1. INTRODUCTION
Currently, energy is crucial for advancing every aspect of life and driving progress in agriculture, industry, and daily activities. Global energy demand continues to rise each year, with fossil fuels serving as the primary energy source. However, fossil fuels are rapidly being depleted, and their consumption releases significant air pollutants, particularly greenhouse gases, which contribute to global climate change. Hydrogen (H2) has gained significant attention as an alternative energy carrier. When consumed, H2 has a heating value of 144 MJ kg-¹ [1]. Additionally, the only byproduct of burning H2 with oxygen (O2) is water, making H2 a clean and renewable energy carrier. H2 can be produced through various processes, including steam-methane reforming, water electrolysis, and biological processes.
H2 production through biological processes can occur in various microorganisms, including bacteria, cyanobacteria, and green algae. These microorganisms use various pathways facilitated by specific enzymes. H2 production by green algae presents significant challenges that span biological, technological, and economical dimensions. H2 production by green algae is influenced by the type of algal strain and the prevailing environmental conditions [2]. In Thailand, H2 production by several green algal strains isolated from various water resources has been investigated [2–5]. However, green algal strains isolated from different geographic locations exhibit variations in robustness to environmental stresses, such as temperature fluctuations, nutrient variability, and high light intensity, which can hinder consistent H2 production [6].
Nutrient deprivation is a factor influencing H2 production in green algae. In Chlorella protothecoides, under nitrogen and sulfur-deficient conditions, cells reduce photosystem II (PSII) activity, leading to decreased O2 evolution. This reduction alleviates the inhibitory effects of O2 on hydrogenase activity, thereby increasing H2 production [7,8]. Additionally, potassium deprivation has been shown to enhance H2 production in Scenedesmus sp. KMITL-OVG1 [9]. Furthermore, various external factors, such as medium pH, incubation temperature, and light intensity, play a critical role in optimizing H2 production by green algae [3,4,10].
In this study, Chlorella strains were isolated from water sources in Thailand, selected, and evaluated for their H2 production capabilities. The selected strain was optimized for H2 production by studying various environmental parameters, including physical factors such as cell age, cell density, light intensity, temperature, and medium pH. Chemical factors, such as nutrient deprivation and the types and concentrations of carbon sources, were adjusted to assess their effects on H2 production. These optimizations improved the H2 production of the selected green algal strain, demonstrating the potential of the newly isolated algae for highly efficient biohydrogen production.
2 . MATERIALS AND METHODS
2.1. Chlorella Strains and Cultivation
Twenty-two Chlorella strains were isolated from various water sources, including rice paddy fields, freshwater ponds, waterfalls, and natural seawater, across six provinces in the central regions and northeastern regions of Thailand: Bangkok, Chai Nat, Chanthaburi, Nakorn Nayok, Nakorn Ratchasima, and Nakorn Sawan. These Chlorella strains were previously identified based on morphological characteristics and genetic analysis using 18S rRNA sequencing. The algal cells were cultivated in a 250-ml flask containing 100 ml of Tris-acetate phosphate (TAP) medium (pH 7.2), which included 17.5 mM acetic acid as a carbon source [11]. The cultures were incubated at 30°C, shaken at 120 rpm, and exposed to a white, fluorescent light intensity of 30 µmol photons m−2 s−1 for 3 days.
2.2. Screening of High H2-Producing Chlorella Strain
Chlorella strains, with an initial cell concentration at OD750 of approximately 0.1, were grown under the previously described conditions at 30°C for 36 hours. Cells were subsequently harvested by centrifugation at 8,000 × g, washed twice, and resuspended in fresh TAP medium. The cell density was adjusted to OD750 at approximately 2.0. Five ml of algal cell suspension was transferred into a glass vial and sealed with a rubber septum. To eliminate O2 from the system, the algal cells in the vial were purged with argon gas for 15 minutes before being shaken at 120 rpm under a light intensity of 30 µmol photons m−2 s−1 at 30°C for 5 days. H2 gas in the headspace was measured using a gas chromatograph (GC) every 24 hours of light incubation.
2.3. Measurements of OD750, Total Chlorophyll Concentration, and Total Cell Number
The optical density of Chlorella was measured at a wavelength of 750 nm using a spectrophotometer (Shimadzu UV-1601, Japan). For total chlorophyll extraction, the cell culture was harvested by centrifugation at 8,000 × g at 4°C for 10 minutes, and the supernatant was discarded. One ml of 90 % (v/v) methanol was added to the cell pellet. The mixture was thoroughly mixed by vortexing and incubated at 70°C for 2 hours. Total chlorophyll content was measured using a spectrophotometer at wavelengths of 650 and 665 nm and calculated according to Becker [12]. The total cell number was determined by a hemocytometer (BOECO, Germany).
2.4. Effect of Cell Age and Cell Optical Density on H2 Production
Chlorella was cultivated in TAP medium under previously described conditions at 30°C for 12, 24, 36, 48, and 60 hours. The cells were then harvested, washed, and resuspended in a fresh TAP medium. Five ml of algal cell suspension was transferred into a glass vial, and O2 was eliminated from the system by purging with argon gas for 15 minutes. H2 production was measured after 2 hours and then every 24 hours of anaerobic light incubation. To investigate the effect of cell optical density on H2 production, the cell density of Chlorella was adjusted to OD750 at 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 2.5, and 3.0.
2.5. Effect of Nutrient Deprivation on H2 Production
Chlorella was cultivated in TAP medium under previously described conditions at 30°C for 36 hours. The cells were harvested by centrifugation and resuspended in 100 ml of various modified media: normal TAP, potassium-deprived TAP (TAP-K), nitrogen-deprived TAP (TAP-N), phosphorus-deprived TAP (TAP-P), and sulfur-deprived TAP (TAP-S). TAP-K was prepared by removing KH2PO4 and K2HPO4 from TAP and adding phosphate in the form of NaH2PO4 and Na2HPO4, respectively, instead. TAP-N was prepared by removing NH4Cl from TAP. TAP-P was prepared by removing KH2PO4 and K2HPO4 from TAP and adding potassium in the form of KCl. TAP-S was prepared by removing MgSO4.6H2O, FeSO4.7H2O, ZnSO4.7H2O, and CuSO4.5H2O, and adding sulfate in the form of MgCl2, FeCl2, ZnCl2, and CuCl2, respectively. The cell suspensions were shaken on a rotary shaker at 120 rpm under light at 30°C for 24 hours. Cells were harvested by centrifugation, washed, and resuspended in fresh media of the same type. Five ml of algal cell suspension with an OD750 of 2.0 was transferred into a glass vial. H2 production was measured under light anaerobic conditions using a GC.
2.6. Effect of Carbon Source and Concentration on H2 Production
Chlorella was cultivated in TAP medium at 30°C for 36 hours before harvesting cells by centrifugation. The cells were then resuspended in nutrient-deprived media and incubated for 24 hours. Subsequently, cells were harvested again and resuspended in fresh nutrient-deprived TAP media containing different carbon sources: acetic acid, sodium acetate, glucose, sucrose, ethanol, propanol, butanol, and glycerol, all at the same C-atom molar concentration (35 mmol C-atom l−1). The optical density of the cell suspension was adjusted to an OD750 of 2.0. Five ml of the cell suspension was transferred into a glass vial, sealed with a rubber septum, and purged with argon for 15 minutes before measuring H2 production. To investigate the effect of carbon concentration on H2 production, the carbon concentrations varied from 0 to 1,750 mmol C-atom l−1.
