1. INTRODUCTION
In Vietnam, consumers can easily purchase chicken meat (slaughtered and unprocessed) at retail markets or supermarkets. However, ensuring food hygiene and safety for chicken meat before, during, and after the slaughter process is a significant concern due to bacterial contamination from various sources. Most retail markets receive chicken meat from small, manual slaughterhouses with unsanitary conditions, posing a high risk of contamination from water sources and chicken feces at both the slaughterhouse and the point of sale [1]. Among the bacteria that cause foodborne illnesses, Salmonella is particularly dangerous, causing severe gastrointestinal diseases and is often associated with chickens produced and processed without adequate food hygiene and safety measures [2].
Salmonella spp. are rod-shaped, nonsporulating, gram-negative bacteria [3]. They are the primary source of food poisoning globally and can cause significant illness in both humans and animals [4]. According to the Centers for Disease Control and Prevention, Salmonella infections affect approximately 1.35 million people annually, resulting in about 420 deaths [5]. In Vietnam, Salmonella has been responsible for a series of food poisoning outbreaks in recent years [6]. Currently, over 2,500 Salmonella serovars have been identified in humans and animals, with approximately 10% isolated from poultry [7].
Among animal-derived food products, chicken meat is considered healthy due to its relatively high protein content and low-fat content [8]. However, raw chickens are a crucial source of Salmonella spp. infection in humans [9]. Poultry meat is linked to about 30% of foodborne salmonellosis infections globally [10]. Nontyphoid Salmonella spp. typically cause mild infections leading to diarrhea, which usually resolves on its own and rarely progresses to septicemia and meningitis [11]. In contrast, typhoid Salmonella spp. can cause severe symptoms, including fever, headache, and fatigue [12].
The pervasive use of antibiotics in veterinary, human, and agricultural medicine has led to the emergence and increase of antibiotic-resistant microorganisms, adversely impacting sustainable food production, agriculture, and the environment. Previous studies have documented antibiotic resistance in various bacterial species that cause diseases in humans [13], poultry [14], and other important animals in aquaculture, including shrimp and fish [15, 16]. Genes for antibiotic resistance can be transferred from bacteria containing these genes to other bacteria in the surrounding environment [17]. Treating bacterial infections has become increasingly challenging due to rising antibiotic resistance [18]. The overuse of antibiotics in veterinary and clinical medicine, as well as animal husbandry, has been linked to the development of antibiotic-resistant Salmonella isolates [19]. Various antibiotic-resistant Salmonella isolates have been collected from animal-derived products, especially poultry meat, and the surrounding environment [20,21]. Numerous studies globally and in Vietnam have recorded multidrug-resistant Salmonella spp. in chicken meat [22,23]. However, data on the antibiotic susceptibility of Salmonella in chicken meat from retail markets are limited.
 | Figure 1. Chicken meat samples were obtained from retail markets in Vinh Long Province (red circles). [Click here to view] |
Ensuring food safety for chicken meat in Vietnam is a significant concern due to the high risk of bacterial contamination, particularly from Salmonella spp. This study aimed to evaluate the antibiotic resistance and contamination rates of Salmonella originating from raw chicken meat at different retail markets in Vinh Long Province. The primary objectives were to determine the prevalence of Salmonella, characterize their antibiotic susceptibility, and identify potential sources of contamination. Using advanced molecular techniques like PCR and 16S rRNA gene sequencing, this research provides a detailed and precise identification of Salmonella strains, offering novel insights into the bacterial contamination landscape in this region. These findings reveal that Salmonella contamination in chicken meat is prevalent and that many strains exhibit significant antibiotic resistance. These results highlight the urgent need for stricter sanitary practices in both slaughterhouses and retail markets to mitigate the spread of antibiotic-resistant bacteria. This study not only adds valuable data to the existing body of knowledge but also has significant implications for public health and food safety. These findings support the development of effective strategies and policies to control bacterial contamination and manage antibiotic resistance, ultimately safeguarding consumer health.
2. MATERIALS AND METHODS
2.1. Study Design
The investigation was conducted between January and December 2023. In total, 83 samples of chicken meat were collected from different retail marketplaces in Vinh Long province. In a controlled environment, samples were collected in sterile plastic bags.
