1. INTRODUCTION
The increased use of fossil fuels has led to significant environmental issues and the depletion of crude oil reserves, driving the search for alternative renewable energy sources. Biodiesel has emerged as a promising alternative, produced from biomass via biochemical or thermochemical processes, which generate glycerol as a by-product. The surge in biodiesel production has consequently resulted in a substantial increase in industrial glycerol waste. Converting this glycerol into valuable chemical compounds, such as 1,3-propanediol (1,3-PDO), is a compelling solution. 1,3-PDO has diverse applications, ranging from polymers and textiles to food, medicine [1], detergents, and cosmetics [2].
The biotechnological production of 1,3-PDO offers significant advantages over conventional chemical methods, including lower costs and environmentally friendly processes [3]. Various bacterial species, such as Clostridium butyricum [4], Enterobacter agglomerans [5], Citrobacter freundii, Klebsiella oxytoca [6], and Klebsiella pneumoniae [7], have been studied for this purpose. Among these, K. pneumoniae is the most extensively researched due to its high substrate tolerance, flexible carbon regulation [8], and excellent 1,3-PDO yield and productivity [9].
Klebsiella pneumoniae metabolizes glycerol via reductive and oxidative pathways. In the reductive pathway, glycerol is converted to 3-hydroxypropionaldehyde by glycerol dehydratase (GDHt) with the help of vitamin B12, and then reduced to 1,3-PDO by NADH and 1,3-propanediol oxidoreductase (dhaT) [9]. In the oxidative pathways, K. pneumoniae produces by-products such as acetic acid, lactic acid, formic acid, 2,3-BDO, and ethanol, which compete for NADH and hinder glycerol bioconversion in the reductive pathway, thus reducing 1,3-PDO levels [10].
Genetic modification, such as overexpressing key enzymes [11] or knocking out genes responsible for unwanted by-products, significantly enhances 1,3-PDO yields [12,13], demonstrating how targeted genetic interventions are likely to improve microbial performances. Excessive by-products during microbial fermentation are a critical challenge for limiting the overall yield of 1,3-PDO. The introduction of heterologous genes to reduce acetate and further reprogramming of carbon metabolism by knocking out lactate dehydrogenase, alcohol dehydrogenase, and succinate dehydrogenase is used to increase 1,3-PDO yield and purity [10]. This not only improves the process efficiency but also reduces the downstream purification costs. To enhance 1,3-PDO production, metabolic engineering has been employed to reduce by-product formation. In K. pneumoniae, eliminating lactic acid production has shown a more significant increase in 1,3-PDO levels compared to eliminating ethanol [14]. Additionally, 2,3-BDO is a major competing by-product [15], and the budB gene (part of the bud operon) is a critical factor in reducing its production [16]. However, there is limited information on the effects of single gene deletions of ldh and budB in a single isolate and their comparative metabolic profiles, particularly in 1,3-PDO production. Therefore, this study aims to identify target genes whose disruption minimally affects microbial growth while reducing by-product formation and enhancing 1,3-PDO production.
