 9.1 software.
3. RESULTS
3.1. Flower Diameter and Stem Circumference
The results presented in Figure 1 indicate an interaction between the Phe treatment concentration and spraying frequency on the diameter of gerbera. At Phe concentrations ranging from 0 to 150 mg/L, the flower diameter increased significantly. However, at concentrations from 150 to 200 mg/L, there was no significant difference in flower diameter across all spraying frequencies, with values ranging from 108.1 to 110.4 mm, approximately 30% higher than the control. The Phe treatments did not contribute to an increase in the circumference of the flower stems.
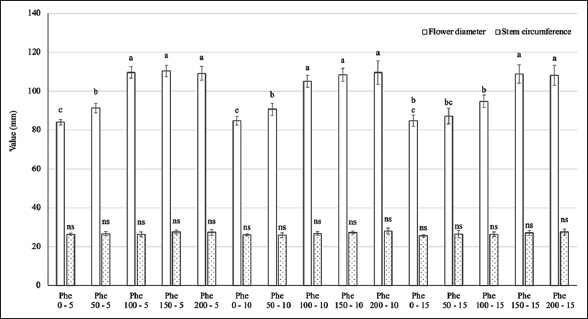 | Figure 1: Effect of phenylalanine on flower diameter and stem circumference in Gerbera after 35 days of treatment. Values are mean ± standard deviation, n=3. Values within treatments with different letters (a-c) in a column differ significantly (P≤0.05). [Click here to view] |
3.2. Stem Firmness
The Phe treatment showed significant differences in the stem hardness of the flowers [Figure 2]. The highest stem hardness values were observed in the Phe treatment groups at concentrations of 150–200 mg/L with spraying frequencies of 5 and 10 days (more than 84% higher than the control). Reducing the spraying frequency to every 15 days at 150–200 mg/L Phe led to a decrease in stem firmness, though it remained above control levels. In treatment groups with lower Phe concentrations ranging from 50 to 100 mg/L, no significant differences in stem hardness were found across all spraying frequencies.
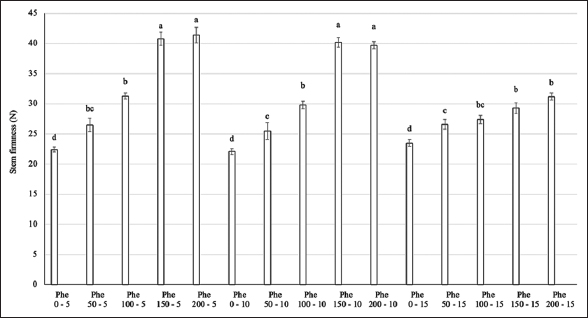 | Figure 2: Effect of phenylalanine on stem firmness in Gerbera after 35 days of treatment. Values are mean ± standard deviation, n=3. Values within treatments with different letters (a-e) in a column differ significantly (P≤0.05). [Click here to view] |
3.3. Vase Life
Regarding the vase life of flower stems, Phe treatment significantly extended the vase life of flowers by up to 10 days in the 150–200 mg/L Phe treatment groups at spraying frequencies of 5 or 10 days (over 70% longer than the control). Other treatment groups were less effective in maintaining flower longevity, averaging 6–8 days [Figures 3 and 4].
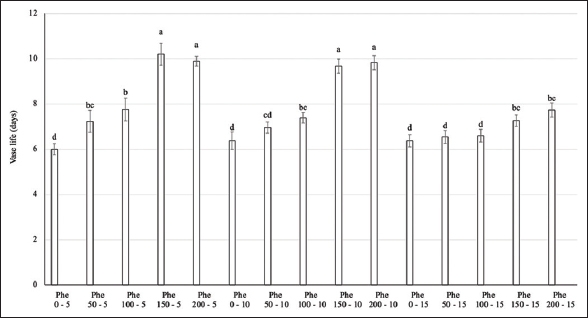 | Figure 3: Effect of phenylalanine on vase life in Gerbera after 35 days of treatment. Values are mean ± standard deviation, n=3. Values within treatments with different letters (a-d) in a column differ significantly (P≤0.05). [Click here to view] |
 | Figure 4: Effect of phenylalanine on the flower morphology of Gerbera during senescence, (a) at harvest time and (b) after 6 days. [Click here to view] |
3.3. Cellulose, Hemicellulose, and Lignin Content
The analysis of the cell wall components, including cellulose, hemicellulose, and lignin, revealed significant differences among the treatments [Figure 5]. The contents of these substances were positively correlated with the Phe concentration and reached a saturation point starting at the treatment level of 150 mg/L in the Phe spraying treatments with frequencies of 5–10 days. However, this correlation was not significant at the spraying frequency of every 15 days.