2.2. Collection and Testing of Chicken Meat Samples
Samples of raw chicken meat were collected from small retail markets in Vinh Long Province (Fig. 1). From each market, three samples of fresh chicken meat, including muscle, skin, and liver, were randomly selected. These samples were stored in foam boxes with ice, and then transported immediately by private vehicle to the laboratory for analysis of bacterial density and isolation of Salmonella.
2.3. Determination of Salmonella Densities
Salmonella bacterial densities in the chicken meat samples were determined using the Vietnamese standard (TCVN) 10780-1:2017 as a guide. Briefly, chicken muscle, skin, and liver samples were pooled and homogenized with sterilized distilled water. The homogenized sample was agitated at 120 rpm for 30 minutes, followed by serial dilution to 10–6. Aliquots (0.1 ml) of each dilution were spread onto Salmonella Shigella agar (SS-agar, HiMedia, India). After 24 hours of incubation at 37°C, the bacterial colonies were counted. The bacterial population was determined using the following formula (log CFU/g): CFU/g = dilution level plated × number of colonies counted/volume plated [24].
2.4. Salmonella Isolation
Salmonella was isolated from the diluted samples. Specifically, 100 μl of each sample was spread on SS agar and incubated at 37°C. Selective colonies were picked and repeatedly cultured in appropriate media to obtain pure colonies. The colony morphology, motility, Gram stain, and catalase activity of the isolated bacterial strains were examined [25].
2.5. Antibiotic Sensitivity of Salmonella Isolates
The antibiotic susceptibility of the isolates was evaluated according to Bauer et al. [26]. Twelve antibiotics were tested: ceftazidime (30 µg), levofloxacin (5 µg), ampicillin (AMP/10 µg), doxycycline (DOX/30 µg), kanamycin (KAN/30 µg), gentamicin (GEN/10 µg), tetracycline (TET/30 µg), sulfamethoxazole-trimethoprim (SXT/23.75/1.25 µg), ciprofloxacin (CIP/5 µg), amoxicillin (AMO/10 µg), and AMP-sulbactam (AMS/10 µg). Colonies were dissolved in sterile 0.85% NaCl to match the turbidity of a McFarland standard, creating a suspension with a density of 108 CFU/ml. A 100 μl aliquot of the bacterial suspension was spread onto TSA media. The plates containing the antibiotic discs were incubated at 37°C for 24 hours. The inhibition zones were then measured and evaluated according to the Clinical and Laboratory Standards Institute [27] guidelines for susceptibility (S), intermediate (I), and resistance (R). Resistance to three or more antibiotic classes was classified as multidrug resistance [28]. Escherichia coli ATCC® 25922 was used as a quality control.
2.6. Multiple Antibiotic Resistance (MAR) Indices
The MAR index for each bacterial strain was calculated using the formula MAR = R/E, where R represents the number of antibiotics to which the strain is resistant, and E represents the total number of antibiotics tested. A MAR value greater than 0.2 indicates frequent antibiotic use, while a value less than 0.2 indicates infrequent or nonuse of antibiotics [29].
2.7. Identification of Salmonella
Bacterial DNA extraction followed the method of Sambrook et al. [30], with slight modifications. Salmonella isolates were enriched in Luria Bertani media (LB media, Himedia, India) and shaken at 110 rpm at room temperature. Two milliliters of bacterial culture were centrifuged at 13,000 rpm for 5 minutes. The bacterial biomass was then resuspended in 1 ml of lysis buffer (1 M Tris-HCl, 0.5 mM EDTA, 5 M NaCl, 0.1% SDS, pH 8.0) and incubated for 30 minutes at room temperature. The supernatant was precipitated with 95% ethanol and resuspended in 70% ethanol. DNA samples meeting the purity and concentration requirements were stored in 100 µl of 0.1X TE solution (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) at −80°C for PCR.
