1. INTRODUCTION
Herbicides are frequently applied agrochemicals to initiate the growth and yield of crops. They are regarded as a coherent tool for controlling numerous odious weed species present around the cultured crop. They are comparatively more economical than hand weeding as it resolves the problem of labor. Weed control in paddy cultivation is a critical issue. It was assessed that weed share nearly 45% of the total yearly loss of agricultural output in India [1]. They are systematically classified based on the degree of selectivity, site of action, time of application, method of application, mode of action, and translocation [Figure 1]. There are various types of herbicides used in paddy fields in India. Each family of herbicide has a unique weed control range. They interact or interfere with weeds through different inhibition pathways such as amino acid synthesis inhibition, lipid synthesis inhibition, and photosynthesis inhibition [2]. The activity of herbicides also depends on soil and environment-born factors such as temperature, moisture, and microbes. Moreover, photodegradation, chemical degradation, and microbial degradation interfere with the activity of herbicides. Hence, it is essential to correlate the soil, environment, and herbicide-born factors before applying it on the agriculture fields.
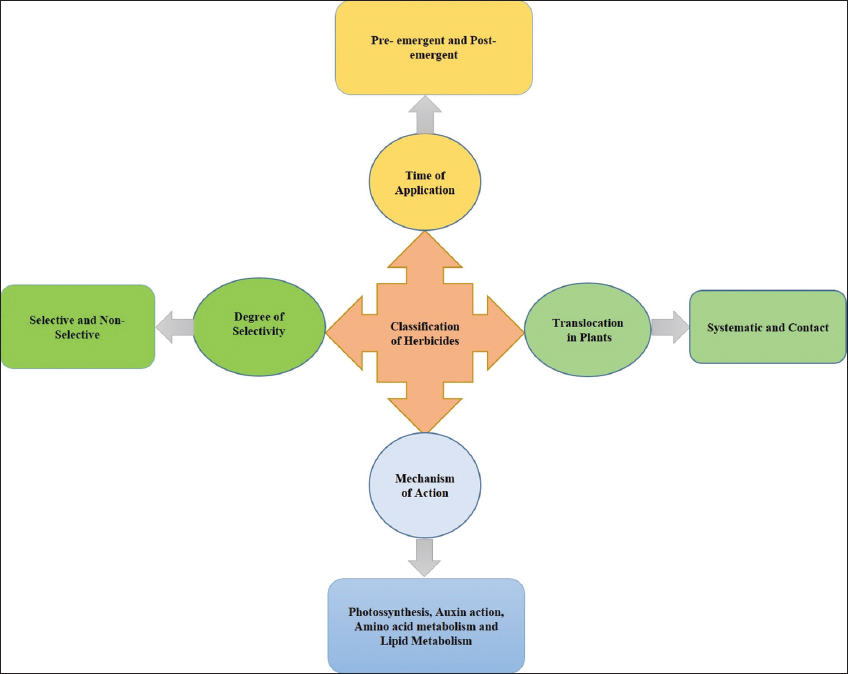 | Figure 1: Diagrammatic representation based on the classification of herbicides. [Click here to view] |
Paddy is the major staple crops overloaded with the starch. The microbes responsible for the growth of paddy crops are cyanobacteria. They are often regarded as natural biofertilizers [3]. They have capacity to degrade herbicides, enrich soil with organic matter, extracellular polysaccharide, vitamins, and hormones and increase the water-holding capacity of the soil [4,5]. Predominant microflora present in the paddy fields is Aulosira, Anabaena, Spirulina, Anabaenopsis, Calothrix, Gloeocapsa sp., Anacystis, Nostoc, Cylindro spermum, Fischereila, Hapalosiphon, and Tolypothrix. The Eastern Belts of India are the major contributor of rice which includes states like Assam, Chhattisgarh, Uttar Pradesh, Bihar, and West Bengal [Table 1]. The application of herbicide cause indiscriminate damage to the paddy field because only a minor portion of applied herbicides actually outreaches the cultivated crop, while a significant portion is discharged into the surrounding ecosystem, potentially having detrimental effects on ecologically important non-target species [6].