2. MATERIALS AND METHODS
2.1. Materials
Klebsiella pneumoniae strain TWO, isolated from tempeh in Indonesia and obtained from the Wilmar Benih Indonesia Collection, was used as the parent strain for glycerol fermentation experiments. Escherichia coli strains DH5α and S17-1λpir were used for plasmid propagation and conjugation, respectively, with bacterial strains, plasmids, and primers listed in Table 1. E. coli strains were cultivated in Luria-Bertani (LB) medium containing 5 g/l yeast extract, 10 g/l tryptone, and 1 g/l NaCl. For the selection of K. pneumoniae mutants, M9 minimal medium was used, which consisted of 6 g/l Na2HPO4, 3 g/l KH2PO4, 1 g/l (NH4)2HPO4, 0.5 g/l NaCl, 15 g/l agar, 2 g/l glucose, 0.25 g/l MgSO4·7H2O, 0.001 g/l thiamine-HCl, and 0.147 g/l CaCl2·2H2O. The seed culture and fermentation media contained 20 g/l glycerol (85% v/v), 0.2 g/l yeast extract, 3 g/l (NH4)2SO4, 0.2 g/l MgSO4·7H2O, 0.012 g/l CoCl2·6H2O, 14 g/l K2HPO4, 6 g/l KH2PO4, 0.2 g/l L-cysteine, and were adjusted to a final pH of 7.0 ± 0.2, with spectinomycin and streptomycin added to the culture medium at final concentrations of 50 µg/ml each for mutant selection. For optimization studies, malic acid, succinic acid, and cyanocobalamin (vitamin B12) were added to the fermentation medium at concentrations of 20 mM, 20 mM, and 0.0025 g/l, respectively. The trace element solution contained 0.7 g/l ZnCl2, 1 g/l MnCl2·4H2O, 0.6 g/l H3BO3, 2 g/l CoCl2·6H2O, 0.2 g/l CuCl2·H2O, and 0.35 g/l Na2MoO4·2H2O, with a separate iron solution prepared by dissolving 5 g/l FeSO4·7H2O in water, and a vitamin solution containing 10 g/l nicotinic acid (C6H5NO2), 5 g/l thiamine-HCl, and 0.1 g/l biotin. DNA fragments were amplified using Q5 High-Fidelity DNA polymerase (New England Biolabs, UK) to ensure accurate and efficient amplification.
 | Table 1. Bacteria strains, plasmids, and primers used. [Click here to view] |
2.2. Construction of Gene Knockouts
Total genomic DNA of K. pneumoniae TWO was extracted using the Wizard® Genomic DNA purification Kit (Promega, USA). For gene knockout, the 945 bp ldh and 1680 bp budB genes were amplified by PCR using the genomic DNA of K. pneumoniae TWO as template DNA, with primers A1-A2 and D1-D2, respectively (Table 1). These primers were designed based on sequence information from K. pneumoniae (GenBank accession numbers CP011976 and CP011313, respectively). The PCR products were cloned into the pUC19 vector, yielding the constructs pNK1 and pNK3.
For ldh gene knockout, the entire plasmid pNK1 was amplified using primers B1-B2 and subsequently ligated to introduce an SmaI restriction site. The spectinomycin/streptomycin-resistance gene (aadA) was amplified from the plasmid pUTminiTn5-Sp/Sm using primers C1-C2. The resulting 1.1-kb PCR product was inserted into the ldh gene in pNK1 at the SmaI site, generating pNK2. The structure of pNK2, extracted from E. coli DH5α, was confirmed through restriction analysis using SacI, NdeI, SacI-NdeI, KpnI, and HindIII. Sequencing with the M13 and universal pUC19 primers confirmed the absence of any introduced mutations.
For budB gene knockout, the 1.1-kb aadA gene was amplified using primers E1-E2 and inserted into the budB gene in pNK3, yielding pNK4. Plasmid pNK4 was transformed into E. coli DH5α, and the insertion was verified by restriction analysis using EcoRV and HindIII, as well as sequencing with M13 primers to confirm the integrity of the construct.
2.3. Modification of Conjugative Plasmid
Plasmid pUTminiTn5-Sp/Sm was modified by digesting with BglII and SmaI to remove the transposase-encoding gene and the transposable element, respectively. Alkaline Phosphatase Calf Intestinal (NEB, UK) was used to dephosphorylate the 5’ ends of the digestion products to improve ligation efficiency. Primers A3-A4 and D3-D4 were employed to amplify the inactive ldh and budB genes (designated as Δldh and ΔbudB) from pNK2 and pNK4, respectively. The PCR products were phosphorylated using T5 polynucleotide kinase (NEB, UK) and subsequently ligated into the linearized, modified pUTmini vector using T4 DNA ligase (NEB, UK), resulting in plasmids pFS5 and pFS6.
The structures of the conjugative plasmids carrying the inactive target genes were transformed into E. coli S17-1 λpir and verified by restriction analysis using BamHI, SalI, and a combination of BamHI and SalI. Sequencing with primers A3-A4 and D3-D4 confirmed that no mutations had been introduced into the constructs.