2.8. PCR Reaction
To amplify the 16S rRNA gene fragment of the bacterial isolates, the primer pairs 27F and 1492R were used [31]. PCRs were performed in a 12.5 μl volume containing distilled water, 1X PCR Buffer, 1.5 mM MgCl2, 150 µM dNTPs, 20 pmol of each primer, 2.0 U Taq DNA polymerase, and the DNA sample. The PCR conditions were as follows: initial denaturation at 94°C for 5 minutes, followed by 30 cycles of denaturation at 94°C for 1 minute, primer annealing at 63°C for 1 minute, extension at 72°C for 2 minutes, and a final extension at 72°C for 10 minutes. The PCR products were electrophoresed on a 1.5% agarose gel, photographed using the Analytik Jena gel imaging system, and sequenced at Macrogen Company (Korea) for bacterial species identification.
2.9. Data Analysis
Descriptive statistical methods were employed to analyze the data, determining the average values and percentages of resistant, susceptible, intermediate, and sensitive bacterial strains, along with the overall bacterial population. The similarity of the DNA sequences of the isolated bacteria to known Salmonella spp. in the NCBI database was assessed using the BLASTn program. For the alignment of bacterial sequences with reference sequences, the CLUSTAL W program was utilized [32].
To construct a phylogenetic tree, the neighbor-joining method, a distance-based algorithm widely used for reconstructing phylogenies, was applied. This method was implemented with bootstrap values calculated from 1,000 replicates to ensure the robustness and reliability of the tree. The phylogenetic analysis was performed using MEGA6 software [33], which provided a detailed evolutionary relationship among the isolated Salmonella isolates and reference strains from the database. This comprehensive analysis not only confirmed the identity of the isolated strains but also offered insights into their genetic relatedness and evolutionary lineage.
 | Table 1. Bacterial population of Salmonella spp. on chicken at traditional markets. [Click here to view] |
3. RESULTS
3.1. Bacterial Population of Salmonella in Chickens
The study revealed that all 83 chicken meat samples collected from various retail markets were contaminated with high densities of Salmonella spp. (Table 1). The bacterial densities ranged from 2.64 ± 0.08 to 2.96 ± 0.03 log CFU/g, with statistically significant differences observed between the surveyed markets.
Notably, the highest bacterial density was recorded in Vinh Long City (2.96 ± 0.03 log CFU/g), which could be attributed to higher market activity and possibly less stringent sanitary conditions. In contrast, the lowest density was found in Binh Minh (2.64 ± 0.08 log CFU/g), suggesting relatively better hygiene practices or lower market turnover. The bacterial densities in other districts, such as Long Ho (2.68 ± 0.12 log CFU/g) and Binh Tan (2.73 ± 0.07 log CFU/g), also showed considerable contamination but were significantly different from the highest and lowest values (p < 0.05).
These results indicate that while Salmonella contamination is a widespread issue across all markets, certain locations present relatively high bacterial loads, highlighting the need for targeted interventions to improve food safety standards. The significant variation in bacterial densities across different markets underscores the impact of local hygiene practices and the need for consistent and effective sanitary measures across all retail locations to mitigate health risks associated with Salmonella in chicken meat.
3.2. Isolation of Salmonella Isolates
Twenty-one Salmonella isolates were obtained from pooled samples of chicken muscle, liver, and skin and cultured on SS-agar media (Fig. 2A). The bacterial colonies on the SS-agar medium were colorless, with a black center, raised, intact, and small (Fig. 2B). The colony size ranged from 3–5 mm after 24–48 hours of incubation at 37°C. All the isolated strains were motile, gram-negative, and short rod-shaped (Fig. 2C), and all the strains were positive for catalase (Fig. 2C).
3.3. Antibiotic Susceptibility of Salmonella Isolates
The results demonstrated that Salmonella spp. strains exhibited varying degrees of sensitivity and resistance to different antibiotics (Fig. 3). The strains were most sensitive to AMS (90%), SXT (67%), and DOX (52%). However, these strains were completely resistant to GEN. Significant resistance was observed against AMP (86%), TET (71%), AMO (62%), and CIP (57%), with the lowest resistance observed for AMS (8%). These findings indicate significant variation in the susceptibility of Salmonella strains to different antibiotics, emphasizing the challenge posed by antibiotic resistance in bacterial infections. The high sensitivity to AMS suggests its potential effectiveness in treating infections caused by these strains. In contrast, the complete resistance to GEN and high resistance to antibiotics such as TET, AMP, CIP, and AMO highlight the pressing necessity for ongoing observation and prudent use of antibiotics to manage and control the spread of resistant Salmonella strains. The varying levels of resistance and susceptibility observed underscore the importance of implementing stringent antibiotic stewardship programs and enhancing hygiene practices in poultry production and processing environments. This approach can help mitigate the risk of antibiotic-resistant bacterial infections and ensure food safety for consumers.