Table 1: The state wise list of herbicides used in the rice fields in Eastern Belts of India [48].
| States | Herbicides |
|---|---|
| Assam | 2,4-D, Butachlor, Paraquat, Pretilachlor |
| Chhattisgarh | 2,4-D, Butachlor, Paraquat, Pretilachlor, Anilofos |
| Bihar and Jharkhand | 2,4-D, Paraquat, Pretilachlor, Anilofos |
| West Bengal and Sikkim | 2,4-D, Butachlor, Paraquat, Pretilachlor |
2. TOXIC EFFECTS OF VARIOUS HERBICIDES USED IN THE PADDY FIELDS
Rice is the most essential food crop grown and consumed all around the globe. They are easily affected by pest, which reduces the overall yield of the crop. To solve this problem several selective and non-selective, pre- and post-emergent inorganic chemical fertilizers are being used at recommended doses. The overreliance and overuse of these inorganic chemical fertilizers tend to pollute the soil and non-target crops by influencing the ability of plant growth promoting microbes and nitrogen fixing cyanobacteria [7]. It may add substantial amount of residual product in the soil ecosystem [8] leading to severe ecological consequences. They also interact and interfere with the aquatic ecosystem through surface runoff and seepage into the water bodies [9]. The herbicides mostly accumulate in the food chain [10] and have been reported to induce mutation and cancer in humans [11]. There are several herbicides applied commonly in the paddy fields all-around the states belonging to Eastern Belts of India [Table 2].
Table 2: Addressing toxicological impacts of several pesticides utilized in the paddy fields of Eastern Belts of India on cyanobacterial proliferation.
| S. No. | Herbicide | Chemical Family | Chemical Name | Trade Name | Species | Effect | References |
|---|---|---|---|---|---|---|---|
| 1. | 2,4-D | Phenoxy | 2,4- Dichlorophenoxyacetic acid | Barrage, Plantgard, lawn-keep, malerbane, weedex, aqua-kleen and weedone | Anabaena fertilissima, Aulosira fertilissima, Aulosira fertilissima and Westiellopsis prolifica | Chlorophyll, Carotenoid, Phycobilin, Carbohydrate and protein content. | [15] |
| Spirulina platensis | Chlorophyll content, growth and development | [16] | |||||
| 2. | Butachlor | Amide | N×-(butoxymethyl) -2-chloro-2 ×, 6×- diethylacetani-lide. | Machete, Butanex, Butataf, Dhanuchlor, Farmachlor and Hiltaklor | Aulosira fertilissima | Photosynthetic process | [23] |
| 3. | Pretilachlor | Chloroacetanilide | 2-chloro-N- (2,6—diethlyphenyl) -N-(2-Propoxyethly) acetamide | Rifit, Pretiherb, Remove, Erase, Prince, Offset, Tatapreet, Hifit, Blade, Sureshot, Shriram pretilachlor, Alchor, Pretit and Pilot | Synechocycystis sp. | Growth , soluble proteins , photosynthetic pigment content, photosynthetic process, respiration activity and nitrogen status | [28] |
| Anabaena sp. and Nostoc sp. | Photosynthetic pigment content, and oxidative stress. | [29] | |||||
| Desmonostoc sp. | Growth , nitrate, nitrite, ammonium, heterocyst, Nitrogenase and Glutathione reductase | [30] | |||||
| 4. | Aniliofos | Organophosphorus | S-4-Chloro- N-isopropyl carbaniloylmethyl-O, O-dimethyl phosphorodithioate. | Aniloguard, Anilophos, Arozin and Rico | Anabaena torulosa | Growth, photosynthetic pigments, photosynthetic pigments, respiration and nitrogen status | [33] |
| Synechocystic sp. | Photosynthetic pigments | [34] | |||||
| 5. | Paraquat | Bipyridylium | 1,1×- dimethyl-4,4 ×- bipyridinium dichloride | Gramoxone and viologen | Nostoc hatei and Anabaena lutea | Dry mass, chlorophyll a and phycocyanin content | [41] |
| Chlorella vulgaris | Chlorophyll a, b and oxidative stress | [44] |
3. 2,4-DICHLOROPHENOXYACETIC ACID