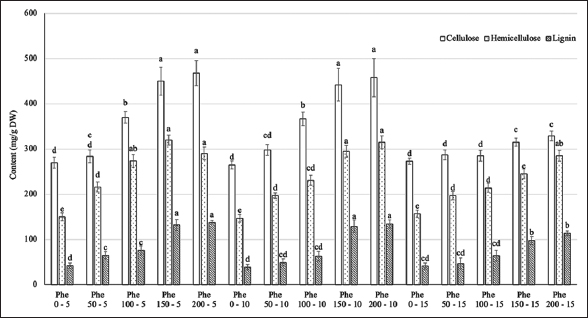 | Figure 5: Effect of phenylalanine on cellulose, hemicellulose, and lignin content in Gerbera after 35 days of treatment. Values are mean ± standard deviation, n=3. Values within treatments with different letters (a-e) in a column differ significantly (P≤0.05). [Click here to view] |
3.4. PAL, Lignin Peroxidase, and Laccase Activity
At harvest time (35 days after treatment), the levels of enzymes including PAL, lignin peroxidase, and laccase showed significant variations among the treatments [Figure 6]. The activity of these enzymes gradually increased with higher Phe concentrations, peaking at 150–200 mg/L. Changing the frequency of Phe treatment did not result in significant alterations in these values. When comparing the activities of the three types of enzymes, it is evident that PAL activity was more predominant than that of the other two enzymes.
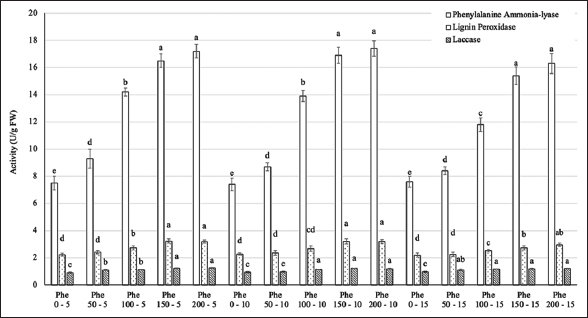 | Figure 6: Effect of phenylalanine on enzyme activity in Gerbera after 35 days of treatment. Values are mean ± standard deviation, n=3. Values within treatments with different letters (a-e) in a column differ significantly (P≤0.05). [Click here to view] |
4. DISCUSSION
Phe plays a vital role in Gerbera’s development by significantly enhancing bloom size, which in turn improves its visual appeal and economic value. Phe’s participation in several metabolic pathways that promote floral growth, especially the regulation of hormone synthesis, is largely responsible for this improvement. Phe, as a pre-cursor of key plant hormones, such as auxins and gibberellins, promotes cell elongation and division, resulting in larger and more visually appealing flowers [15]. As a result, larger, more esthetically pleasing flowers are produced. Larger flowers do have obvious esthetic and commercial advantages, but it’s important to weigh the possible trade-offs of higher cell growth and cell wall weakening. Excessive auxin synthesis can cause cell wall weakening, which permits cells to expand and absorb more water [16]. This occurrence may result in a delicate structure that is more prone to wilting and damage during handling or transit, even though it is advantageous for short-term esthetics. This is one of the drawbacks of this strategy, albeit the extent of the effect varies depending on a number of variables, including humidity, light, and temperature [17]. Environments with high temperatures can hasten transpiration, which causes wilting and a shorter vase life. Conversely, maintaining flowers in high humidity conditions can help alleviate these issues by reducing water loss, thus enhancing longevity despite the increased cell expansion. This adverse effect can be lessened, though, because Phe plays a part in the synthesis of structural proteins, such as expansins and extensins that are necessary for the integrity of the cell wall in addition to its involvement in increasing size. These proteins improve the cell walls’ elasticity and flexibility, which promotes structural resilience and better expansion during flower development [18]. As a result, the manner the applied Phe is processed by the plant’s metabolic pathways greatly affects the outcomes of Phe therapy. This is one example of a dual-edge strategy in floriculture. Even though it has several benefits in terms of size and appearance, the effects of enhanced cell expansion and wall thinning on structural integrity and post-harvest durability must be carefully considered.