 | Figure 2. Salmonella strains isolated on SS-agar medium with A. chicken meat samples from the retail market; B. Salmonella spp. was grown on SS agar media; C. gram stain results; D. catalase activity of the isolated strain. [Click here to view] |
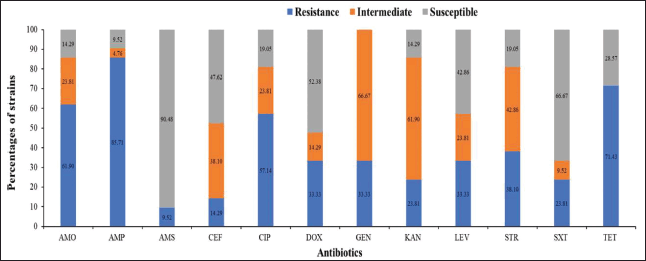 | Figure 3. Proportion of Salmonella isolates susceptible to antibiotics. Note: AMO, AMP, AMS, CEF, CIP, DOX, GEN, KAN, LEV, STR, SXT, and TET. [Click here to view] |
3.4. Multidrug Resistance in Bacteria
Research has indicated that 86% of Salmonella spp. strains exhibit multidrug resistance (Fig. 4). The most common resistance pattern involved three antibiotics, with the highest rate of 19%. This was followed by resistance to four, five, eight, and nine antibiotics, each at 14%, while the lowest resistance was observed with six antibiotics at 10%. Additionally, out of the 21 bacterial strains identified in the present study, 20 presented multidrug-resistant phenotypes (Table 2). Notably, two strains, STH_10 and SVL_21, exhibited the same resistance phenotype to AMP and TET.
The high prevalence of MAR among Salmonella isolates highlights a critical public health issue. The diversity in resistance patterns suggests that these bacteria have been exposed to multiple antibiotics, resulting in the selection of resistant strains. The most common resistance to these three antibiotics underscores the need for stringent antibiotic usage policies and the importance of regular surveillance to monitor resistance trends. The identification of common resistance phenotypes, such as AMP-TET, provides valuable information for developing targeted treatment strategies and informs public health interventions to mitigate the spread of resistant isolates.
 | Figure 4. Multidrug resistance rate of isolated Salmonella strains. [Click here to view] |
3.5. MAR Indices of Salmonella
The results demonstrated that the multidrug resistance index of Salmonella isolates ranged from 0.17 to 0.67 (Table 2). Notably, the majority of bacterial strains (eighteen out of twenty-one) presented a MAR index >0.2, indicating frequent exposure to antibiotics. The highest multidrug resistance indices were observed in strains SP8_8, SP9_9, and SBM_20, each with a MAR index of 0.67.
The high MAR indices observed among the Salmonella isolates suggest widespread and frequent use of antibiotics, leading to significant multidrug resistance. Strains exhibiting the highest MAR indices, such as SP8_8, SP9_9, and SBM_20, pose a substantial public health risk due to their potential resistance to multiple commonly used antibiotics. These results underline the importance of enforcing strict antibiotic stewardship and improving antibiotic usage monitoring in poultry production and retail settings. The presence of multidrug-resistant phenotypes underscores the need for developing and implementing effective antibiotic management policies to mitigate the spread of resistant strains and ensure food safety.
3.6. Salmonella Identification via PCR
The findings demonstrated that the 16S rRNA gene segment was effectively amplified from all the isolated bacterial strains via PCR, resulting in a product size of approximately 1,500 bp (Fig. 5).