One of the extensively utilized herbicide in the paddy fields is 2,4-D. It belong member of the phenoxy family [12]. It is a selective herbicide, sensitive to change in pH and temperature. It is applied to control the problem of broadleaf weed infestation in rice fields [13]. The high concentration of 2,4-D influence the activity of many photosynthetic and nitrogen fixing cyanobacteria [14]. It was reported that the exposure of three strains of filamentous-heterocyst forming cyanobacteria Anabaena fertilissima, Aulosira fertilissima, and Westiellopsis prolific to different concentration, that is, 60 ppm, 80 ppm, and 120 ppm, respectively, leads to reduction in carbohydrate, amino acid, and pigment content of the cell [15]. It induced damaging effects on growth and pigment content in cyanobacteria Spirulina platensis [16]. It is reported to decrease the organic carbon content and the dehydrogenase activity (DHA) of the soil [17]. Hence, its application in paddy field reduces the overall quality and yield of rice [18]. Due to its overuse, it had been reported to cause cancer in human [19]. Moreover, severe damaging symptoms were also visible on fish Channa punctatus, at 25–75 mg/mL concentration causing micronucleus inhibition [20].
4. BUTACHLOR
Butachlor is the member of Chloroacetanilide family. It is widely applied as pre-emergent, systematic herbicide used for the growth of paddy crops [21]. It suppress nitrogenase, nitrate reductase and glutamine synthetase activities in Nostoc muscorum while fluctuate photosynthetic efficiency in both Gloeocapsa sp. and Nostoc muscorum [22].The accumulation of 65µM of butachlor caused a decline in the photosynthetic efficacy by 24–48% in strain A. fertilissima after 15 days application [23]. Furthermore, butachlor has been reported to inhibit the growth and reproduction of the earthworm Eisenia fetida. [24] and Perionyx sansibaricus [25]. It also leads to DNA damage, affects survival, development, and metamorphosis stage in tadpole of frog Fejervarya limnocharis at high concentration [26]. The toxicity of butachlor on African catfish (Clarias gariepinus) at different concentrations of 1, 2, and 2.5 ppm impose oxidative stress notably on their kidney and liver cell [27]. Therefore, to protect cell from apoptosis, the enzymatic scavengers play a crucial role [Figure 2] [28].
 | Figure 2: Diagrammatic representation of site of action of Butachlor in catfish [27]. [Click here to view] |
5. PRETILACHLOR
Pretilachlor is the member of Chloroacetanilide family. It widely used in paddy fields as pre-emergent and early post-emergence herbicide. It is used to manage broad-leaved weeds and annual grasses. The high concentration of pretilachlor decrease growth, soluble proteins, photosynthetic pigments content (chlorophyll a, carotenoids, phycocyanin, allophycocyanin, and phycoerythrin), photosynthetic process, respiration activity, and nitrogen status in a dose dependent (10,15 and 20 mgL-1) manner in unicellular cyanobacterium Synechocystis sp. [29]. The exposure of light intensity (suboptimum, 25 µM photon m-² s-¹) at different dose of pretilachlor (3 µg/mL and 6 µg/mL) act as a limiting factor on the growth of cyanobacteria Anabaena sp. and N. muscorum, it decline the photosynthetic pigment content and induce oxidative stress notably during sub optimal light intensity. However, Anabaena sp. was more severely damaged than N. muscorum [30]. The successive decline in the growth, nitrate, nitrite, ammonium, heterocyst formation, nitrogenase (10 ppm), and glutathione synthetase activity of cyanobacteria Desmonostoc muscorum PUPCCC405.10 was reported at different graded concentration above 2.5–15 ppm [31].