2.4. Biparental Mating
Biparental mating was conducted using E. coli S17-1 λpir as the donor and K. pneumoniae TWO as the recipient to transfer each conjugative plasmid carrying the inactive target gene. Homologous recombination occurred between the plasmid and the target gene in the K. pneumoniae genome. For the selection of transconjugants, K. pneumoniae ΔLDH (inactive ldh) and ΔBUDB (inactive budB), M9 minimal medium containing spectinomycin and streptomycin (50 µg/ml each) was used. Spectinomycin- and streptomycin-resistant colonies were verified using colony PCR to confirm correct recombination and obtain mutants.
2.5. Shake-Flask Fermentation
Shake-flask experiments were conducted under controlled conditions: agitation speed at 250 rpm, initial pH at 7.0 ± 0.2, and temperature at 30°C. Both K. pneumoniae TWO and its mutants were initially cultured in 10 ml of LB medium (Inoculum-I) and incubated for 6 hours. Two percent of Inoculum-I was then transferred to 10 ml of seed culture medium and incubated for 16 hours. Subsequently, 2% of the seed culture was inoculated into 100 ml of fermentation medium in a 250 ml shake flask and cultured for 30 hours under microaerobic conditions with the flask closed by a screw cap.
To optimize the production of 1,3-PDO, the fermentation medium was supplemented with 20 mM malic acid, 20 mM succinic acid, and 0.0025 g/l vitamin B12 coenzyme. Additionally, to enhance glycerol consumption, 1 ml each of a trace element solution, Fe solution, vitamin solution, and their combination were added to the fermentation medium.
2.6. Analytical Methods
Cell optical density was measured at 600 nm using a spectrophotometer (Bio-Rad, USA) with appropriate dilution. Supernatants were collected by centrifuging culture samples at 13,000 rpm for 2 minutes, followed by filtration through a 0.22 µm membrane (Nylon Whatman, Uniflo). Secondary metabolites, including lactic acid, formic acid, acetic acid, 1,3-PDO, 2,3-BDO, ethanol, and glycerol, were quantified using High-Performance Liquid Chromatography (Agilent 1200). The analysis was performed with an organic acid analysis column (300 mm × 7.8 mm; Aminex HPX-87H; Bio-Rad, USA) at 60°C, and detection was done with a refractive index detector. The mobile phase used was 5 mM H2SO4 with a flow rate of 0.5 ml/minute.
3. RESULTS AND DISCUSSION
3.1. Confirmation of Genomic Gene Knockouts
Homologous recombination was used to knock out the 945 bp ldh and 1680 bp budB genes in the K. pneumoniae TWO genome. The 1138 bp aadA gene, encoding spectinomycin-streptomycin (Sp/Sm) resistance, was inserted into each gene, resulting in fragments of 445 bp and 499 bp for ldh and 969 bp and 422 bp for budB. The Δldh and ΔbudB genes were cloned into pUTminiTn5.Sp/Sm, modified by eliminating the transposable element and transposase gene. This vector is a suicide vector due to the R6K origin of replication. The 5.8 kb pFS5 and 6.7 kb pFS6 plasmids were transmitted from E. coli S17-1λpir into K. pneumoniae TWO, facilitating double-crossover events. Sp/Sm-resistant colonies were confirmed by colony PCR, producing 2 kb and 2.5 kb PCR products representing Δldh and ΔbudB mutants, respectively (Fig. 2). Sequencing analysis confirmed that the inactive genes replaced the target genes (data not shown).