The sequencing results indicated that three strains, SP5_7, SP8_8, and SP9_9, shared 95.31%, 97.89%, and 93.60% similarity, respectively, with S. enterica strain KS7 (KT270313.1), S. enterica strain GS-31 16S (OP382468.1), and S. enterica isolate SV68221 (LR792423.1) from GenBank. Phylogenetic tree analysis revealed that these three bacterial isolates were genetically closely related and grouped with known Salmonella strains in the GenBank database (Fig. 6). These results confirm the identity of the isolated strains as Salmonella spp. and highlight their genetic similarity to previously characterized strains. The effective amplification and sequencing of the 16S rRNA gene segment provide solid evidence for the presence and identification of Salmonella in the samples, supporting the conclusion that these isolates belong to the same phylogenetic group as other Salmonella strains recorded in GenBank. This genetic information is crucial for understanding the epidemiology and potential pathogenicity of these isolates, contributing to broader efforts in monitoring and controlling Salmonella contamination in food products.
 | Table 2. Phenotypes and multi-resistance indexes of Salmonella isolates. [Click here to view] |
4. DISCUSSION
Salmonella spp. are critical indicators of contamination in fresh meat, and their presence is prohibited by Vietnamese standards (TCVN 7046:2009). However, this study indicates an increased frequency of Salmonella infection in chicken meat sold at traditional markets, with significant differences observed among the surveyed markets. Specifically, the highest contamination density was recorded in Vinh Long city, while the lowest was found in Binh Minh (p < 0.05). These results surpass those of earlier research; for example, Huong et al. [34] reported a 48.9% contamination rate in chicken meat from retail markets in Hanoi, and Manh et al. [35] reported a 41.7% contamination rate in Ben Tre city. Additionally, a study in Egypt by El-Aziz [36] detected S. typhimurium in 44% of fresh chicken meat, 40% of liver, and 48% of heart samples. The high contamination rates observed in the present study may be attributed to poor hygienic conditions throughout the chicken production chain like storage conditions or the hygiene practices at the markets, including inadequate sanitation of water sources [37]. Furthermore, the high rate of Salmonella contamination in this study might be the result of possible limitations, such as the representativeness of the poultry meat samples or the sampling size; the samples were not taken from a variety of market types, such as supermarkets, small retail stores, or wet markets; and there are variations in the handling procedures or hygiene standards among market vendors during transportation.
Regarding antibiotic resistance, the results revealed that Salmonella strains exhibited high resistance rates to AMP (86%), TET (71%), AMO (62%), and CIP (57%). These findings align with those of Akinola et al. [38], who reported significant resistance in Salmonella strains from broilers and egg-laying chickens in South Africa to AMP (56%), TET (69%), and CIP (30%). Similar patterns of resistance have been observed in other regions. For example, Kanaan [39] reported that Salmonella isolates from frozen and raw chickens in Iraq exhibited high resistance to nalidixic acid (73.7%), TET (63.2%), and SXT (63.2%). In Morocco, Salmonella isolates were resistant to TET (57.50%), CIP (57.50%), and KAN (27.50%), but no resistance to GEN was detected [40]. In Vietnam, Nhat et al. [41] reported resistance rates of 68.8% for AMP, 67.7% for TET, and 57.3% for trimethoprim in Salmonella isolates from pig and poultry meat. Additionally, Huong et al. [42] demonstrated high resistance rates to AMP (59.5%), TET (83.8%), and streptomycin (89.2%) in Salmonella strains from poultry farms in North Vietnam. Antibiotic resistance is likely a result of the indiscriminate use of antimicrobials as growth promoters and medicinal agents on farms [43]. Resistance can originate at various stages, from breeding sites [44] to animal feed [45], and within farming environments for broilers and egg-laying chickens [46]. This ultimately leads to the circulation of drug-resistant bacteria in products, presenting a serious risk to consumer health [47]. Many previous studies established a relationship between agricultural antibiotic use and resistance patterns in foodborne pathogens [48,49].