The accumulation of pretilachlor is reported to pollute aquatic ecosystem by triggering various behavioral changes such as buccal movement, feeding attempts, and swimming speed in Clarias batrachus (Linnaeus) fish [32].
6. ANILOFOS
Anilofos is member of organophosphate family. It is extensively applied as a pre-emergent and early post-emergent herbicide to manage annual grasses, sedges, and some broad-leaved weeds in transplanted and direct seeded rice crops [33].
The treatment of anilofos at concentration of 2.5, 5.0, 7.5, and 10.0 mg/L successively affected the growth, photosynthetic pigments, photosynthesis, respiration, and nitrogen status and activated stress enzyme in heterocyst forming photoautotrophic cyanobacterium Anabaena torulosa [34]. Its exposure in concentration dependent manner (5, 10, and 20 mg/L) declines the level of photosynthetic pigments trigger oxidative stress in the Synechocystis sp. [35]. Moreover, the toxicity of anilofos has been well assessed in rats [36]; it was reported to showcase noteworthy retardation in embryo growth in pregnant rats [37]. Moreover, it leads to cytotoxic and genotoxic effect in human cells [38].
7. PARAQUAT
Paraquat is member of bipyridylium chemical family. It is a non-selective, post-emergent herbicide applied to eradicate broadleaf weeds [39]. It prevents the formation of NADP to NADPH in the Photosystem I and inhibits the conversion of active oxygen to molecular oxygen which in turn triggers the formation of reactive oxygen species (ROS) in the chloroplast [40] [Figure 3]. Thereby, it is significantly used in the cultivation of paddy fields surrounding the eastern belts of India.
 | Figure 3: Diagrammatic representation of Paraquat Site of Action (Co-Q: Co- enzyme Q, Pq: Plastoquinone, Cyt b6: Cytochrome b6, Pc: Plastocyanin, FRS: Ferrodoxin reducing substances, Fd: Ferredoxin) [40]. [Click here to view] |
However, its exposure in dose dependent concentration is reported to cause severe toxicity such as decline in dry mass, chlorophyll a, and phycocyanin contents in two essential species of heterocyst forming cyanobacteria Nostoc hatei and Anabaena lutea [41], predominantly present in the paddy fields and is responsible for increasing the fertility of the soil by fixing atmospheric nitrogen [42]. Moreover, the unicellular marine water algae Chlorella vulgaris reported to increase the growth of paddy crop [43] at different concentration (0.5 µM and 0.75 µM) of paraquat subsequently decreased the photosynthetic content (chlorophyll a, b) and induced the production of free radical scavenging enzymes [44]. Moreover, it shares a structural similarity with dopaminergic neurotoxin [45]. It is also reported to cause several neurodegenerative ailments such as Alzheimer’s disease due to oxidative stress leading to neuronal cell death in humans [46]. Furthermore, the interaction of paraquat with dynamic α-synuclein protein increased their aggregation in the neurons of brain cells in mice and cause Parkinson’s disease [47].
8. CONCLUSION
Weed management is the primary concern of farmers. To eliminate the weeds for obtaining high yield and good quality crops there are several techniques being employed such as mechanical, chemical and biological. However, because of ease of application and cost effectiveness chemical methods remained the cornerstone in agricultural practices. However, its environmental and toxicological impacts on sustaining life cannot be ignored. This review article focuses on the residual effects of various herbicides persisting on paddy fields around the eastern belts of India. It also draws attention towards the unintentional side effects of herbicides on non-targeted life forms such as aquatic life, human beings and animals through surface run-off from the targeted fields. Subsequently, these synthetic chemicals alleviate burden on the environment by degrading the quality of soil and water. Furthermore, they tend to cause serious alteration in environment and trigger toxicity in the microbe. There overuse may emerge out as genotoxic, neurotoxic, and immunologic towards various life forms including human and animals. They accumulate in the waterbodies through surface runoff from the agricultural fields and influence the overall physiology of the aquatic life. Hence, it is essential to head on toward organic modes of farming. Organic farming aims to provide quality food and are source-efficient, animal-friendly, and socially responsible manner. For higher agricultural productivity, weed management strategies must be improved. As a result, we must use environmentally friendly weed-killing agents such as plant hormones or select herbicide-resistant crops. Herbicide safeners are another possibility. These are chemical mixture that are used in conjunction with herbicides to make them safer by lowering herbicide toxicity on crop plants and improving selectivity between agricultural crops and weed species that herbicides target.
