1. INTRODUCTION
Coir is a versatile lignocellulosic natural fiber [1], with lignins and hemicelluloses forming the strengthening constituents of the fiber cells. The tough fiber taken out from the husk of coconut is used to make well-designed floor endowing furnishings in coir division set up in various coconut cultivating regions of India. These divisions supply to the domestic and export marketplace [2]. The coir fiber used to make mats, matting, runners, rugs, and carpets is whitened using a variety of reagents such as hypochlorites, peroxides, peracids, chlorites, sulfites, borohydrides, bisulfites, and others. Alkaline treatment is one of the most effective and low-cost procedures for softening coir fiber; however, it has been proven to be detrimental to the yarn’s strength. Biosoftening uses specialized microorganisms with definite enzyme specificity for topical cell wall elements to generate a biopolishing effect on coir fibers. Unlike chemically softened fibers, the microbial treatment produced soft, brighter fibers with higher tensile strength and elongation qualities, as well as superior durability [3]. Biosoftened coir fiber is spinnable and may be combined with other natural fibers such as sisal, jute, and banana to create fabrics, textiles, and other products [4].
The color as well as the rigid property of the coir fiber are due to the presence of a lignin network between fiber cells and are extremely impervious to biodegradation [5]. The high flexural rigidity due to the lignin content in coir fibers renders it unsuitable for a variety of applications. Thus, lessening of flexural rigidity and toughness is vital for making coir fiber, anoutstanding material for numerous applications including geotextiles and Geomashes. Various chemical approaches are employed for upgrading the quality of coir fiber, namely, smoothness, light fastness, and tensile strength for advanced enactment of the coir fiber for commercial uses [6]. Biological cures are more practicable and ecofriendly which concurrently augments softening and bleaching. Although lignin deprivation by bacteria has been researched extensively in white-rot and brown-rot fungus, it is not wellunderstood in bacteria. Termites are one of the possible sources of lignin-degrading machinery in nature. Few studies are only reported regarding the digestive processes and the microbial associates residing the digestive gut of the fungus cultivating termites [7]. The microflora in the termite gut digests lignocellulose into by-products such as molecular hydrogen and acetate and is consequently taken up by the termite or other microorganisms in the community. The previous research has found that termite microflora removes 5–83% of the lignin, along with most of the neutral polysaccharides and the best part of the acidic saccharides [8,9].
Averrhoa bilimbi L., commonly called bimbling plum, blimblin; English: bilimbi, cucumber tree, tree sorrel; Filipino: Kamias; French: Blimblim, blinblin, and carambolier bilimbi [10], is a member of the Oxalidaceae family [11], which is extensively grown in tropical areas. The fruit juice contains more oxalic acid and hence used to eliminate iron-rust colors from clothing and gives brassware, a lustrous sheen [12,13]. Biosoftened coir fibers find use in a variety of industries since it is spinnable and effortlessly combined with other natural fibers [4]. The current work seeks to bleach and soften coir fiber using a microbial consortium derived from termite gut microflora, followed by A. bilimbi extracts, and then assess the hue and quality of the coir fiber. Research has been done on the processing of coir fibers using ligninolytic fungi and bacteria, but no reports have been made yet on exploiting the termite gut microflora in combination with the natural fruit extracts to soften and brighten the coir fibers. This is the first time Kosakonia oryzendophytica and Pseudomonas chengduensis, both isolated from termite guts, which have been used to improve coir fiber bleaching and softening. The present investigation leads to the development of a bioformulation which could be used as an alternative to the present chemical methods of bleaching as well as softening employed in the coir industry to soften the coir fibers.
2. MATERIALS AND METHODS
2.1. Coir Fiber Treatment
Coir fiber (5 g) was weighed for each treatment, rinsed well in running water, and dehydrated extensively. Nutrient broth was prepared for 300 mL and inoculated with bacterial strains K. oryzendophytica (Accession No. OM943747) and P. chengduensis (Accession No. OM943748) isolated fromthe gut of termite, Odontotermes obesus, collected from the vicinity of Central Coir Research Institute, Kerala, followed by the addition of coir fiber [14], which acts as a lignin substrate for the microbes’ growth in the medium. The culture was then incubated at 37°C for up to 72 h. The treated fibers, after incubation, were completely washed, dried, and surface sterilized under UV light before being treated with A. bilimbi.