.gif) | Figure 5. Amplification of the 16S rRNA gene segment of representative Salmonella isolates via PCR. Note: L: 100 bp DNA standard ladder; Lanes 1-11: bacterial strains SP5_7, SP3_4, SP4_6, STH_12, SMT_19, SP3_3, SP4_5, STH_13, STO_16, SP8_8, and SP9_9, respectively; Lane 12: Negative control. [Click here to view] |
 | Figure 6. Phylogenetic tree showing isolates belonging to the same group as Salmonella spp. in Genbank. [Click here to view] |
The multidrug resistance phenomenon of Salmonella has been extensively documented in prior research [50]. In the current investigation, 86% of Salmonella isolates exhibited multidrug resistance, which is greater than the 29.4% reported by Fanissa et al. [51] in chicken meat from traditional markets in Indonesia. In Malaysia, Sukri et al. [52] reported that all Salmonella isolates from raw chicken and contact surfaces in wet markets were multidrug resistant to erythromycin, penicillin, TET, and chloramphenicol. Similarly, Zhang et al. [53] reported an 81.1% multidrug resistance rate in Salmonella spp. from chicken meat in China. Furthermore, Ali et al. [54] revealed that 86% of Salmonella strains from retail chickens in Ethiopia were resistant to 2–7 antibiotics. Guran et al. [55] illustrated that 86.3% of Salmonella strains from organic chickens in Turkey were multidrug resistant. In summary, the high rate of multidrug resistance and Salmonella infection found in this study highlights the critical need for improved hygienic procedures in the retail and chicken production industries. These findings call for stringent antibiotic stewardship and continuous monitoring to mitigate the spread of resistant strains and ensure consumer safety; for instance, suggestions for better farm management techniques, surveillance, or legislative adjustments may be helpful.
5. CONCLUSION
This study revealed that all tested chicken meat samples from traditional markets in Vinh Long were heavily contaminated with Salmonella spp., with significant variations in contamination levels across different markets. Three bacterial strains (SP5_7, SP8_8, and SP9_9) were confirmed as Salmonella spp. through PCR techniques and 16S rRNA gene sequencing. The antibiogram results revealed extensive resistance to multiple antibiotics, with a multidrug resistance rate of 86%, and a majority of the strains exhibited frequent antibiotic exposure, as indicated by a MAR index >0.2. The novel application of advanced molecular techniques in this study provides important new information about the state of bacterial contamination and emphasizes the critical need for focused interventions and improved sanitary practices in slaughterhouses and retail markets. Future research should concentrate on stringent antibiotic stewardship programs and continuous surveillance to manage and control the spread of antibiotic-resistant Salmonella strains, ensuring the sustainability of poultry farming practices and safeguarding public health.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Klaharn K, Pichpol D, Meeyam T, Harintharanon T, Lohaanukul P, Punyapornwithaya V. Bacterial contamination of chicken meat in slaughterhouses and the associated risk factors: a nationwide study in Thailand. PLoS One 2022;17(6):e0269416.
2. Ehuwa O, Jaiswal AK, Jaiswal S. Salmonella, food safety, and food gandling practices. Foods 2021;10(5):907.
3. Andino A, Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci World J 2015;2015:520179.
4. Rahman HS, Othman HH. Salmonella infection: the common cause of human food poisoning. J Biosci Bioeng 2017;1(1):5–10.
5. Lamichhane B, Mawad AMM, Saleh M, Kelley WG, Harrington PJII, Lovestad CW, et al. Salmonellosis: an overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024;13:76.
6. Phuoc TP. Salmonella, E. coli bacteria responsible for mass banh mi poisoning in southern Vietnam. 2024. VN Express International, Hanoi, Vietnam. Available via https://e.vnexpress.net/news/news/salmonella-e-coli-bacteria-responsible-for-mass-banh-mi-poisoning-in-southern-vietnam-4743248 (Accessed 10 May 2024).
7. Nair VTD, Venkitanarayanan K, Kollanoor JA. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018;7(10):167.
8. Jilo SA, Hasan LA. The importance of poultry meat in medicine: a review. JWPR 2022;12(4):258–62.
9. Shah DH, Paul NC, Sischo WC, Crespo R, Guard J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult Sci 2017;96(3):687–702.
10. Hoffmann S, Devleesschauwer B, Aspinall W, Cooke WR, Corrigan T, Havelaar A, et al. Attribution of global foodborne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS One 2017;12(9):e0183641.
11. Popa GL, Popa MI. Salmonella spp. Infection–a continuous threat worldwide. Germs 2021;11(1):88–96.
12. Wattiau P, Boland C, Bertrand S. Methodologies for Salmonella enterica subsp. enterica subtyping: gold standards and alternatives. Appl Environ Microbiol 2011;77(22):7877–85.
13. Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS. Antibiotic resistance and persistence—implications for human health and treatment perspectives. EMBO Rep 2020;21(12):e51034.