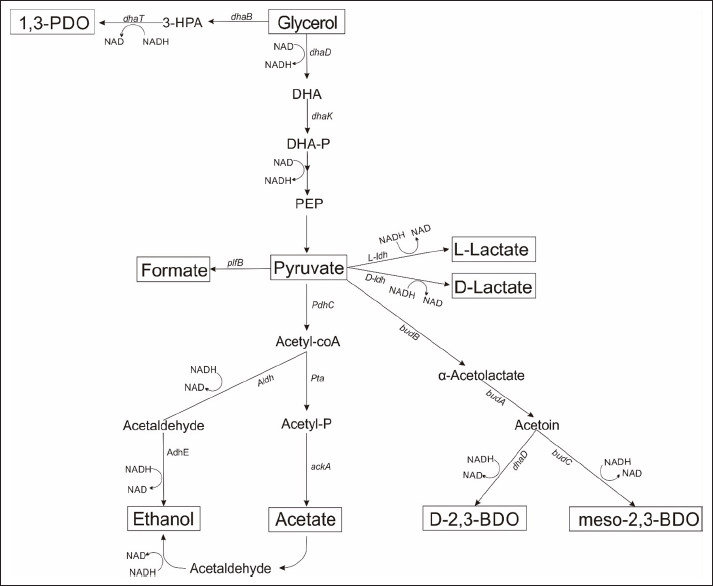 | Figure 1. The metabolic pathway of glycerol in K. pneumoniae TWO under microaerobic condition. [Click here to view] |
 | Figure 2. Verification of K. pneumoniae TWO wildtype and mutants by colony PCR identification. Lanes: M, 10 kb DNA Ladder Marker (SmoBio, Taiwan); 1, ldh (945 bp); 2, ?ldh (2083 bp); 3, budB (1680 bp); 4, ?budB (2529 bp). [Click here to view] |
3.2. Impact of Argeted Gene Knockouts on Glycerol Metabolism and Cell Growth
All strains consumed 17 g/l of glycerol over varying periods. Both mutants, however, displayed slower glycerol consumption, taking 20 hours to fully deplete it due to changes in metabolic flux (Fig. 3). In the ΔLDH mutant, cell growth decreased to an OD600 of 4.5 at 12 hours, showing no significant difference from the wild type at the end of fermentation. In contrast, the ΔBUDB mutant exhibited the lowest cell growth, with OD600 values of 3.7 and 3.4 at 12 and 30 hours, respectively (Fig. 4). The decreased cell growth in the ΔBUDB mutant was associated with increased acetic acid levels [16–18]. This study confirmed that the continuous accumulation of acetic acid, a by-product of the ΔBUDB mutant’s fermentation, negatively impacted cell growth. This finding was also supported by the acetic acid levels and OD600 results from the ΔLDH mutant’s fermentation. These results are further discussed in detail in the by-product level section below.
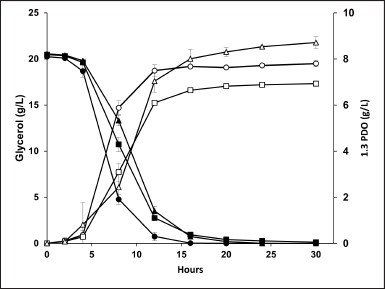 | Figure 3. Glycerol consumed (black) and 1,3-PDO production (white) in a shake flask under anaerobic condition for 30 hours of K. pneumoniae; (?) TWO, (?) ?LDH, and (?) ?BUDB. [Click here to view] |
 | Figure 4. Cell growth and acetate production of K. pneumoniae; (?) TWO, (?) ?LDH, dan (?) ?BUDB. [Click here to view] |
3.3. Impact of Targeted Gene Knockouts on 1,3-PDO Production
The ΔBUDB mutant achieved the highest 1,3-PDO level, increasing by 11.6% with a yield of 0.51 mol/mol glycerol (Table 2). This significant increase was observed from the 16 hour of fermentation (Fig. 3). In comparison, another study reported a 1,3-PDO yield of 0.47 mol/mol glycerol in a K. pneumoniae mutant with triple deletions in lactate dehydrogenase (ldh), formate acetyltransferase (pflB), and acetolactate decarboxylase (budA) [19]. Similarly, a 1,3-PDO yield of 0.50 mol/mol glycerol was achieved by deleting multiple genes related to the production of 2,3-BDO, ethanol, and acetic acid (Table 3) [12]. Our study indicates that a single knockout of the budB gene is more efficient for increasing the 1,3-PDO level and yield. The increased 1,3-PDO level was achieved by blocking α-acetolactate production in the 2,3-BDO biosynthetic pathway and improving NADH availability, thereby redirecting the metabolic flux to the reductive pathway.