9. AUTHORS’ CONTRIBUTIONS
All authors made noteworthy contributions to plan and design, acquisition of data; took part in framing the article or rechecking it critically for essential intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be account for all aspects of the work.
10. FUNDING
There is no funding to report.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This particular study does not involve any animals or human studies.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gharde Y, Singh PK, Dubey RP, Gupta PK. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot 2018;107:12-8. [CrossRef]
2. Tomlin SC. The pesticide manual:A world compendium. 12th ed., Vol. 14. Surry:British Crop Protection Council;2000. 502-4.
3. Chittora D, Meena M, Barupal T, Swapnil P, Sharma K. Cyanobacteria as a source of biofertilizer for sustainable agriculture. Biochem Biophys Rep 2020;22:100737. [CrossRef]
4. Singh DP, Khattar JI, Nadda J, Singh Y, Garg A, Kaur N, et al. Chlorpyrifos degradation by the cyanobacterium Synechocystis sp. strain PUPCCC 64. Environ Sci Pollut Res 2011;18:1351-9. [CrossRef]
5. Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M. Soil microalgae and cyanobacteria:The biotechnological potential in the maintenance of soil fertility and health. Crit Rev Biotechnol 2019;39:981-98. [CrossRef]
6. Prado R, Rioboo C, Herrero C, Cid A. The herbicide paraquat induces alterations in the elemental and biochemical composition of non-target microalgal species. Chemosphere 2009;76:1440-4. [CrossRef]
7. Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, et al. Impact of agrochemicals on soil microbiota and management:A review. Land 2020;9:34. [CrossRef]
8. Kumar A, Nayak AK, Shukla AK, Panda BB, Raja R, Shahid M, et al. Microbial biomass and carbon mineralization in agricultural soils as affected by pesticide addition. Bull Environ Contam Toxicol 2012;88:538-42. [CrossRef]
9. Sudo M, Goto Y, Iwama K, Hida Y. Herbicide discharge from rice paddy fields by surface runoff and percolation flow:A case study in paddy fields in the Lake Biwa basin, Japan. Pestic Sci 2018;43 [CrossRef]
10. Waring RH, Mitchell SC, Brown I. Agrochemicals in the food chain. In:Present Knowledge in Food Safety. United States:Academic Press;2023. 44-61. [CrossRef]
11. Nguyen-Ngoc H, Durrieu C, Tran-Minh C. Synchronous-scan fluorescence of algal cells for toxicity assessment of heavy metals and herbicides. Ecotoxicol Environ Saf 2009;72:316-20. [CrossRef]
12. Teixeira MC, Duque P, Sa-Correia I. Environmental genomics:Mechanistic insights into toxicity of and resistance to the herbicide 2, 4-D. Trends Biotechnol 2007;25:363-70. [CrossRef]
13. Wright TR, Shan G, Walsh TA, Lira JM, Cui C, Song P, et al. Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoate dioxygenase transgenes. Proc Natl Acad Sci 2010;107:20240-5. [CrossRef]
14. Panigrahi S, Padhy S, Padhy RN. Toxicity of parathion?methyl to cells, akinetes and heterocysts of the cyanobacterium Cylindrospermum sp. and the probit analysis of toxicity. Ann Appl Biol 2003;143:195-202. [CrossRef]
15. Kumar NJ, Amb MK, Kumar RN, Bora A. Consequences of 2, 4-D and pencycuron treatment on three different cyanobacterial species-Anabaena fertilissima Rao, Aulosira fertilissima Ghose and Westiellopsis prolifica Janet. Electron J Environ Agric Food Chem 2010;1:9.