2.7. Effect of Temperature, Initial Medium pH, and Light Intensity on H2 Production
Chlorella adapted in nutrient-deprived media for 24 hours were harvested by centrifugation and resuspended in fresh nutrient-deprived media containing the selected carbon source and concentration before being transferred into vials. To study the effect of initial medium pH on H2 production, the medium pH was adjusted to 5.0, 6.0, 7.0, 7.2, 8.0, and 9.0. To examine the effects of temperature and light intensity on H2 production, the vials were incubated at temperatures of 25°C, 30°C, 35°C, 40°C, 45°C, and 50°C and exposed to light intensities of 0, 30, 90, 150, 210, 300, and 390 µmol photon m−2 s−1. H2 production was measured every 24 hours using GC.
2.8. H2 and O2 Measurement
H2 and O2 levels in the headspace gas phase were measured using a GC (Hewlett-Packard HP5890A, Japan). A 0.5 ml sample of headspace gas was collected with a gas-tight syringe and injected into the GC. H2 and O2 were separated in a packed column with a molecular sieve (5 °A, 60/80 mesh), using argon as the carrier gas, and detected by a Thermal Conductivity Detector (TCD). The GC-TCD conditions were as previously reported [13]. H2 and O2 production was expressed as the amount of hydrogen produced (µmol H2) per chlorophyll content (mg chl). The production rate was expressed as the amount of production per incubation time (hour).
2.9. In Vivo Hydrogenase Activity Measurement
In vivo H2ase activity was assayed in a 12.5 ml glass-tight vial. The reaction mixture consisted of 25 mM potassium phosphate buffer containing 1% (v/v) Triton X-100, 5 mM methyl viologen, 20 mM sodium dithionate, and the Chlorella culture [14]. The reaction mixture was incubated at 30°C in darkness for 30 minutes before H2 production was measured using a GC. Hydrogenase activity was expressed as the amount of H2 produced (µmol H2) per chlorophyll content (mg chl) per incubation time in minutes.
2.10. Statistical Analysis
Data from each experiment were analyzed using one-way analysis of variance (ANOVA) with IBM SPSS Statistics version 28.0 (SPSS software, New York, NY). Results are presented as mean ± SE. The Duncan multiple range test was employed to identify statistically significant differences between the parameters under study, with the level of significance set at a p-value < 0.05. Different English letters in figures or tables indicate significant differences.
3. RESULTS
3.1. Screening of High H2-Producing Chlorella Strain
In this study, all 22 strains of Chlorella exhibited H2 production under anaerobic light conditions. Among them, Chlorella sp. ChiW1, isolated from a rice paddy field in Chai Nat province, showed the highest H2 production rate with 5.07 ± 0.23 µmol H2 mg chl−1 h−1 and reached a maximum H2 production of 169.46 ± 7.48 µmol H2 mg chl−1 after 48 hours of incubation in TAP medium (Table 1). This result indicates that each Chlorella strain exhibited different H2 production rates. Notably, Chlorella sp. ChiW1 showed the highest H2 production rate, while Chlorella sp. KLM146 exhibited the lowest rate, with an H2 production rate 11.3 times lower. Therefore, Chlorella sp. ChiW1 was selected as the highest H2-producing Chlorella strain for optimization of H2 production.
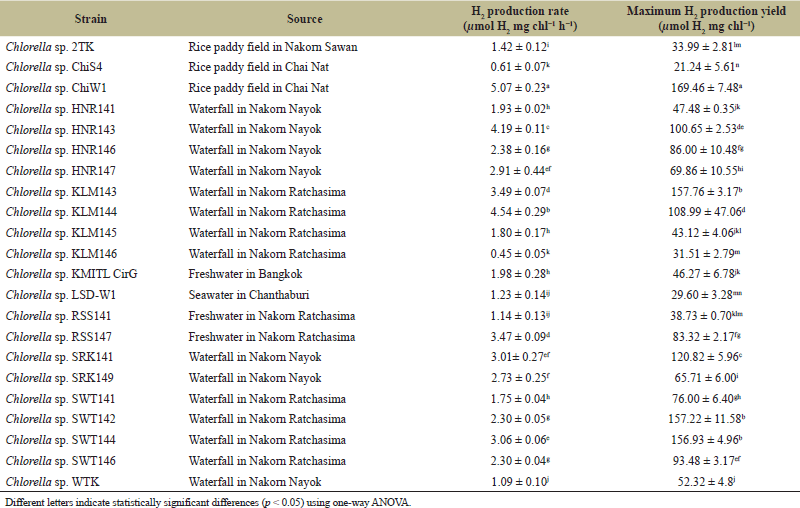 | Table 1. H2 production rate and maximum H2 production yield of Chlorella sp. isolated from various water sources in Thailand. [Click here to view] |
3.2. Effect of Cell Age and Cell Density on H2 Production by Chlorella sp. ChiW1
Chlorella sp. ChiW1, cultured for 36 hours in the mid-logarithmic phase, demonstrated the highest H2 production rate with 5.12 ± 0.13 µmol H2 mg chl−1 h−1 and a maximum H2 production of 169.58 ± 9.77 µmol H2 mg chl−1 after 48 hours of incubation in TAP medium under anaerobic light conditions (Fig. 1). Cells grown in the lag (12 hours) and early logarithmic (24 hours), late logarithmic (48 hours), and stationary (60 hours) phases showed lower H2 production rates. This result indicates the significant role of cell age on H2 production in Chlorella sp. ChiW1.
To investigate the effect of cell density on H2 production, H2 production was measured in cell cultures of Chlorella sp. ChiW1 was grown for 36 hours and adjusted to various OD750 values. Cultures with higher cell densities contained higher total cell numbers and total chlorophyll concentrations (Table 2). The Chlorella sp. ChiW1 culture with an OD750 of 2.0 exhibited the highest H2 production rate with 5.07 ± 0.18 µmol H2 mg chl−1 h−1 and a maximum H2 production of 169.60 ± 6.80 µmol H2 mg chl−1 at 48 hours of incubation (Table 2). H2 production in Chlorella sp. ChiW1 increased with increasing cell densities, total cell numbers, and chlorophyll contents (Table 2). However, H2 production decreased in cultures with OD750 values higher than 2.0. For further investigation on the optimization of H2 production, Chlorella sp. ChiW1 with a cell age of 36 hours and an OD750 of 2.0 was chosen.
3.3. Effect of Nutrient Deprivation on H2 Production by Chlorella sp. ChiW1
To enhance H2 production, Chlorella sp. ChiW1 cells grown in TAP medium for 36 hours were adapted in different nutrient-deprived TAP media for 24 hours before adjusting the cell density to an OD750 of 2.0. The highest H2 production rate of 10.57 ± 0.39 µmol H2 mg chl−1 h−1 was observed under nitrogen-deprived conditions (Table 3). Chlorella sp. ChiW1 incubated in TAP-N reached a maximum H2 production of 321.18 ± 6.92 µmol H2 mg chl−1 after 48 hours of incubation (Table 3). This H2 production was approximately twice that observed under normal TAP conditions. Sulfur deprivation in TAP also promoted H2 production in Chlorella sp. ChiW1, resulting in an H2 production rate of 8.45 ± 049 µmol H2 mg chl−1 h−1 and a maximum H2 production of 270.36 ± 9.36 µmol H2 mg chl−1, about 1.6 times higher than under normal TAP conditions (Table 3). However, H2 production in Chlorella sp. ChiW1 incubated in phosphorus-deprived and potassium-deprived media was not significantly different from that observed under normal TAP conditions (Table 3).