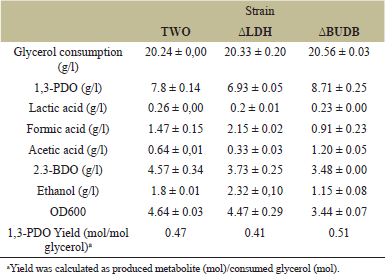 | Table 2. Overview of cell growth, metabolites, consumed glycerol, product yield of wildtype and mutants K. pneumoniae TWO in shake flask under microaerobic condition at 30 hours. [Click here to view] |
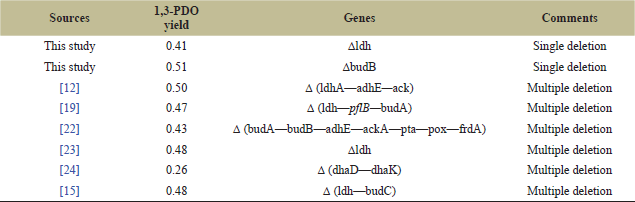 | Table 3. Comparison of knockout studies in K. pneumoniae for improving 1,3-PDO production from glycerol. [Click here to view] |
Consistent with another finding [16], K. pneumoniae TWO used in this study also has an operon with four genes: budB, budA, budC, and budR, encoding α-acetolactate decarboxylase, acetolactate synthase, acetoin reductase, and a transcriptional regulator, respectively (Fig. 1), based on our existing whole-genome database. However, the knockout of budC did not affect the 1,3-PDO level [15], while the knockout of budA decreased the 1,3-PDO level by 18% [16].
In contrast, the ΔLDH mutant resulted in an 11.2% reduction in the 1,3-PDO level, with a yield of 0.41 mol/mol glycerol. This result indicates that not all competitor genes in the by-product biosynthetic pathway can increase the level of 1,3-PDO.
3.4. Impact of Targeted Gene Knockouts on by-Product Levels
To analyze the by-products of glycerol fermentation, HPLC analysis was conducted, focusing on lactic acid, formic acid, acetic acid, 2,3-BDO, and ethanol levels. Knockout of the ldh gene was confirmed by a 23% reduction in lactic acid levels (0.20 g/l). Acetic acid levels decreased by 53.1% (Fig. 4), while ethanol levels increased by 18.2% at the 12 hour (Fig. 5). The ΔLDH mutant ultimately exhibited the highest ethanol level (2.32 g/l) and the lowest acetic acid level (0.33 g/l). Acetic acid, an intermediate in ethanol production, is reduced to acetaldehyde by aldehyde dehydrogenase and then converted to ethanol [20]. Pyruvate accumulation led to increased ethanol production and decreased 1,3-PDO production due to NADH utilization for converting acetyl-CoA to ethanol [21]. This resulted in reduced acetic acid and 1,3-PDO levels, aligning with studies that show ethanol formation influences 1,3-PDO production [14,21]. The pyruvate accumulation also produced formic acid without NADH consumption, confirmed by the highest formic acid level (2.15 g/l) (Table 2).
 | Figure 5. Lactic acid, 2,3-BDO, ethanol and formic acid, production of K. pneumoniae; (?) TWO, (?) ?LDH, dan (?) ?BUDB. [Click here to view] |
The ΔBUDB mutant exhibited different by-product production behaviors from the eighth hour onward. Knockout of the budB gene reduced 2,3-BDO production by 87% from the fourth hour, stabilizing at 3.48 g/l by the end of fermentation (a 24% reduction) (Table 2). Consequently, the increased availability of NADH facilitated higher 1,3-PDO production, which suppressed ethanol production by 36% (1.15 g/l) and induced an 87% increase in acetic acid levels (1.20 g/l) (Table 2), leading to the low OD600 of the ΔBUDB mutant. Acetic acid production competed with the ethanol biosynthetic pathway for carbon flux [25]. A low formic acid level (0.91 g/l) was maintained until the end of fermentation (Fig. 5).