16. Singh HS, Singh SG, Kumar D. Effect of Aspartic acid and glucose on growth rate and pigmentation of Spirulina platensis:An aquatic source of food. Int J Fish Aquat 2018;6:377-9.
17. Arora S, Arora S, Sahni D, Sehgal M, Srivastava DS, Singh A. Pesticides use and its effect on soil bacteria and fungal populations, microbial biomass carbon and enzymatic activity. Curr Sci 2019;116:643-9. [CrossRef]
18. Scott BJ, Norsworthy J, Barber T, Hardke J. Rice weed control. In:Arkansas Rice Production Handbook. MP192 Revised. Fayetteville, AR:University of Arkansas Cooperative Extension Service;2013. 53-62.
19. Beladghame O, Bouchikhi N, Lerari D, Charif IE, Soppera O, Maschke U, et al. Elaboration and characterization of molecularly imprinted polymer films based on acrylate for recognition of 2, 4-D herbicide analogue. Iran Polym J 2023;?;?32:483-97. [CrossRef]
20. Farah MA, Ateeq B, Ali MN, Ahmad W. Evaluation of genotoxicity of PCP and 2, 4-D by micronucleus test in freshwater fish Channa punctatus. Ecotoxicol Environ Saf 2003;54:25-9. [CrossRef]
21. Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J. Butachlor induced dissipation of mitochondrial membrane potential, oxidative DNA damage and necrosis in human peripheral blood mononuclear cells. Toxicology 2012;302:77-87. [CrossRef]
22. Singh LJ, Tiwari DN. Effects of selected rice-field herbicides on photosynthesis, respiration, and nitrogen assimilating enzyme systems of paddy soil diazotrophic cyanobacteria. Pestic Biochem Physiol 1988;31:120-8. [CrossRef]
23. Kumari N, Narayan OP, Rai LC. Understanding butachlor toxicity in Aulosira fertilissima using physiological, biochemical and proteomic approaches. Chemosphere 2009;77:1501-7. [CrossRef]
24. Gobi M, Gunasekaran P. Effect of butachlor herbicide on earthworm Eisenia fetida-its histological perspicuity. Appl Environ Soil Sci 2010;2010:850758. [CrossRef]
25. Muthukaruppan G, Janardhanan S, Vijayalakshmi G. Sublethal toxicity of the herbicide butachlor on the earthworm Perionyx sansibaricus and its histological changes. J Soils Sediments 2005;5:82-6. [CrossRef]
26. Liu WY, Wang CY, Wang TS, Fellers GM, Lai BC, Kam YC. Impacts of the herbicide butachlor on the larvae of a paddy field breeding frog (Fejervarya limnocharis) in subtropical Taiwan. Ecotoxicology 2011;20:377-84. [CrossRef]
27. Farombi EO, Ajimoko YR, Adelowo OA. Effect of butachlor on antioxidant enzyme status and lipid peroxidation in fresh water African catfish (Clarias gariepinus). Int J Environ Res Public Health 2008;5:423-7. [CrossRef]
28. Lopes PA, Pinheiro T, Santos MC, da Luz Mathias M, Collares-Pereira MJ, Viegas-Crespo AM. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. Sci Total Environ 2001;280:153-63. [CrossRef]
29. Singh DP, Khattar JS, Kaur G, Singh Y. Toxicological effect of pretilachlor on some physiological processes of cyanobacterium Synechocystis sp. strain PUPCCC 64. J Appl Biol Biotechnol 2016;4:12-9.