2.2. Treatment with A. bilimbi Extracts
In a rotary shaker, the microbially treated fibers were immersed in A. bilimbi extracts and cultured for 72 h at room temperature. The volume of extract used for the treatment was weighed for 150 mL to ensure that all of the fibers were entirely soaked. The raw extract was used in triplicate for the treatment. The material was rinsed in distilled water, dried at room temperature, then analyzed, and then characterized.
2.3. Estimation of Lignin
The Chesson method was executed to calculate the amount of lignin in the samples [15]. After 30 min of drying in a hot air oven at 105°C, 1 g of coir fiber was weighed. After shaking for 10 min with 5 mL of 98% sulphuric acid, the sample was shifted to a 1000 mL Erlenmeyer flask with 450 mL distilled water. The mixture was boiled for 10 min and filtered through a grade 1 glass filter. The residue was then washed to neutrality and dried at 105°C and the final weight was measured.
2.4. Estimation of Cellulose
One gram of the coir fiber sample was mixed with 3 mL acetic/nitric mixture (150 mL of 80% acetic acid + 15 mL concentrated nitric acid) utilizing a vortex mixture and kept in a waterbath for 30 min at 100°C. After cooling, the mixture was centrifuged for 15 to 20 min. After the supernatant was drained, the residue was carefully washed with distilled water. The washed residue was mixed with 10 mL of 67% sulfuric acid and allowed to stand for 1 h and later diluted 1 mL of thesolution to 100 mL. To 1 ml of the above-diluted solution, 10 mL of anthrone reagent (200 mg anthrone was dissolved in 100 mL ice-cold 95% sulfuric acid and chilled for 2 h before use) was added and cooled and the absorbance was read at 630 nm [16,17].
2.5. Surface Morphology
The external modifications of the raw (untreated) and processed fibers were checked with the help of FESEM (Carl Zeiss (USA), Model: Sigma with Gemini column, resolution 1.5 nm).
2.6. Flexural Rigidity
The flexural rigidity of untreated and coir fibers was tested using a flexural rigidity tester designed by CCRI. The raw and treated fibers, 25 samples each, were tied around a 2-inch diameter PVC pipe to make a ring. A Flexural Rigidity Tester was employed to test the rings created by the fibers with and without stress (1 g). This information was used to calculate the ring diameter and deformation during load suspension. Then, the flexural rigidity was calculated by the following mathematical equation
Flexural Rigidity (gcm2) = 0.0047 • mg • (2πr)2• (cosθ/tanθ) [18].
Where, mg =weight of the load in grams
r=radius of the ring in cm
d=deformation of lower endofthe ringincm
θ=493d/2πr
2.7. Tensile Strength
The tensile behavior of the fiber were tested employing the Universal Testing Machine (UTM) Shimadzu AG-X/R with different parameters, namely, strain rate = 10 mm/min, gripping length = 5 cm at atmospheric pressure.
2.8. Lightfastness
The Xeno test (Colorfastness to light, air-cooled xenon arc lamp), IS standard AATCC 16 H-1998, was used to determine the lightfastness of the treated fibers. It was done by exposing a fiber sample to a light source for a certain amount of time and then comparing it to an unexposed sample. The Xenotest can also be used to determine fiber quality by evaluating lightfastness ratings [16]. This is a permanent specification for evaluating the light fastness and weather fastness of target samples at a faster rate than is possible. A 1500-watt Xenon arc lamp is utilized in the Xenotest to provide radiation; the cleared spectrum of this lamp employed in the Xenotest is equivalent to sunlight. The fiber samples were exposed to light and dark for alternating intervals. This method comes close to simulating real day and night circumstances. To assess color change, exposure was stopped when the contrast between the exposed and unexposed portions of the specimen was equivalent to the greyscale grades. The tested and original fabrics were compared under a white light using the blue standard as a reference to determine color change [Table 1] [19].
Table 1: The light fastness grades [15].
| Grade | Degree of fading | Light fastness type |
|---|---|---|
| 8 | No fading | Outstanding |
| 7 | Very slight fading | Excellent |
| 6 | Slight fading | Very good |
| 5 | Moderate fading | Good |
| 4 | Appreciable fading | Moderate |
| 3 | Significant fading | Fair |
| 2 | Extensive fading | Poor |
| 1 | Very extensive fading | Very poor |
2.9. Brightness Index
The brightness index of the fibers was verified as per the TAPPI-452 method with the help of premier color scan spectrophotometer (SS 5100A).