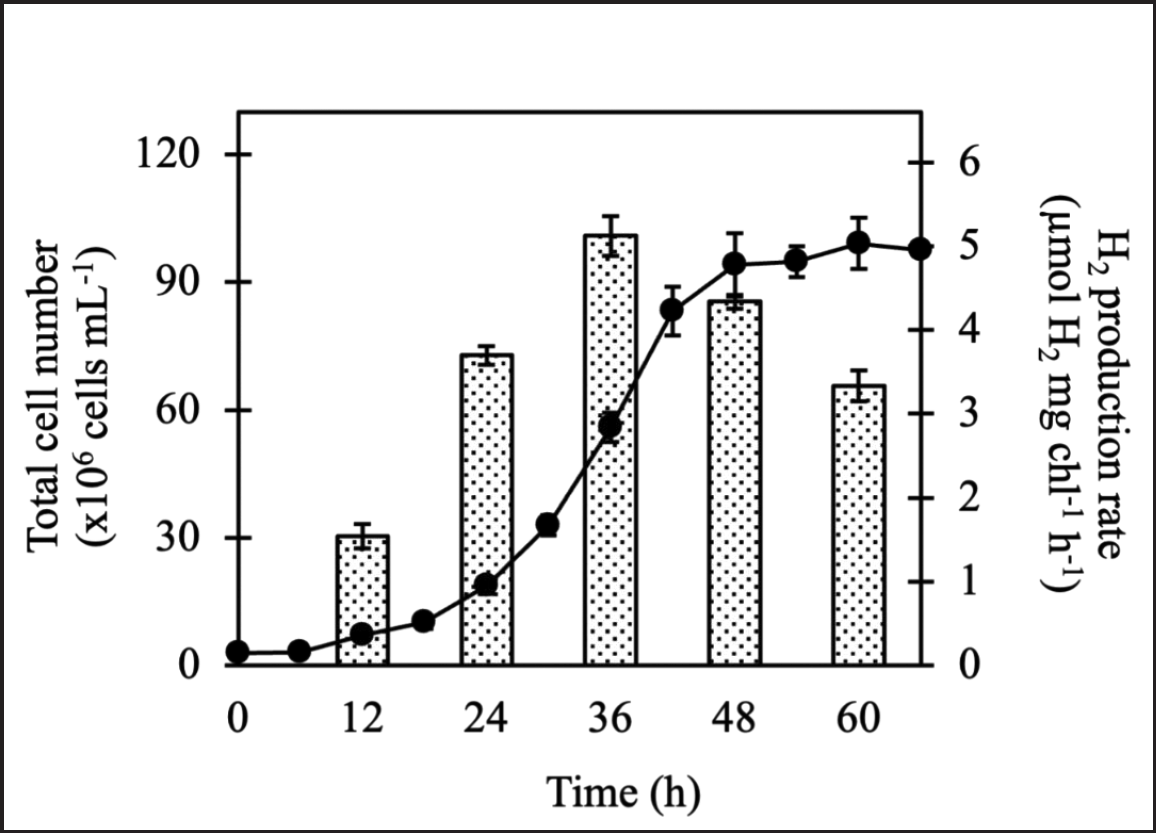 | Figure 1. Growth by cell number measurement and H2 production rate of Chlorella sp. ChiW1 cultivated in TAP medium for different cultivation times. [Click here to view] |
 | Table 2. H2 production rate and maximum H2 production yield of Chlorella sp. ChiW1 cultivated in TAP for 36 hours and adjusted the initial optical cell densities at 750 nm from 0.2 to 3.0. Each different initial OD750 showed different total cell numbers and total chlorophyll concentrations. [Click here to view] |
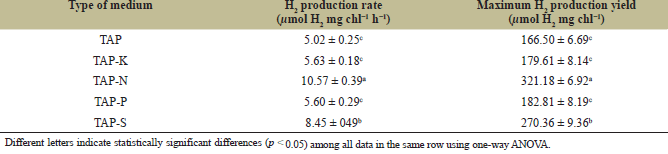 | Table 3. H2 production rate and maximum H2 production yield of 36 hours old Chlorella sp. ChiW1 cells incubated in different nutrient-deprived TAP media for 24 hours before adjusting the cell density to an OD750 of 2.0. [Click here to view] |
3.4. Effect of Nitrogen Deprivation on H2 and O2 Production, and In Vivo Hydrogenase Activity by Chlorella sp. ChiW1
To investigate the effects of nitrogen-deprived metabolism on H2 production, Chlorella sp. ChiW1 cells were incubated in TAP-N medium under light aerobic conditions for 24 hours before harvesting cells to determine H2 and O2 production, as well as in vivo H2ase activity under anaerobic conditions. The nitrogen-deprived cells produced approximately two-fold higher H2 production and H2ase activity than normal cells during 120 hours of light anaerobic conditions (Fig. 2A and B). In contrast, O2 production was significantly decreased in nitrogen-deprived cells (Fig. 2C). The increased H2 production was attributed to an increase in H2ase activity, which resulted from the decreased O2 in the system.
3.5. Effect of Carbon Source and Concentration on H2 Production by N-Deprived Cells of Chlorella sp. ChiW1
H2 production was measured in N-deprived cells of Chlorella sp. ChiW1 incubated in TAP-N medium containing various carbon sources, including acetic acid, butanol, ethanol, glucose, glycerol, propanol, sodium acetate, and sucrose, all at the same C-atom concentration of 35 mmol C-atom l−1. The result showed that Chlorella sp. ChiW1 incubated in TAP-N medium containing acetic acid as the carbon source exhibited the highest H2 production rate with 10.90 ± 0.95 µmol H2 mg chl−1 h−1 (Table 4) and reached a maximum H2 production yield of 330.52 ± 15.80 µmol H2 mg chl−1 after 72 hours of incubation (Table 4). On the other hand, the lowest H2 production rates were observed in cells incubated in TAP-N medium containing either glycerol or sucrose (Table 4). Thus, acetic acid was identified as the most effective carbon source for H2 production by Chlorella sp. ChiW1.