30. Kumar J, Patel A, Tiwari S, Tiwari S, Srivastava PK, Prasad SM. Pretilachlor toxicity is decided by discrete photo-acclimatizing conditions:Physiological and biochemical evidence from Anabaena sp. and Nostoc muscorum. Ecotoxicol Environ Saf 2018;156:344-53. [CrossRef]
31. Singh DP, Khattar JI, Kaur G, Gupta M, Singh Y, Gulati A. Effect of pretilachlor on nitrogen uptake and assimilation by the cyanobacterium Desmonostoc muscorum PUPCCC 405.10. Acta Physiol Plant 2015;37:177. [CrossRef]
32. Soni R, Verma SK. Acute toxicity and behavioural responses in Clarias batrachus (Linnaeus) exposed to herbicide pretilachlor. Heliyon 2018;4:e01090. [CrossRef]
33. Hazarika A, Sarkar SN, Kataria M. Subacute toxicity of anilofos, a new organophosphorus herbicide in male rats:Effect on lipid peroxidation and ATPase activity. Indian J Exp Biol 2001;39:1112-7.
34. Singh DP, Khattar JS, Kaur K, Sandhu BS, Singh Y. Toxicological impact of anilofos on some physiological processes of a rice field cyanobacterium Anabaena torulosa. Environ Toxicol Chem 2012;94:1304-18. [CrossRef]
35. Singh DP, Khattar JIS, Kaur M, Kaur G, Gupta M. Anilofos tolerance and its mineralization by the cyanobacterium Synechocystis sp. strain PUPCCC 64. PLoS One 2013;8:e53445. [CrossRef]
36. Hazarika A, Sarkar SN, Hajare S, Kataria M. Influence of malathion pretreatment on the toxicity of anilofos in male rats:A biochemical interaction study. Toxicology 2003;185:1-8. [CrossRef]
37. Aggarwal M, Wangikar PB, Sarkar SN. Effects of low level arsenic exposure on the developmental toxicity of anilofos in rats. J Appl Toxicol 2007;27:255-61. [CrossRef]
38. Akyil D, Konuk M, Eren Y. Mutagenic and genotoxic effects of Anilofos with micronucleus, chromosome aberrations, sister chromatid exchanges and Ames test. Cytotechnology 2017;69: [CrossRef]
39. Fuerst EP, Vaughn KC. Mechanisms of paraquat resistance. Weed Technol 1990;4:150-6. [CrossRef]
40. Autor AP. Reduction of paraquat toxicity by superoxide dismutase. Life Sci 1974;14:1309-19. [CrossRef]
41. Tansai S, Issakul K, Ngearnpat N. Toxicity of Paraquat on Growth of Cyanobacteria (Nostoc sp. N1 and Anabaena sp. A1) and Germination of Rice Seed (San-Pah-Twang 1). In:The 6th International Conference on Biochemistry and Molecular Biology, Thailand;2018.
42. Kim JD, Lee CG. Diversity of heterocystous filamentous cyanobacteria (blue-green algae) from rice paddy fields and their differential susceptibility to ten fungicides used in Korea. J Microbiol Biotechnol 2006;16:240-6.
43. Dineshkumar R, Kumaravel R, Gopalsamy J. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 2018;9:793-800. [CrossRef]
44. Qian H, Chen W, Sun L, Jin Y, Liu W, Fu Z. Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 2009;18:537-43. [CrossRef]
45. Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, et al. Carrier-mediated processes in blood-brain barrier penetration and neural uptake of paraquat. Brain Res J 2001;906: [CrossRef]
46. McCarthy S, Somayajulu M, Sikorska M. Paraquat induces oxidative stress and neuronal cell death;neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol 2004;201:21-31. [CrossRef]
47. Manning-Bog AB, McCormack AL, Li J. The herbicide paraquat causes up-regulation and aggregation of a-synuclein in mice:paraquat and a-synuclein. J Biol Chem 2002;277:1641-4. [CrossRef]
48. Choudhury PP, Singh R, Ghosh D, Sharma AR. Herbicide Use in India Agriculture. Jabalpur, Madhya Pradesh:I.C.A.R-Directorate of Weed Research;2016. 110.