2.10. Fourier-Transform InfraRed (FTIR) Spectroscopy
Comparative analysis and identification of surface structural groups of control and treated coir fibers wereperformed by Thermo Nicolet, Avatar 370 FTIR spectrometer. With a resolution of 4 cm−1 and 32 scans per sample, FTIR spectrum of the raw fiber and microbial treated fibers was taken and the absorbance spectra were recorded at wavenumbers from 500 to 4000 cm−1.
2.11. Statistical Analysis
Two-way ANOVA was performed using IBM SPSS v 20 (IBM Corporation, Armonk, NY) to analyze the significant difference (Tukey test, LSD; P < 0.05) in cellulose and lignin contents retrieved from coir fiber after different treatments (P. chengduensis, K. oryzendophytica and Consortium) tested at different hours (24, 48, and 72).
3. RESULTS AND DISCUSSION
Changes in the degree of softness and vividness of the coir fibers are approved by the treatment. The hard lignin network that connects fiber cells inhibits the fiber from becoming flexible. Since lignin is the component responsible for color and stiffness, fiber softening with negligible impact on fiber strength can be attained in conjunction with the biobleaching treatment [20]. The surface of coir is plentiful with amorphous lignin, and the inside structure is sculpted by cell walls, which are abundant in crystalline cellulose, according to the previous reports [16]. Researches have reported the development of plasma treatment for coir fibers that involved etching the fibers [21]. In nature, lignin breakdown occurs as a result of collaboration between fungi and bacteria in natural microbial consortia [22]. The production of extracellular enzymes and secondary metabolites by the bacterial species makes them, the major lignocelluloses degraders in soils [23,24]. Researchers reported that the wood-feeding termites found in tropical savanna and forests are able to digest lignocellulosic substrates efficiently and, hence, likely have a lignin-preconditioning machinery that allows them to cope such proficient deprivation of timbered floras [25]. Based on the reports by Rajan et al., the bilimbi fruit extract has the potential to lower the stiffness of coir fibers [Figure 1] [15].
 | Figure 1: Averrhoa bilimbi fruit (extract) used to obtain softened and bleached coir fibers. [Click here to view] |
3.1. Lignin and Cellulose
Estimated cellulose and lignin contents in coir fiber after treatment using P. chengduensis + A. bilimbi (Pc+Ab), K. oryzendophytica + A. bilimbi (Ko+Ab), and Consortium + A. bilimbi (C+Ab) were compared among the treatments and the performance of individual treatments at different hours. After being treated with the microbial consortium and then bilimbi, it was discovered that coir fiber had less lignin on the surface, resulting in changes in softness. The lignin in coir fiber (control) was lowered on the combined treatment at various intervals of incubation, as shown in Figure 2. The Pc+Ab performed significantly at 24 h of treatment, and the percentage of cellulose content estimated from coir fiber was significantly high compared with Ko+Ab and C+Ab treatments [Figure 2a]. The treatments were extended to 48 and 72 h and did not significantly differ; loss in cellulose percentage is significantly low [Figure 2a]. On the other hand, the treatments of coir fiber with C+Ab showed a significant decrease in the percentage of lignin when compared to individual treatments using Pc+Ab and Ko+Ab [Figure 2b].