Phe is crucial for improving the structural integrity of floral stems because it affects the deposition of lignin, cellulose, and hemicellulose. These elements are essential for giving the stem solidity and strength so that the flower can be supported by it effectively. Plant cell walls are strengthened by the complex polymer lignin, which gives them rigidity and resistance to mechanical stress [19]. In addition, the buildup of these substances may result in the xylem vessels becoming smaller, which improves the capillary action in the vascular system. This enhancement makes it easier for water to pass continuously from the stem to the flower petals, keeping the blooms hydrated and bright [20]. For the bloom to be healthy and long-lived, turgor pressure must be maintained, which is made possible by effective water transport. In addition to improving water transport, lignin, cellulose, and hemicellulose cause the stem to become more fibrillated, which lessens its brittleness. Because of its enhanced fibrousness, the stem is able to endure external stresses, such as wind and handling during shipping, making it a more durable structure [8].
Phe is an essential pre-cursor in the production of phenolic chemicals, and a critical enzyme in this process is PAL. The synthesis of lignin depends on the creation of phenolic acids, which are increased when Phe is more readily available because it increases PAL activity [21]. Phe also increases the activity of other lignin biosynthesis-related enzymes, including laccase and lignin peroxidase. By catalyzing the oxidation of lignin monomers, lignin peroxidase helps them polymerize into a strong three-dimensional network [22]. Increased Phe levels cause lignin peroxidase activity to rise, which in turn causes lignin to accumulate more in the cell wall. This improves the stem’s structural integrity and tolerance to environmental stressors and pathogens. Lignin deposition is further aided by laccase, which encourages the polymerization of phenolic chemicals into lignin [23]. Cellulose and hemicellulose, in addition to lignin, are essential elements of the plant cell wall that support its general elasticity and strength. For tensile strength, cellulose – which is made up of lengthy chains of glucose units – is necessary [24]. The presence of Phe stimulates the activity of cellulose synthase, which polymerizes glucose into cellulose fibers. Increased cellulose content enhances the stem’s ability to withstand mechanical forces, such as wind and the weight of the flower. By connecting with cellulose fibers, hemicellulose provides flexibility and support as a filler substance inside the cell wall [25]. By triggering particular enzymes, including as xylanase and glucanase, which aid in the breakdown of complex carbohydrates and permit the creation of hemicellulose structures that combine with cellulose to improve the structural integrity of the stem, Phe affects the production of hemicellulose [25].
Indeed, the application of Phe in this study has been shown to significantly boost the synthesis of polysaccharides, such as lignin, cellulose, and hemicellulose, which are essential for reinforcing the structural integrity of flower stems [Figures 5 and 6]. This enhancement contributes to increased stem hardness [Figure 2], which in turn leads to extended flower longevity [Figures 3 and 4]. Because of their stems’ increased mechanical strength and resistance, which helps them resist wilting and breaking, the flowers maintain their esthetic appeal for a longer amount of time. Particularly with regard to the improvement of cut flower quality, these findings are expected to have a major impact on the floriculture sector. By incorporating Phe treatments into cultivation practices, growers can enhance the structural integrity of flower stems, thereby reducing the risk of damage during transportation and display. This increased durability not only leads to greater customer satisfaction but also minimizes waste, as flowers maintain their esthetic appeal for extended periods. Furthermore, the enhanced water transport capabilities associated with reinforced stems ensure that the petals stay hydrated, contributing to the overall health and longevity of the flowers. In addition to the immediate benefits observed in Gerbera, future studies could explore the application of Phe on other ornamental plant species. By improving flower quality in a range of species, these programs could significantly boost the marketability of ornamental plants in the floriculture industry, ultimately helping growers and consumers.
5. CONCLUSION
By promoting natural lignin synthesis, Phe provides a more sustainable alternative to traditional techniques, such as chemical preservatives and artificial growth regulators, which mainly postpone the senescence process. This improvement makes gerbera blooms more resilient by fortifying their structural integrity. Phe treatment significantly boosts flower quality by elevating enzyme activity, particularly PAL, which enhances levels of cellulose, hemicellulose, and lignin. Applying Phe at concentrations of 150–200 mg/L every 5–10 days results in improved flower diameter, stem firmness, and an extension of vase life by up to 10 days. This underscores Phe’s potential as an innovative approach to enhancing post-harvest quality in floriculture, highlighting its significance for both flower health and environmental sustainability.