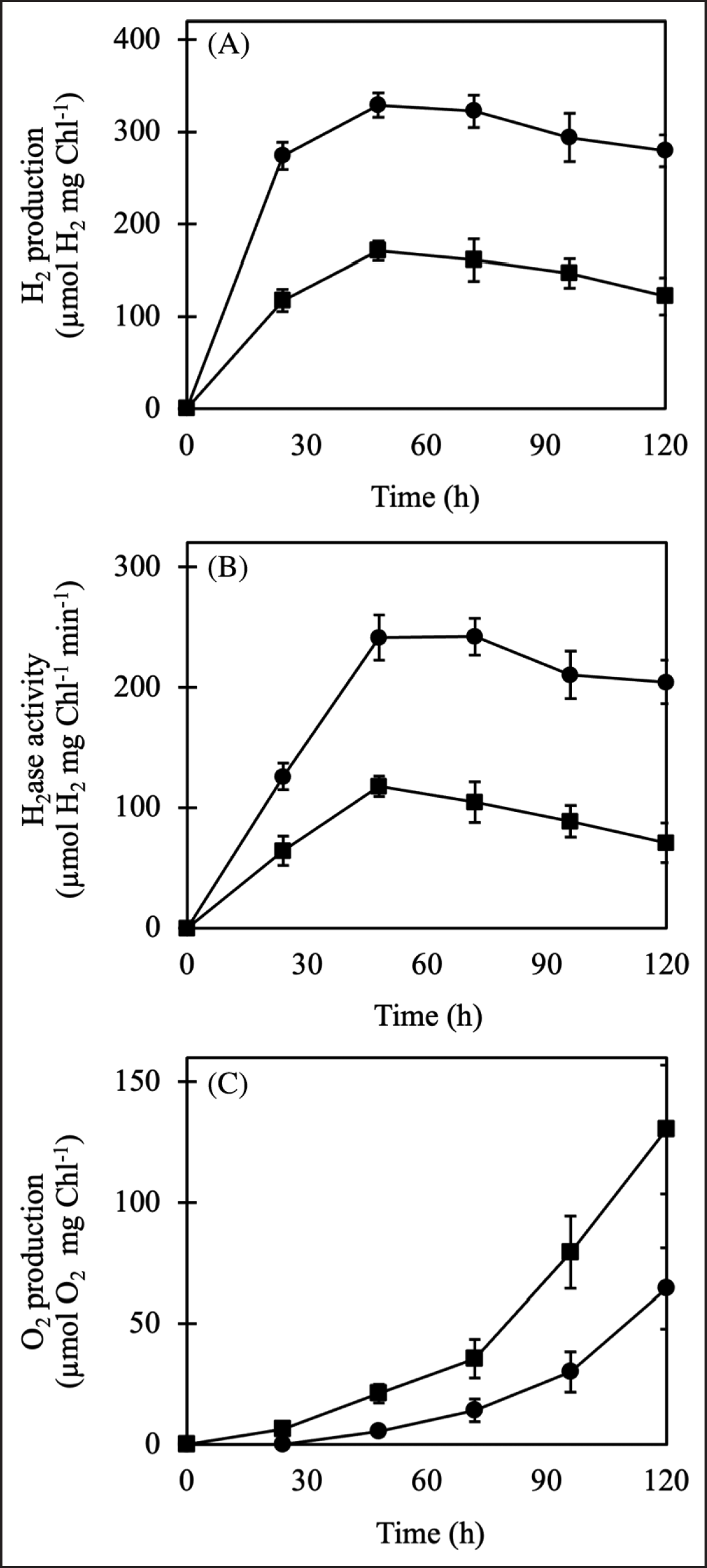 | Figure 2. H2 production (A), H2ase activity (B), O2 production (C) of Chlorella sp. ChiW1 cells incubated in normal TAP (?) and TAP-N medium (?). [Click here to view] |
Additionally, H2 production was determined in nitrogen-deprived cells incubated in TAP-N medium containing various concentrations of acetic acid, with TAP-N containing 35 mmol C-atom l−1 acetic acid used as the control medium. The result showed that the maximum H2 production rate of 14.66 ± 0.53 µmol H2 mg Chl−1 h−1, and maximum H2 production yield of 445.31 ± 21.53 µmol H2 mg Chl−1 were obtained in cells incubated in TAP-N containing 175 mmol C-atom l−1 acetic acid, which is five times the carbon concentration of TAP-N (Table 5). Concentrations lower or higher than this decreased H2 production (Table 5). Without the addition of acetic acid, Chlorella sp. ChiW1 had the lowest H2 production rate with only 0.34 ± 0.06 µmol H2 mg Chl−1 h−1 (Table 5), indicating the significance of carbon source on H2 metabolism in Chlorella sp. ChiW1.
3.6. Effect of Medium pH, Incubation Temperature, and Light Intensity on H2 Production by Chlorella sp. ChiW1
H2 production was measured in nitrogen-deprived Chlorella sp. ChiW1 cells were incubated in TAP-N medium containing 175 mmol C-atom l−1 acetic acid, with pH varied in the range of 5.0–9.0. The highest H2 production rate with 14.46 ± 1.19 µmol H2 mg Chl−1 h−1 was found in cells incubated at pH 7.2 (Fig. 3A). This rate did not show significant differences compared to the H2 production rates of cells incubated at pH 7.0. Cells at pH 7.2 reached a maximum H2 production yield of 454.94 ± 14.28 µmol H2 mg Chl−1 after 96 hours of incubation. Under various incubation temperatures, Chlorella sp. ChiW1 cells incubated in TAP-N medium containing 175 mmol C-atom l−1 acetic acid with pH 7.2 at incubation temperature 35°C demonstrated the highest production rate with 16.89 ± 0.80 µmol H2 mg Chl−1 h−1 (Fig. 3B) and reached the highest production rate with 517.42 ± 20.50 µmol H2 mg Chl−1 after 96 hours of incubation. To investigate the effect of light intensity on H2 production by Chlorella sp. ChiW1, light intensity was varied in the range of 0–390 µmol photons m−2 s−1. The result indicated that cells incubated in TAP-N medium containing 175 mmol C-atom l−1 acetic acid with pH 7.2 at incubation temperature 35°C under a light intensity of 210 µmol photons m−2 s−1 showed the highest H2 production rate with 31.28 ± 1.73 µmol H2 mg Chl−1 h−1 (Fig. 3C) and achieved the maximum H2 production yield of 952.38 ± 25.55 µmol H2 mg Chl−1. This H2 production rate was approximately 2 times higher than that of cells incubated at 30 µmol photons m−2 s−1. In contrast, cells incubated in darkness provided the lowest H2 production rate with 3.20 ± 0.54 µmol H2 mg Chl−1 h−1 (Fig. 3C).
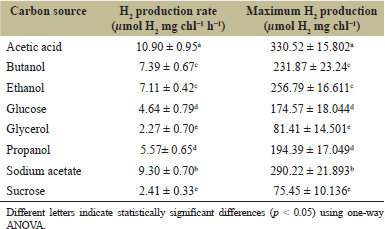 | Table 4. H2 production rate and maximum H2 production yield of N-deprived adapted cells of Chlorella sp. ChiW1 incubated in TAP-N medium containing various carbon sources. [Click here to view] |
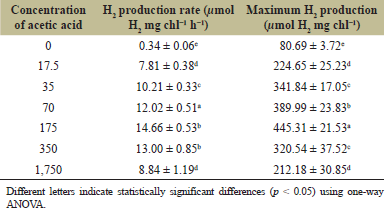 | Table 5. H2 production rate and maximum H2 production yield of N-deprived adapted cells of Chlorella sp. ChiW1 incubated in TAP-N medium containing acetic acid concentrations. [Click here to view] |
4. DISCUSSION
4.1. Screening of Potential H2-Producing Chlorella Strain
Among the 22 Chlorella strains investigated, Chlorella sp. ChiW1, isolated from a rice paddy field in Chai Nat province, exhibited the highest H2 production rate and yield (Table 1). This could be attributed to the unique composition of water in rice paddy fields, which may contain primary macronutrients (N, P, and K) essential for both plant and algal growth [15]. Additionally, the presence of Mg ions, vital for photosynthesis [16], and Fe ions, which act as cofactors for the hydrogenase enzyme [17], likely contribute to this strain's efficiency. Moreover, rice paddy fields are exposed to high light intensities and elevated temperatures, which may have favored the development of Chlorella sp. ChiW1 with an enhanced photosynthetic rate. Its hydrogenase enzyme might also exhibit greater tolerance to O2 compared to strains isolated from other water sources.