3.5. Redistribution of Glycerol into by-Products
The effect of deleting the ldh and budB genes on the percentage of by-products relative to glycerol consumption was investigated (g/g). All strains, including the budB deficient mutant, produced the highest levels of 2,3-BDO. Blocking the 2,3-BDO biosynthetic pathway significantly increased the acetic acid ratio to 5.8%, with 23.6% of the carbon flux being redirected towards other by-products (Fig. 6). There was a notable decrease in the carbon flux ratio for ethanol, formic acid, and 2,3-BDO production. However, 2,3-BDO production persisted because acetolactate synthase, a key enzyme in this pathway, has eight isozymes. The budB gene, a major gene in this pathway (1680 bp) with 99% similarity to Accession Number CP011313, plays a crucial role (Fig. 7).
 | Figure 6. Conversion ratio of by-product level of consumed glycerol by K. pneumoniae; Black—TWO, White—?LDH, Gray—?BUDB. [Click here to view] |
 | Figure 7. Phylogenetic tree of the isozymes of K. penumoniae TWO: A, Lactate dehydrogenase (LDH); B, Acetolactate synthase (ASL). Asterisk: target gene that inactivated. All sequences not published. Based on similarity genes from GenBank. [Click here to view] |
In contrast, the knockout of the ldh gene significantly increased the carbon flux towards ethanol and formic acid production, each by approximately 11%. Additionally, 1.6% and 18% of the glycerol flux were directed towards acetic acid and 2,3-BDO, respectively (Fig. 6). Blocking lactic acid production via ldh deletion was less effective in reducing carbon flux. The ldh gene (954 bp), classified under enzyme commission 1.1.1.27, catalyzes the production of L-lactate. Our study identified two isozymes of L-lactate dehydrogenase in the K. pneumoniae TWO genome. The ldh target gene in this study was highly similar to Accession Number CP011976, indicating that L-lactate was a minor component in the lactic acid production of K. pneumoniae TWO.
3.6. Impact of Malic Acid, Succinic Acid, and Vitamin B12 Supplementation
As previously explained, the NADH required for converting glycerol to 1,3-PDO is generated not only from the oxidative pathway but also from the TCA cycle. Optimizing the TCA cycle could augment the supply of reducing equivalents and promote the synthesis of 1,3-PDO [26]. This can be achieved by supplementing the TCA cycle with intermediates such as succinic acid and malic acid.
Additionally, vitamin B12 (cyanocobalamin) plays a crucial role in increasing glycerol carbon flux towards 1,3-PDO. In this study, malic acid, succinic acid, and vitamin B12 were supplemented at concentrations of 20 mM, 20 mM, and 0.0025 g/l, respectively. The results showed a decrease in both glycerol consumption and 1,3-PDO production across all treatments. However, supplementation with malic acid and vitamin B12 increased the 1,3-PDO yield to 0.55 mol/mol glycerol and 0.60 mol/mol glycerol, respectively, compared to the previous yield of 0.53 mol/mol glycerol. Additionally, 2,3-BDO and lactic acid levels decreased with vitamin B12 supplementation, reducing competition for glycerol. Ethanol was not produced at all during fermentation with vitamin B12 (Table 4). These findings suggest that optimizing glycerol consumption remains a key area for further study.
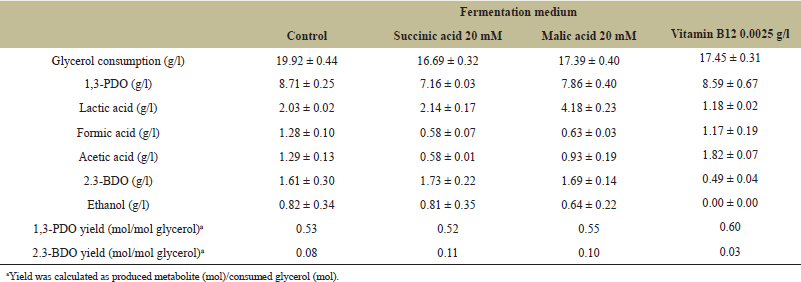 | Table 4. The effect of malic acid, succinic acid, and vitamin B12 on 1,3-PDO levels and by-products of ?BUDB mutant. [Click here to view] |
3.7. Impact of Trace elements, Vitamin Solution, Iron Solution, and their Combinations
To enhance glycerol consumption, we supplemented the fermentation medium with trace elements, vitamin solution, Fe solution, and their combinations, along with vitamin B12. Only trace element supplementation alone did not reduce residual glycerol levels. The combination of 0.0025 g/l vitamin B12 with the vitamin solution significantly increased glycerol consumption, resulting in a residual glycerol level of 0.08 g/l. This treatment also produced the highest level of 1,3-PDO at 10.08 g/l, achieving a yield of 0.62 mol/mol glycerol (Table 5). Klebsiella pneumoniae needs vitamin B12 as a coenzyme for the GDHt enzyme to convert glycerol into 1,3-PDO.