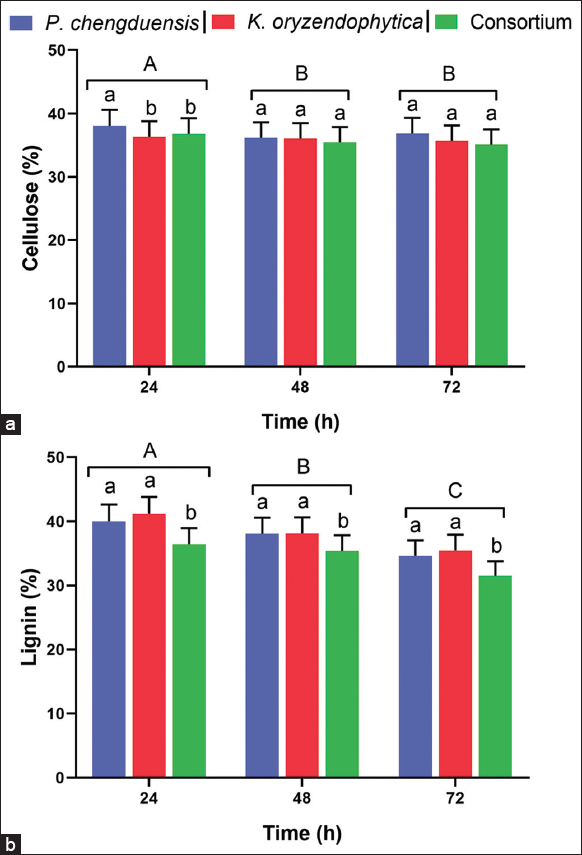 | Figure 2: Estimated cellulose and lignin contents in coir fiber after treatment using Pseudomonas chengduensis + Averrhoa bilimbi, Kozakonia oryzendophytica + Averrhoa bilimbi, and Consortium + Averrhoa bilimbi at different hours. (a) Cellulose and (b) Lignin percentages were obtained after every treatment tested at different hours. Six replications (Mean ± SE) were maintained for both estimations. Vertical bars under different small letters were statistically significant, and the same letters indicate statistically insignificant (Tukey test, LSD; P < 0.05) among the treatments (Pseudomonas chengduensis, Kozakonia oryzendophytica, and Consortium). Different capital letters on the top of the vertical bar indicate that every treatment tested at different hours (24, 48, and 72) was statistically significant (Tukey test, LSD; P < 0.05), and the same capital letters indicate statistically insignificant. [Click here to view] |
The cellulose composition was found to be reduced insignificantly which proves that the method effectively softens and brightens the fibers with negligible loss of the cellulose content of the fibers [Figure 2a]. Meanwhile, the lignin concentration was observed to be reduced by up to 32% after treatment with microbial consortia and bilimbi extracts after 72 h of incubation. The reciprocal action of the lignolytic enzymes produced by the microbial consortia as well as the oxalate present in the bilimbi extracts may be accountable for the partial delignification of coir fibers. The outcome disclosed the ability of the method to diminish the inflexible arrangement of coir fibers.
3.2. Surface Morphology
External micropore protrusions on coir fibers may provide defianceand toughen the coir fiber surface. It is evident from the results that the apparent deposit of the coir fiber has been wiped on treatment and the internal packs of micro fibrils are observable. Thus, the external micropore protrusions have been cleared through the process resulting the fiber getting smoother. The SEM pictures and descriptions disclose the exclusion of the external cover which is abundant with cellulosic groups and amorphous lignin that uncover the core arrangement of packs of cellulosic microfibrils [Figure 3].
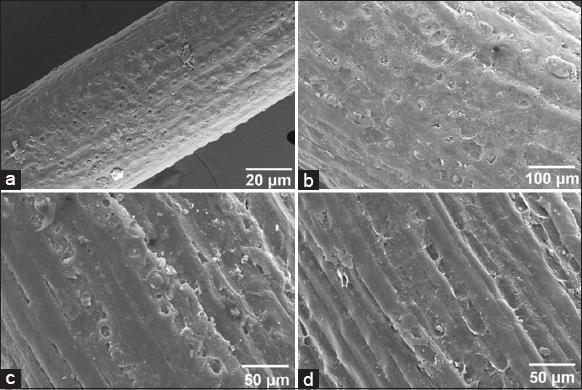 | Figure 3: Scanning electron micrographs of raw and treated fibers. (a) Raw fibers. (b) Pseudomonas chengduensis + Averrhoa bilimbi extract treated fiber, (c) Kozakonia oryzendophytica + Averrhoa bilimbi extract treated fiber, and (d) Microbial consortium + Averrhoa bilimbi extract treated fiber. (Magnification at ×500). [Click here to view] |
3.3. Flexural Rigidity
When the fibers were treated with the microbial consortium followed by A. bilimbi treatment at 72 h of incubation, the flexural rigidity decreased significantly from 1.12 (for untreated fiber) to the lowest of 0.53 [Table 2]. The outcomes show that treatment with the microbial consortia modifies the apparent structures of coir fibers without affecting the core structure or strength. Thus, the treatment is a favorable, economical, and biological technique for attaining softened coir fibers.