In Thailand, previous research focused on the isolation of green algae from diverse freshwater sources, resulting in the identification of 43 strains across six genera [2]. These strains exhibited variations in their capacity for H2 production. Moreover, within the same genus, differences in H2 production were observed under various TAP conditions. Most Chlorella strains and all Chlamydomonas strains enhanced H2 production under simultaneous nitrogen limitation and sulfur deprivation conditions [2]. Additionally, a previous report on the isolation of green algae from rice paddies in Thailand, cultured in BG11 medium, identified nine green algal strains that demonstrated varying levels of H2 production [15]. From screening H2-producing green algae isolated from natural seawater in Thailand, Chlorella sp. LSD-W2 showed the highest H2 production rate with 1.52 μmol H2 mg chl−1 h−1 when incubated in TAP medium under light anaerobic conditions. Additionally, Chlorella sp. LSD-W2 under N-deprived conditions increased its H2 production rate by up to 20 times compared to that under normal TAP conditions [5].
These findings underscore that variations in H2 production can occur even within the same strain of green algae under similar or different cultivation conditions. This variability highlights the importance of not only the inherent characteristics of the algae strain but also the specific environmental and nutritional factors during cultivation. Despite these variations, our study specifically demonstrated that Chlorella sp. ChiW1 shows promising potential for H2 production. Therefore, Chlorella sp. ChiW1 emerges as an intriguing candidate deserving further investigation and optimization of cultivation conditions and other influencing factors to enhance H2 production. By studying and optimizing these conditions and factors, such as cell age, cell density, nutrient availability, medium pH, incubation temperature, and light intensity, it may be possible to maximize the H2 production capabilities of Chlorella sp. ChiW1, thus contributing to its potential application in sustainable biofuel production or other industrial processes.
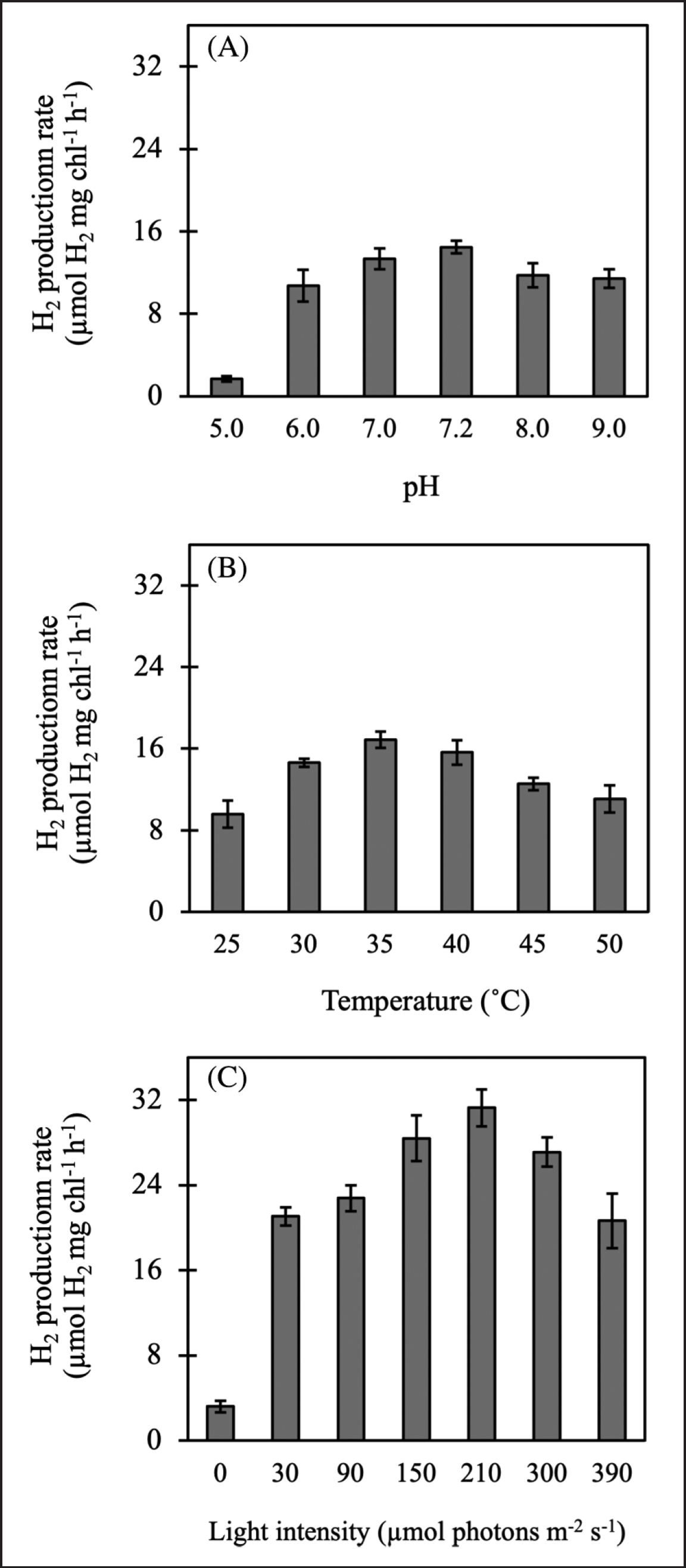 | Figure 3. Effect of medium pH (A), incubation temperature (B), and light intensity (C) on H2 production by Chlorella sp. ChiW1 incubated in TAP-N containing 175 mmol C-atom l−1 acetic acid. [Click here to view] |
4.2. Effect of Cell Age and Cell Density on H2 Production by Chlorella sp. ChiW1
The suitability of algal cells is one of the first aspects studied for optimizing H2 productivity. The age and density of algal cells were varied to determine optimal conditions for H2 production by Chlorella sp. ChiW1. Experimental results indicated that Chlorella sp. ChiW1 produced H2 most efficiently at a cell age of 36 hours (Fig. 1) and a cell density of OD750 equal to 2.0 (Table 2). At 36 hours of cultivation, Chlorella sp. ChiW1 was in the mid-logarithmic growth phase (Fig. 1), a stage where the cells grew well, divided very quickly, and produced high chlorophyll content. The chlorophyll content is a crucial factor for H2 production by green algal cells under light conditions since chlorophyll is responsible for absorbing light energy used in the photosynthesis process at PSII. When chlorophyll absorbs light energy, it initiates the water-splitting reaction, producing protons, electrons, and oxygen (O2). The electrons and protons generated from water splitting can be utilized for H2 production, mediated by hydrogenase (H2ase) [18]. Therefore, as chlorophyll content increased, the substrates for H2 production also increased, leading to higher H2 output. In Chlamydomonas reinhardtii UTEX 90, cells at the late-exponential phase containing a chlorophyll content of 39.29 mg l−1 showed the highest H2 production of 159 ml H2 g−1 cell under anaerobic sulfur-deprived conditions [19].
However, the water-splitting reaction in photosynthesis produces O2 as a byproduct, which is a potent inhibitor of H2ase [20]. Excessive chlorophyll content resulted in higher residual O2 in the system, inhibiting enzyme activity. Thus, optimal cell concentration was critical to avoid excessive chlorophyll levels. This aligns with the experimental results showing that cell density higher than an OD750 of 2.0 resulted in decreased H2 production despite the increased cell density. In addition, an excess number of cells might lead to lower electron availability from the photosynthetic process due to cell shading [18]. Previous research on the effect of cell density on H2 production by Scenedesmus sp. KMITL-OVG1 demonstrated that H2 production decreased from 0.79 to 0.48 ml l−1 h−1 when OD750 increased from 0.8 to 1.0 [9].