 | Table 5. The effects of trace element, vitamin solution, Fe solution, and its combination on the consumed glycerols and by-products of ?BUDB mutant. [Click here to view] |
4. CONCLUSION
In conclusion, the single knockout of the budB gene in the K. pneumoniae TWO genome proved to be an effective strategy for enhancing 1,3-PDO production, achieving a concentration of 8.71 g/l with a yield of 0.51 mol/mol glycerol. Supplementing the fermentation medium with 0.0025 g/l vitamin B12 and a vitamin solution further increased glycerol conversion efficiency, resulting in 62% of glycerol being converted into 1,3-PDO. This combination yielded a 1,3-PDO concentration of 10.08 g/l with an improved yield of 0.62 mol/mol glycerol, accompanied by the lowest residual glycerol levels.
5. ACKNOWLEDGMENT
This study is part of the authors Master Degree work. The authors would like to thank Prof. Antonius Suwanto. The authors also acknowledge the support and technical assistance of the Enzyme and Bioprospecting Team, Research and Development Department, Wilmar Benih Indonesia.
6. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
7. FUNDING
There is no funding to report.
8. AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data are available with the authors and shall be provided upon request.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors, and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Chen J, Xia Q, Wang Y, Huang Y. Progress in production of 1, 3-propanediol from selective hydrogenolysis of glycerol. Front Chem Eng 2020;2(1):604624–31; CrossRef
2. Zhu Y, Wang Y, Gao H, Wang H, Wan Z, Jiang Y, et al. Current advances in microbial production of 1,3-propanediol. Biofpr 2021;15(5):1566–83; CrossRef
3. Biebl H, Menzel K, Zeng AP, Deckwer WD. Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 1999;52(1):289–97; CrossRef
4. Szymanowska-Powa?owska D. 1,3-propanediol production from crude glycerol by Clostridium butyricum DSP1 in repeated batch. Electron J Biotechnol 2014;17:322–28; CrossRef
5. Drozdzy?ska A, Pawlicka J, Kubiak P, Ko?mider A, Pranke D, Olejnik-Schmidt A, et al. Conversion of glycerol to 1,3-propanediol by Citrobacter freundii and Hafnia alvei—newly isolated strains from the Enterobacteriaceae. N Biotechnol 2014;31(5):402–10; CrossRef
6. Wojtusik M, Rodríguez A, Ripoll V, Santos VE, García JL, García-Ochoa F. 1,3-Propanediol production by Klebsiella oxytoca NRRL-B199 from glycerol. Medium composition and operational conditions. Biotechnol Rep 2015;6:100–07; CrossRef
7. Kong DS, Kim M, Li S, Mutyala S, Jang M, Kim C, et al. Bioconversion of glycerol to 1,3-propanediol using Klebsiella pneumoniae L17 with the microbially influenced corrosion of zero-valent iron. Ferment 2023;9(3):233–45; CrossRef
8. Hao J, Lin R, Zheng Z, Liu H, Liu D. Isolation and characterization of microorganisms able to produce 1,3-propanediol under aerobic conditions. World J Microbiol Biotechnol 2008;24:1731–40; CrossRef
9. Celi?ska E. Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv 2010;28(4):519–30; CrossRef
10. Wang W, Yu X, Wei Y, Ledesma-Amaro R, Ji XJ. Reprogramming the metabolism of Klebsiella pneumoniae for efficient 1,3-propanediol production. Chem Eng Sci 2021;236(8):116539; CrossRef