14. Tan MF, Li HQ, Yang Q, Zhang FF, Tan J, Zeng YB, et al. Prevalence and antimicrobial resistance profile of bacterial pathogens isolated from poultry in Jiangxi Province, China from 2020 to 2022. Poult Sci 2023;102(8):102830.
15. Marijani E. Prevalence and antimicrobial resistance of bacteria isolated from marine and freshwater fish in Tanzania. Int J Microbiol 2022;2022:4652326.
16. Okeke ES, Chukwudozie KI, Nyaruaba R, Ita RE, Oladipo A, Ejeromedoghene O, et al. Antibiotic resistance in aquaculture and aquatic organisms: a review of current nanotechnology applications for sustainable management. ESPR 2022;29(46):69241–74.
17. Ajayi AO, Odeyemi AT, Akinjogunla OJ, Adeyeye AB, Ayo-Ajayi I. Review of antibiotic-resistant bacteria and antibiotic resistance genes within the one health framework. Infect Ecol Epidemiol 2024;13-14(1):2312953.
18. Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare 2023;11:1946.
19. Karatzas KAG, Randall LP, Webber M, Piddock LJV, Humphrey TJ, Woodward MJ. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella enterica serovar typhimurium selected following exposure to disinfectants. Appl Environ Microbiol 2008;74(5):1508–16.
20. Nidaullah H, Abirami N, Shamila-Syuhada AK, Chuah L, Nurul H, Tan TP, et al. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. Vet World 2017;10(3):286–92.
21. Simoes M, Pereira MO, Vieira MJ. Effect of mechanical stress on biofilms challenged by different chemicals. Water Res 2005;39(20):5142–52.
22. Vu THA, Tu NHK, Hai CV, Ha HY. Antimicrobial susceptibility of Salmonella spp. isolated from raw meats at traditional markets in Ho Chi Minh City. Vietnam J Sci and Technol 2021;63(8):55–9.
23. Molina A, Thye T, Muñoz-Vargas L, Zamora-Sanabria R, Chercos DH, Hernández-Rojas R, et al. Molecular characterization of antibiotic resistant Salmonella enterica across the poultry production chain in Costa Rica: a cross-sectional study. Int J Microbiol 2024;16:110663.
24. Adeyanju GT, Ishola O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria. Springerplus 2014;12(3):139.
25. Cappuccino J, Welsh C. Microbiology: a laboratory manual. 11th edition, Pearson Education, London, UK, 2017.
26. Bauer AL, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45(4):493–6.
27. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing for bacteria isolated from aquatic animals. In: CLSI supplement VET04. 3rd edition, Clinical and Laboratory Standards Institute, Wayne, PA, 2020.
28. Davis GS, Waits K, Nordstrom L, Grande H, Weaver B, Papp K, et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol 2018;18:174.
29. Sandhu R, Dahiya S, Sayal P. Evaluation of multiple antibiotic resistance (MAR) index and doxycycline susceptibility of Acinetobacter species among inpatients. Indian J Microbiol 2016;3(3):299.
30. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual.Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989.
31. Heuer H, Krsek M, Baker P, Smalla K, Wellington EM. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel electrophoretic separation in denaturing gradients. Appl Environ Microbiol 1997;63(8):3233–41.
32. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997;25(24):4876–82.
33. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30(12):2725–9.
34. Huong LH, Hanh TT, Reinhard F, Padungtod P. Typing results of Salmonella strains isolated from chicken meat in Hanoi. J Vet Sci Technol 2006;XIII(1):50–3.
35. Manh LH, Dao TX, Dung NNX, Minh BTL. Survey of bacterial infected levels in poultry meat in slaughterhouse and retail markets at Ben Tre city. CTU J Sci 2016;(2):56–60.
36. El-Aziz DMA. Detection of Salmonella typhimurium in retail chicken meat and chicken giblets. Asian Pac J Trop Biomed 2013;3(9):678–81.
37. Mpundu P, Mbewe AR, Muma JB, Zgambo J, Munyeme M. Evaluation of bacterial contamination in dressed chickens in lusaka abattoirs. Front Public Health 2019;7:19.
38. Akinola SA, Mwanza M, Ateba CN. Occurrence, genetic diversities and antibiotic resistance profiles of Salmonella serovars isolated from chickens. Infect Drug Resist 2019;12:3327–42.