4.3. Effect of Nutrient Deprivation on H2 Production
H2 production by Chlorella sp. ChiW1 increased under N-deprived and S-deprived conditions (Fig. 2), consistent with several studies promoting H2 production by green algae. Under nitrogen deficiency, algal cells adapt by altering their metabolism, for example, by inhibiting growth and protein synthesis while promoting starch accumulation [21–23]. When cells enter anaerobic conditions, starch is degraded into glucose molecules, which then generate numerous electrons that serve as substrates for H2ase activity [24]. Consequently, H2 production by algal cells under nitrogen deprivation is induced. Moreover, nitrogen deficiency also restricts the synthesis of the D1 protein, thereby inhibiting the repair of PSII [25]. This reduces PSII activity, leading to the establishment of anaerobiosis and the induction of H2ase activity, eventually enhancing H2 production [7,8]. Previous reports showed similar results with N-deprivation enhancing H2 production by Chlorella sp. LSD-W2 and Chlorella pyrenoidosa IOAC707S [5,26]. Thus, N-deprived conditions, which generated the highest H2 production by Chlorella sp. ChiW1, were selected for further optimization.
In addition to nitrogen deprivation, sulfur deficiency could also induce H2 evolution in several green algal strains, including Chlorella sp. ChiW1. Sulfur, as a crucial component of amino acids, proteins, and enzymes, plays a significant role in cellular metabolism [27]. In C. reinhardtii, sulfur deprivation led to the rapid degradation of the PSII complex, which severely inhibited electron transport in PSII [28]. This reduction in PSII activity decreased O2 photoevolution, thereby increasing H2ase activity, and ultimately enhancing H2 production.
Consistent with previous reports on C. reinhardtii CC-125 and Chlorella sp. LSD-W2, sulfur deprivation enhanced H2 production compared to normal TAP conditions [5,29]. Incubating algal cells under nutrient-deprived conditions can reduce medium costs; however, it poses significant limitations when scaling up to large-scale systems. Scaling up nutrient deprivation processes requires precise control and additional resources to monitor and adjust nutrient levels, which increases operational costs and reduces the economic feasibility of large-scale H2 production systems. Furthermore, prolonged nutrient deprivation can stress the cells, leading to reduced viability and potentially causing system instability. This is especially critical in open or semi-continuous systems where maintaining a stable culture is challenging.
4.4. H2 Metabolism Under Nitrogen Deprivation
Under nitrogen deficiency, Chlorella sp. ChiW1 increased H2 production and H2ase activity but decreased O2 production (Fig. 2). The decreased O2 in the system might be caused by a lower O2 evolution rate due to the reduced photosynthesis and increased respiration rate. Previous studies showed that C. pyrenoidosa and C. protothecoides exhibited increased respiration rates while O2 evolution and the efficiency of PSII activity decreased under N-deprivation [7,8,30]. As a result, N-deprivation could prolong the anaerobic state of the incubation system and promote H2ase activity.
Besides PSII, starch is the main endogenous electron source for H2 production by green algae [8]. During nitrogen deprivation, intracellular starch in Chlorella sp. ChiW1 accumulates approximately 2–3 times higher than under normal conditions (data not shown). Under anaerobic conditions, cells degrade starch rather than accumulate it, subsequently releasing a large number of electrons. A previous study indicated that starch concentration increased in C. reinhardtii cells incubated in both sulfur-deprived and nitrogen-deprived media. Specifically, cells under nitrogen-deprived conditions demonstrated approximately twice the starch accumulation compared to those under sulfur-deprived conditions [21]. This increased starch accumulation was subsequently catabolized, releasing a high level of electrons, which could promote hydrogenase activity and enhance H2 production.
4.5. Effect of Carbon Source and Concentration on H2 Production by Chlorella sp. ChiW1
The carbon source and its concentration have been shown to play a crucial role in enhancing H2 production by green algae. In Chlorella sp. ChiW1, acetic acid available in TAP medium was identified as a potential carbon source for H2 production (Table 4), with the optimal acetic acid concentration at 175 mmol C-atom l−1 or 87.5 mM (Table 5). Acetic acid provided the highest H2 production by Chlorella sp. ChiW1, suggesting that it might be easily metabolized in carbohydrate metabolism, ultimately establishing anoxia in the cultures through its oxidation in the tricarboxylic acid cycle and oxidative phosphorylation. In C. reinhardtii strain 704 (cw15 arg7+ Nia1:Ars mt+), the addition of acetate to cultures decreased photosynthetic efficiency and promoted mitochondrial respiration, leading to a reduction in O2 evolution [31].
Furthermore, studies on the fermentative metabolism in C. reinhardtii F-60 have supported the conversion of acetate into H2 under anaerobic and light-dependent conditions via the citric acid and glyoxylate cycles [32]. Thus, the availability of acetic acid is assumed to reduce O2 in the system and subsequently promote H2ase activity. Several previous studies have reported that acetate as a carbon source provided the highest H2 yield in C. reinhardtii strain CC124 and Parachlorella kessleri [33,34].
In addition, Chlorella sp. ChiW1 incubated in acetic acid-free TAP medium produced less H2 than cells in an acetic acid-containing TAP medium (Table 5). This observation is consistent with previous studies that highlighted the importance of using acetate as a carbon source in culture media, compared to autotrophic cultivation. It was found that H2 production by C. reinhardtii cultured in acetate-containing media was higher than that of autotrophic cultures [31]. The concentration of acetic acid is also crucial for H2 production, as excessively high concentrations could affect various factors, such as pH balance and inhibition of initial substrates [35,36].
4.6. Effect of Medium pH, Incubation Temperature, and Light Intensity on H2 Production by Chlorella sp. ChiW1
The pH of the culture medium significantly influences H2 production by green algae, including various strains of Chlorella. In this study, the pH levels of 7.0 and 7.2 provided the highest H2 production by Chlorella sp. ChiW1 (Fig. 3). This indicates that a neutral pH is optimal for H2ase activity, whereas acidic and alkaline pH levels are not suitable. This finding is consistent with a study on Chlorella sp. KLSc59, which demonstrated that the highest H2 production was found at pH 7.2 [4]. Other studies have reported that the maximum H2 production by Chlorella vulgaris and other Chlorella species was obtained at pH 8.0 [37,38]. This suggests that most green microalgal strains including Chlorella sp. ChiW1, can efficiently produce H2 within a range of neutral pH conditions, while inhibition of H2-producing enzymes occurs in both acidic and extremely alkaline conditions [39]. In addition, maintaining a pH at the optimal level is another crucial factor for sustainable H2 production in green algae [40].