11. Wang X, Zhang L, Liang S, Yin Y, Wang P, Li Y, et al. Enhancing the capability of Klebsiella pneumoniae to produce 1, 3-propanediol by overexpression and regulation through CRISPR-dCas9. Microbial Biotech 2022;15(7):2112–25; CrossRef
12. Wang Y, Mu J, Liao Y, Wang Y, Yin X, Xu B, et al. Klebsiella pneumoniae metabolic regulation and its application in 1,3-propanediol production. Res Square 2021;4:1–17; CrossRef
13. Park J, Rathnasingh C, Song H. Metabolic engineering of Klebsiella pneumoniae based on in silico analysis and its pilot-scale application for 1,3-propanediol and 2,3-butanediol co-production. J Ind Microbiol Biotechnol 2017;44(33):431–41; CrossRef
14. Chen L, Ma C, Wang R, Yang J, Zheng H. Deletion of ldhA and aldH genes in Klebsiella pneumoniae to enhance 1,3-propanediol production. Biotechnol Lett 2016;38:1769–74; CrossRef
15. Kumar V, Durgapal M, Sankaranarayanan M, Somasundar A, Rathnasingh C, Song HH, et al. Effects of mutation of 2,3-butanediol formation pathway on glycerol metabolism and 1,3-propanediol production by Klebsiella pneumoniae J2B. Bioresour Technol 2016;214:432–40; CrossRef
16. Lu X, Ji G. The role of budABC on 1,3-propanediol and 2,3-butanediol production from glycerol in Klebsiella pneumoniae CICIM B0057. Bioeng 2016;7(6):439–44; CrossRef
17. Xue X, Li W, Li Z, Xia Y, Ye Q. Enhanced 1,3-propanediol production by supply of organic acids and repeated fed-batch culture. J Ind Microbiol Biotechnol 2010;37(7):681–87; CrossRef
18. Cui YL, Zhou JJ, Gao LR, Zhu CQ, Jiang X, Fu SL, et al. Utilization of excess NADH in 2,3-butanediol-deficient Klebsiella pneumoniae for 1,3-propanediol production. J Appl Microbiol. 2014;117(3):690–98; CrossRef
19. Lee JH, Jung MY, Oh MK. High-yield production of 1,3-propanediol from glycerol by metabolically engineered Klebsiella pneumoniae. Biotechnol Biofuels 2018;11:104–17; CrossRef
20. Krivoruchko A, Zhang Y, Siewers V, Chen Y, Nielsen J. Microbial acetyl-CoA metabolism and metabolic engineering. Metab Eng 2015;28:28–42; CrossRef
21. Zhang Y, Li Y, Du C, Liu M, Cao Z. Inactivation of aldehyde dehydrogenase: a key factor for engineering 1,3-propanediol production by Klebsiella pneumoniae. Metab Eng 2006;8(6):578–86; CrossRef
22. Xin B, Tao F, Wang Y, Liu H, Ma C, Xu P. Coordination of metabolic pathways: enhanced carbon conservation in 1,3-propanediol production by coupling with optically pure lactate biosynthesis. Metab Eng 2017;41:102–14; CrossRef
23. Durgapal M, Kumar V, Yang TH, Lee HJ, Seung D, Park S. Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour Technol 2014;159:223–31; CrossRef
24. Horng YT, Chang KC, Chou TC, Yu CJ, Chien CC, Wei YH, et al. Inactivation of dhaD and dhaK abolishes by-product accumulation during 1,3-propanediol production in Klebsiella pneumoniae. J Ind Microbiol Biotechnol 2010;37(7):707–16; CrossRef
25. Lee SM, Hong WK, Heo SY, Park JM, Jung YR, Oh BR, et al. Enhancement of 1,3-propanediol production by expression of pyruvate decarboxylase and aldehyde dehydrogenase from Zymomonas mobilis in the acetolactate-synthase-deficient mutant of Klebsiella pneumoniae. J Ind Microbiol Biotechnol 2014;41(8):1259–66; CrossRef
26. Lu XY, Len SL, Lu JZ, Zong H, Song J, Zhuge B. Enhanced 1,3-Propanediol production in Klebsiella pneumoniae by a combined strategy of strengthening the TCA Cycle and weakening the glucose effect. J Appl Microbiol 2018;124(3):628–90; CrossRef