39. Kanaan MHG. Prevalence and antimicrobial resistance of Salmonella enterica serovars Enteritidis and typhimurium isolated from retail chicken meat in Wasit markets, Iraq. Vet world 2023;16(3):455–63.
40. Nacer S, El Ftouhy FZ, Derqaoui S, Khayli M, Nassik S, Lkhider M. Prevalence and antibiotic resistance of Salmonella spp. and Staphylococcus aureus isolated from broiler chicken meat in modern and traditional slaughterhouses of Morocco. World J Vet Sci 2022;12(4):430–9.
41. Nhat TT, Duong TTQ, Giang TTH, Hue VK, Son DTT. Prevalence and antibiotic-resistance of Salmonella isolated from pork, chicken meat in Ha Noi, Bac Ninh and Nghe An. NASATI 2019;XXVI(5):30–7.
42. Huong CTT, Ngoc PT, Thai TH. Prevalence and antibiotic resistance of Salmonella spp. and Escherichia coli isolated from poultry farms in the North Vietnam. J Dairy Vet Anim Res 2023;12(1):70–5.
43. Wibisono FM, Wibisono FJ, Effendi MH. A review of salmonellosis on poultry farms: public health importance. Sys Rev Pharm 2020;11(9):481–6.
44. Osman KM, Kappell AD, Elhadidy M, Elmougy F, El-Ghany WAA, Orabi A, et al. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: a risk topublic health and food safety. Sci Rep 2018;8:5859.
45. Ngai DG, Nyamache AK, Ombori O. Prevalence and antimicrobial resistance profiles of Salmonella species and Escherichia coli isolates from poultry feeds in Ruiru Sub-County, Kenya. BMC Res Notes 2021;14(1):41.
46. Assoumy MA, Bedekelabou AP, Teko-Agbo A, Ossebi W, Akoda K, Nimbona F, et al. Antibiotic resistance of Escherichia coli and Salmonella spp. strains isolated from healthy poultry farms in the districts of Abidjan and Agnibilékrou (Côte d’Ivoire). Vet World 2021;14(4):1020–7.
47. Phiri N, Mainda G, Mukuma M, Sinyangwe NN, Banda LJ, Kwenda G, et al. Antibiotic-resistant Salmonella species and Escherichia coli in broiler chickens from farms, abattoirs and open markets in selected districts of Zambia. J Epidemiol Res 2020;6(1):13–21.
48. Rafiq K, Islam MR, Siddiky NA, Samad MA, Chowdhury S, Hossain KMM, et al. Antimicrobial resistance profile of common foodborne pathogens recovered from livestock and poultry in Bangladesh. Antibiotics (Basel) 2022;11(11):1551.
49. Kim J, Ahn J. Emergence and spread of antibiotic-resistant foodborne pathogens from farm to table. Food Sci Biotechnol 2022;31(12):1481–99.
50. Telsaç R, Tuncay RM. Prevalence and antibiotic resistance profiles of Salmonella spp. in chicken meat. Turk J Vet Anim Sci 2022;46(5):5.
51. Fanissa F, Effendi MH, Tyasningsih W, Ugbo EN. Multidrug-resistant Salmonella species from chicken meat sold at Surabaya Traditional Markets, Indonesia. Biodiversitas 2022;23:2823–9.
52. Sukri A, Zulfakar SS, Taib ISM, Omar NF, Zin NM. The high occurrence of multidrug-resistant Salmonella spp. isolated from raw chicken meat and contact surfaces at wet market in Malaysia. Sains Malays 2021;50(12):3765–72.
53. Zhang L, Fu Y, Xiong Z, Ma Y, Wei Y, Qu X, et al. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front Microbiol 2018;9:2104.
54. Ali AD, Tadesse B, Ebabu A. Prevalence and antibiotic resistance pattern of Salmonella isolated from Cecal contents of exotic chicken in Debre Zeit and Modjo, Ethiopia. Int J Microbiol 2020;2020:1910630.
55. Guran HS, Ciftci R, Gursoy NC, Ozekinci T. Prevalence of antibiotic-resistant Salmonella in retail organic chicken. Br Food J 2020;122(4):1238–51.