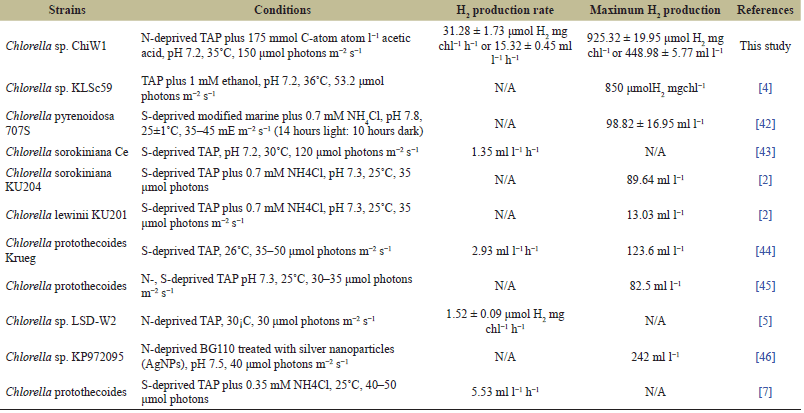 | Table 6. H2 production of various Chlorella strains under different incubation conditions. [Click here to view] |
Microorganisms can produce H2 efficiently at different optimal temperatures, depending on the strain. The high H2 production by Chlorella sp. ChiW1 occurred within the temperature range of 30°C–40°C, with the maximum H2 production at 35°C (Fig. 3), suggesting this is the optimal temperature for H2ase activity. However, Chlorococcum minutum and C. reinhardtii exhibited efficient H2 production at 25°C, and a significant decrease in growth and H2 production was obtained at 35°C [41]. In Chlorella sp. NIER-10003, higher temperatures reduced the adaptation period and increased the H2 production rate. However, temperatures above 40°C were unsuitable, as the cells began to die. Conversely, temperatures below 25°C resulted in a prolonged adaptation period and a significantly reduced hydrogen production rate [47].
Light intensity plays a crucial role in H2 production by green algae since electrons, which are substrates for H2ase, are obtained from light energy via water photolysis. Theoretically, green algae can utilize sunlight as an energy source to generate H2, reaching up to 13% efficiency [39,48]. H2 production by Chlorella sp. ChiW1 increased with higher light intensities until it reached the optimal light intensity at 210 µmol photons m−2 s−1 (Fig. 3). Similarly, C. reinhardtii UTEX 90 cultivated in sulfur-deficient conditions demonstrated the impact of light on H2 production, achieving the highest H2 production under a light intensity of 200 µmol photons m−2 s−1 [49]. Increased light intensity affects internal mechanisms, one of which involves the inhibition of the synthesis of the D1 protein in PSII [50], contributing to the prolonged maintenance of an anaerobic system for higher H2 production [51]. In contrast, Chlorella sp. KLSc59 and Chlorella sp LSD-W2 gave the highest H2 production under a light intensity of only 53.2 and 60 µmol photons m−2 s−1 [4,52]. Thus, light-dependent H2 production varies based on the type of strains and species. Excessive light intensities can result in cell damage by contributing to heat accumulation within the cultivation system, potentially leading to cell death [10].
In this study, Chlorella sp. ChiW1 showed the highest H2 production rate with 31.28 ± 1.73 µmol H2 mg chl−1 h−1 or 15.32 ± 0.45 ml l−1 h−1 and reached the maximum H2 production yield with 925.32 ± 19.95 µmol H2 mg chl−1 or 448.98 ± 5.77 ml l−1. These results were observed in cells grown in TAP medium for 36 hours, subsequently incubated in N-deprived TAP medium for 2 days before harvesting the cells and adjusting the cell density to an OD750 of about 2.0 in N-deprived TAP medium containing 175 mmol C-atom l−1 acetic acid at pH 7.2, and incubating at 35°C under light intensity of 150 µmol photons m−2 s−1 (Table 6). These H2 production rates and yields were higher than those of other Chlorella strains, due to the strain dependence and environmental factors, including medium composition, medium pH, incubation temperature, and light intensity [2,4,5,7,42–46]. Therefore, using conditions that are appropriate and harmonious within the system is crucial in developing a more efficient and sustainable H2 production process.
5. CONCLUSION
In summary, the green alga Chlorella sp. ChiW1, isolated from a rice paddy field in Chai Nat province, Thailand, demonstrated significant potential for efficient H2 production compared to other investigated Chlorella strains. Nitrogen deprivation doubled H2 production in Chlorella sp. ChiW1 by enhancing H2ase activity due to reduced O2 levels. Acetic acid, at a concentration of 175 m mol C-atom l−1 (or 87.5 mM), was identified as the optimal carbon source for H2 production by Chlorella sp. ChiW1. Light intensity also played a crucial role in H2 production. Future research directions for improving H2 production by Chlorella sp. ChiW1 should focus on scaling up the process, which may be challenged by the reliance on nitrogen deprivation. Additionally, the supplementation of acetic acid and exposure to high light intensity should be optimized to enhance economic feasibility.
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
This research is a result of the project entitled “Hydrogen Production by Green Algae by Co-Cultivation with Bacteria Grant N0. RE-KRIS/FF67/034” by King Mongkut’s Institute of Technology Ladkrabang (KMITL), which has been received funding support from the NSRF. TS is grateful for the scholarship received from School of Science, King Mongkut’s Institute of Technology Ladkrabang (RA/TA-2564-M-010).
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Fischer M. Safety aspects of hydrogen combustion in hydrogen energy systems. Int J Hydrogen Energy 1986;11(09):593–601; CrossRef
2. Pongpadung P, Liu J, Yokthongwattana K, Techapinyawat S, Juntawong N. Screening for hydrogen-producing strains of green microalgae in phosphorus or sulphur deprived medium under nitrogen limitation. Sci Asia 2015;41(2):97–107; CrossRef
3. Maneeruttanarungroj C, Lindblad P, Incharoensakdi A. A newly isolated green alga, Tetraspora sp. CU2551, from Thailand with efficient hydrogen production. Int J Hydrogen Energy 2010;35(4):13193–9; CrossRef
4. Sirawattanamongkol T, Maswanna T, Maneeruttanarungroj C. A newly isolated green alga Chlorella sp. KLSc59: potential for biohydrogen production. J Appl Phycol 2020;32:2927–36; CrossRef
5. Tinpranee N, Incharoensakdi A, Phunpruch S. Hydrogen production by unicellular green alga Chlorella sp. LSD-W2 isolated from seawater in Thailand. KKU Res J 2016;22(1):256–66; CrossRef
6. Javed MA, Zafar AM, Hassan AA, Zaidi AA, Farooq M, Badawy AE, et al. The role of oxygen regulation and algal growth parameters in hydrogen production via biophotolysis. J Environ Chem Eng 2022;10(1):107003–18; CrossRef
7. He M, Li L, Zhang L, Liu J. The enhancement of hydrogen photoproduction in Chlorella protothecoides exposed to nitrogen limitation and sulfur deprivation. Int J Hydrogen Energy 2012;37(22):16903–15; CrossRef
8. Shastik E, Li L, Zhang L, Qin R, Yu W, Liu J. Some molecular aspects of hydrogen production by marine Chlorella pyrenoidosa under nitrogen deprivation condition in natural seawater. Int J Hydrogen Energy 2020;45(27):13876–83; CrossRef
9. Warichanan K, Phunpruch S. Effect of cell density and nutrient deprivation on hydrogen production by unicellular green alga Scenedesmus sp. KMITL-OVG1. Asia Pac J Sci Technol 2019;24(2):APST–24; CrossRef
10. Rashid N, Lee K, Han JI, Gross M. Hydrogen production by immobilized Chlorella vulgaris: optimizing pH, carbon source and light. Bioprocess Biosyst Eng 2013;36:867–72; CrossRef
11. Harris EH. The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. Academic Press, San Diego, CA, 1989.
12. Becker EW. Microalgae: biotechnology and microbiology. Cambridge University Press, Cambridge, UK, 1994.
13. Baebprasert W, Lindblad P, Incharoensakdi A. Response of H2 production and Hox-hydrogenase activity to external factors in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Int J Hydrogen Energy 2010;351(3):6611–6; CrossRef
14. Taikhao S, Junyapoon S, Incharoensakdi S, Phunpruch S. Factors affecting biohydrogen production by unicellular halotolerant cyanobacterium Aphanothece halophytica. J Appl Phycol 2013;25:575–85; CrossRef
15. Phunpruch S, Puangplub A, Incharoensakdi A. Biohydrogen production by microalgae isolated from the rice paddle field in Thailand. KKU Res J 2016;21(2):236–47; CrossRef
16. Carvalho AP, Silva SO, Baptista JM, Malcata FX. Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 2011;89:1275–88; CrossRef
17. Happe T, Naber JD. Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur J Biochem 1993;214:475–81; CrossRef
18. Kosourov S, Tsygankov A, Seibert M, Ghirardi ML. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnol Bioeng 2002;78(7):731–40; CrossRef
19. Kim JP, Kang CD, Sim SJ, Kim MS, Park TH, Lee D, et al. Cell age optimization for hydrogen production induced by sulfur deprivation using a green alga Chlamydomonas reinhardtii UTEX 90. J Microbiol Biotechnol 2005;15(1):131–5.