Table 2: Tensile strength and flexural rigidity of raw and treated coir fibers after 24, 48, and 72 h of incubation.
| Sample | Time of incubation (hours) | Tensile strength | Flexural rigidity | |
|---|---|---|---|---|
| Breaking stress (N/mm2) | Break strain (%) | Flexural rigidity (Gf) | ||
| Raw coir fiber | 245.351 | 25.024 | 1.12 | |
| Pseudomonas chengduensis+Averrhoa bilimbi | 24 | 241.346 | 25.425 | 0.89 |
| 48 | 227.943 | 24.764 | 0.81 | |
| 72 | 213.048 | 26.946 | 0.78 | |
| Kozakonia oryzendophytica+Averrhoa bilimbi | 24 | 239.907 | 25.678 | 0.91 |
| 48 | 224.053 | 26.543 | 0.87 | |
| 72 | 217.092 | 26.386 | 0.77 | |
| Microbial Consortium+Averrhoa bilimbi | 24 | 245.610 | 25.703 | 0.65 |
| 48 | 212.145 | 26.079 | 0.59 | |
| 72 | 230.999 | 24.243 | 0.53 | |
3.4. Tensile Strength, Brightness Index, and Light Fastness
The breaking stress imparted on raw fiber and treated fibers was found to be lowered from 245.35 to 230.999, indicating that the treatment had relatively minor effects on fiber strength [Table 2]. The brightness index of the coir fibers increased from 9.45 to 13.49 after treatment with the microbial consortia and A. bilimbi [Table 3]. Due to lignin build-up on the fiber surface, the fiber darkens, necessitating bleaching, which makes the fiber brittle. The strength of chemically softened fibers is dramatically diminished, which has a negative impact on spinning characteristics and yarn strength. The light fastness of the fibers improved from Grade 2/3 to Grade 3/4 after the combination treatment [Table 3]. Studies on biobleaching of jute (Indian Jute Industries Research Association, Annual report, 1984–1985) found that in situ growth of selected lignolytic white-rot fungus Polyporus sanguineus on raw jute and grey fabrics improved the light fastness of the samples (Indian Jute Industries Research Association, Annual report, 1984–1985) [4]. Biosoftening softens, thins, and bleaches the fiber while avoiding the use of caustic chemicals, resulting in less pollution [3].
Table 3: Light fastness and brightness index of raw and treated fibers after 72 h of incubation.
| Sample | Light fastness type | Brightness index |
|---|---|---|
| Raw fiber | 2/3 | 9.45 |
| Treated fiber at 72 h incubation | ||
| Pseudomonas chengduensis+Averrhoa bilimbi | 3 | 10.25 |
| Kozakonia oryzendophytica+Averrhoa bilimbi | 3 | 10.49 |
| Consortium+Averrhoa bilimbi | 3/4 | 13.49 |
3.5. FTIR Spectroscopy
Primarily, the peak ranging from 3000 cm−1 to 3700 cm−1 is related to the stretching of hydroxyl groups [26]. Given the spectrum of the untreated coir fiber as a citation to check, the efficiency of the microbial + bilimbi cures performed on the fiber, for the spectrum of treated fibers, the deepened bands such as a 1024-cm−1 bond bound to a C–H stretching vibration of the cellulose backbone and 2908 bonded to an ester bond, and the band 3328 cm−1 associated to the existence of hydroxyl groups (–OH) of cellulose. Meanwhile, the absorption band corresponding to the C = O stretching of carboxyl and acetyl groups in the hemicellulose generated the untreated coir fiber peak band at 1738 cm−1. However, it shrank when the fibers were treated with the consortium + A. bilimbi which relates to the elimination of hemicellulose, and also the band near 1228 cm−1 representing the presence of CH stretching of acetyl groups of lignin exhibited a direreduction in the intensity in the treated fibers [27]. The region between 1252 and 1439 cm−1 is associated with the lignin content category of the C = H group [28,29]. Fascinatingly, the reduced peak intensity at 1368 cm−1 specifies the elimination of surface lignin in the treated fibers. These alterations show the exclusion of lignin and hemicellulose from the coir fiber surface and, hence, attest that the treatments were done effectively to alter the physiognomies of the fiber surface [Figure 4] [22].
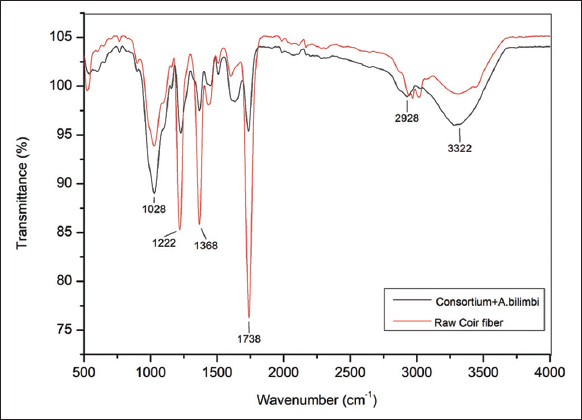 | Figure 4: FTIR spectra of raw fiber and coir fiber treated with microbial consortium together with Averrhoa bilimbi extract. [Click here to view] |
4. CONCLUSION
Coir, a resourceful ecofriendly fiber, the basic stuff for the coir industry, provides income for a huge zone of rural artisans in the tropical regions. The present study exploits the incredible potential of the gut micro flora of termites to delignify and soften the coir fibers and the bleaching property of A. bilimbi to impart brightness to the fibers. The combined treatments using the consortium and fruit extracts have functioned well in transforming the surface topography, drop in the lignin proportion, flexural rigidity, and augmentation of the brightness index. The technique commends that the cure does not affect the inner assembly of coir fiber but modifies the external alignments of lignin thereby refining the coir fiber eminence without losing strength. Thus, the treatment will slash its trajectory to a bio method, an alternative for the prevailing chemical methods, and thus benefits the future coir trade to be more environmentally friendly.
5. AUTHOR’S CONTRIBUTIONS
All the authors have made extensive scholarly contributions to mount this manuscript in the following areas: Conceptualization: Revathy Rajan, Ajith Sudhakaran, Anita Ravindranath, Rajathy Sivalingam and Ratheesh Kumar; data acquisition/analysis/interpretation/drafting manuscript: Revathy Rajan and Ajith Sudhakaran; Critical Revision of the manuscript: Revathy Rajan, Ajith Sudhakaran, Anita Ravindranath, Rajathy Sivalingam and Ratheesh Kumar; supervision and final approval: Anita Ravindranath, Rajathy Sivalingam and Ratheesh Kumar.
6. FUNDING
No funding to report.
7. CONFLICTS OF INTEREST
The authors announce that they have no conflicts of interest.
8. ETHICAL APPROVALS
The research work does not include experimentations on animals or human subjects.
9. DATA AVAILABILITY STATEMENT
All datasets were generated and analyzed in the present study.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. ACKNOWLEDGMENTS
The University Grants Commission, New Delhi, India, and the Kerala State Council for Science Technology and Environment, a self-governing agency of the Government of Kerala, have both generously supported the authors. The authors also acknowledge Shri. D. Kuppuramu, Chairman, Coir Board, for expanding CCRI’s laboratory capabilities. The School of Environmental Studies at Cochin University of Science and Technology, Cochin, India, also provided assistance and facilities, in which the authors gratefully acknowledge. We sincerely appreciate the time and assistance given to mount the paper by Shri. Sivaprasath Prabhu, Chinese Academy of Agricultural Sciences Institute of Plant Protection, Beijing, China.
REFERENCES
1. Ansera S, Sudhakaran A, Ravindranath A. Enhancement of fire retardancy in coir fibers using phosphorylation treatment. J Nat Fibers 2021;19:1-9. [CrossRef]
2. Sudhakaran A, Rajan R, Ravindranath A. Characterization and application of biochar from spent fermentation sludge of coir wastes in removing malachite green from effluent water. Pollut2022;8:1026-37.
3. Rajan A, Senan RC, Pavithran C, Abraham TE. Biosoftening of coir fiber using selected microorganisms. Bioprocess Biosyst Eng 2005;28:165-73. [CrossRef]
4. Rajan A, Abraham TE. Coir-fiber process and opportunities. J Nat Fibers 2007;4:1-11. [CrossRef]
5. Tchin BL, Ho WS, Pang SL. Isolation and in silico characterization of full-length cinnamyl alcohol dehydrogenase gene involved in lignin biosynthesis in Neolamarckia cadamba. J Appl Biol Biotechnol 2018;6:1-5.