20. Das D, Dutta T, Nath K, Kotay SM, Das, AK, Veziroglu TN. Role of Fe-hydrogenase in biological hydrogen production. Curr Sci 2006;90(12):1627–37.
21. Philipps G, Happe T, Hemschemeier A. Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta 2012;235(4):729–45; CrossRef
22. Martin NC, Goodenough UW. Gametic differentiation: in Chlamydomonas reinhardtii. J Cell Biol 1975;67:587–605; CrossRef
23. Manoyan J, Samovich T, Kozel N, Demidchik V, Gabrielyan L. Growth characteristics, biohydrogen production and photochemical activity of photosystems in green microalgae Parachlorella kessleri exposed to nitrogen deprivation. Int J Hydrogen Energy 2022;47(38):16815–23; CrossRef
24. Krasna AI. Hydrogenase: properties and applications. Enzyme Microb Technol 1979;1(3):165–72; CrossRef
25. Nishiyama Y, Allakhverdiev SI, Murata N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiologia Plantarum 2011;142(1):35–46; CrossRef
26. Li L, Zhang L, Liu J. The enhancement of hydrogen photoproduction in marine Chlorella pyrenoidosa under nitrogen deprivation. Int J Hydrogen Energy 2015;40(43):14784–9; CrossRef
27. Gonzalez-Ballester D, Jurado-Oller JL, Fernandez E. Relevance of nutrient media composition for hydrogen production in Chlamydomonas. Photosynth Res 2015;125:395–406; CrossRef
28. Wykoff DD, Davies JP, Melis A, Grossman AR, The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 1998;117(1):129–39; CrossRef
29. Sereetrakul K, Phunpruch S. Factors affecting hydrogen production by unicellular green alga Chlamydomonas reinhardtii CC-125. Chiang Mai J Sci 2021;48(4):979–95.
30. Li L, Zhang L, Gong F, Liu J. Transcriptomic analysis of hydrogen photoproduction in Chlorella pyrenoidosa under nitrogen deprivation. Algal Res 2020;47:101827–38; CrossRef
31. Jurado–Oller JL, Dubini A, Galván A, Fernández E, González–Ballester D. Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol Biofuels 2015;8:149; CrossRef
32. Gibbs M, Gfeller RP, Chen C. Fermentative metabolism of Chlamydomonas reinhardii: III. Photoassimilation of acetate. Plant Physiol 1986;82(1):160–6; CrossRef
33. Kosourov SN, Seibert M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng 2009;102(1):50–8; CrossRef
34. Gabrielyan L, Hakobyan L, Trchounian A. Characterization of light-dependent hydrogen production by new green microalga Parachlorella kessleri in various conditions. J Photochem Photobiol B 2017;175:207–10; CrossRef
35. Nath K, Kumar A, Das D. Effect of some environmental parameters on fermentative hydrogen production by Enterobacter cloacae DM11. Can J Microbiol 2006;52(6):525–32; CrossRef
36. Rashid N, Lee K, Mahmood Q. Bio-hydrogen production by Chlorella vulgaris under diverse photoperiods. Bioresour Technol 2011;102(2):2101–4; CrossRef
37. Alalayah WM, Alhamed YA, Al-zahrani A, Edris G. Influence of culture parameters on biological hydrogen production using green algae Chlorella vulgari. Rev Chim (Bucharest) 2015;66(6):788–91.
38. Jiménez-Llanos J, Rez-Carmona MR, Rendón-Castrillón L, Ocampo-López C. Sustainable biohydrogen production by Chlorella Sp. Microalgae. Int J Hydrogen Energy 2020;45(15):8310–28; CrossRef
39. Mandotra SK, Sharma C, Srivastava N, Ahluwalia AS, Ramteke PW. Current prospects and future developments in algal bio-hydrogen production. Biomass Conv Bioref 2023;13:8575–92; CrossRef
40. Saifuddin N, Priatharsini P. Developments in bio-hydrogen production from algae. Res J Appl Sci Eng Technol 2016;12(9):968–82; CrossRef
41. Paramesh K, Reddy NL, Shankar MV, Chandrasekhar T. Enhancement of biological hydrogen production using green alga Chlorococcum minutum. Int J Hydrogen Energy 2018;43(8):3957–66; CrossRef
42. He M, Li L, Liu J, Zhang J. Improvement of H2 photoproduction in Chlorella pyrenoidosa in artificial and natural seawater by addition of acetic acid and control of nutrients. Algal Res 2015;10:104–9; CrossRef
43. Chader S, Hacene H, Agathos SN. Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int J Hydrogen Energy 2009;34(11):4941–6; CrossRef
44. He M, Li L, Liu J. Isolation of wild microalgae from natural water bodies for high hydrogen producing strains. Int J Hydrogen Energy 2012;37(5):4046–56; CrossRef
45. Zhang L, He M, Liu J, Li L. Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta 2015;241(4):1005–14; CrossRef
46. Subramani K, Wutthithien P, Saha R, Lindblad P, Incharoensakdi A. Characterization and potentiality of plant-derived silver nanoparticles for enhancement of biomass and hydrogen production in Chlorella sp. under nitrogen deprived condition. Chemosphere 2024;361:142514–23; CrossRef
47. Song W, Rashid N, Choi W, Lee K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Bioresour Technol 2011;102(18):8676–81; CrossRef
48. Boboescu IZ, Gherman VD, Lakatos G, Pap B, Bíró T, Maróti G. Surpassing the current limitations of biohydrogen production systems: the case for a novel hybrid approach. Bioresour Technol 2016;204:192–20; CrossRef
49. Kim JP, Kang CD, Park TH, Kim MS, Sim SJ. Enhanced hydrogen production by controlling light intensity in sulfur-deprived Chlamydomonas reinhardtii culture. Int J Hydrogen Energy 2006;31(11):1585–90; CrossRef
50. Skjånes K, Rebours C, Lindblad P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit Rev Biotechnol 2013;33(2):172–215; CrossRef
51. Ghirardi ML, King PW, Posewitz MC, Maness PC, Fedorov A, Kim K, et al. Approaches to developing biological H2-photoproducing organisms and processes. Biochem Soc Trans 2005;33(1):70–2; CrossRef
52. Puangplub A, Phunpruch S. Effect of light intensity and light pattern on hydrogen production by unicellular green alga Chlorella sp. LSD-W2. Asia Pac J Sci Technol 2019;24(2):APST–24; CrossRef