6. Simimol AS, Sebastian S, Ravindranath DA, Jacob A. Enhancement of physical, mechanical and morphological properties of coir by mercerization and acetylation. Int J Innov Sci Res Technol 2020;5:530-535.
7. Ayitso AS, Onyango DM, Wagai SO. Antimicrobial activities of microorganisms obtained from the gut of Macrotermes michaelseni in Maseno, Kenya. J Appl Biol Biotechnol 2015;3:048-52.
8. Breznak JA, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol 1994;39:453-87. [CrossRef]
9. König H, Fröhlich J, Hertel H. Diversity and lignocellulolytic activities of cultured microorganisms. In:Intestinal Microorganisms of Termites and Other Invertebrates. Berlin, Heidelberg:Springer;2006. 271-301.
10. Ali MR, Hossain M, Runa JF, Hasanuzzaman M. Preliminary cytotoxic activity of different extracts of Averrhoa bilimbi (fruits). Int Curr Pharm J 2013;2:83-4. [CrossRef]
11. Kumar KA, Gousia SK, Anupama M, Latha JN. A review on phytochemical constituents and biological assays of Averrhoa bilimbi. Int J Pharm Pharm Sci Res 2013;3:136-9.
12. Lim TK. Averrhoa bilimbi. In:Edible Medicinal and Non-Medicinal Plants. Dordrecht:Springer;2012. 448-53. [CrossRef]
13. Lennox A, Ragoonath J. Carambola and Bilimbi Fruits 1990;45:497-501.
14. Rajan R, Sudhakaran A, Ravindranath A, Sivalingam R, Kumar R. Isolation, screening, and identification of lignin degraders from the gut of termites Odontotermes obesus. J Pure Appl Microbiol2022;16:1696-704. [CrossRef]
15. Rajan R, Ajith S, Joshy A, Ravindranath AD. Bleaching and softening of coir fiber using Averrhoa Bilimbi extract. J Nat Fibers 2020;?19:4477-84. [CrossRef]
16. Bauer S, Ibáñez AB. Rapid determination of cellulose. Biotechnol Bioeng 2014;111:2355-7. [CrossRef]
17. Dampanaboina L, Yuan N, Mendu V. Estimation of crystalline cellulose content of plant biomass using the updegraff method. J Vis Exp 2021;171:e62031. [CrossRef]
18. Lakshmi NS, Babu S, Sebastian S, Ravi PK. Improvement in physical properties of MMA grafted coir fibres. CORD Coconut Res Dev J 2015;31:6. [CrossRef]
19. Lawal AS, Nnadiwa C. Evaluation of wash and light fastness of some selected printed fabrics. J Polym Text Eng 2014;1:1-4.
20. Ravindranath AD, Chithraleka M. Bio-softening of coir yarn for ecofriendly wet processing. CORD 2010;26:10. [CrossRef]
21. de Farias JG, Cavalcante RC, Canabarro BR, Viana HM, Scholz S, Simão RA. Surface lignin removal on coir fibers by plasma treatment for improved adhesion in thermoplastic starch composites. Carbohydr Polym 2017;165:429-36. [CrossRef]
22. Zhang W, Ren X, Lei Q, Wang L. Screening and comparison of lignin degradation microbial consortia from wooden antiques. Molecules 2021;26:2862. [CrossRef]
23. Janusz G, Pawlik A, Sulej J, ?widerska-Burek U, Jarosz-Wilko?azka A, Paszczy?ski A. Lignin degradation:Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 2017;41:941-62. [CrossRef]
24. Sonia MT, Jedidi N, Hassen A. Studies on the ecology of actinomycetes in an agricultural soil amended with organic residues:Identification of the dominant groups of Actinomycetales. World J Microbiol Biotechnol 2011;27:2239-49. [CrossRef]
25. Ke J, Laskar DD, Singh D, Chen, S. In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites:Crucial lignin modification. Biotechnol Biofuels 2011;4:17. [CrossRef]
26. Begum K, Islam MA, Huque MM. Investigation on the tensile and flexural properties of coir-fibre-reinforced polypropylene composites. J Sci Res 2015;7:97-111. [CrossRef]
27. Abdellaoui H, Raji M, Essabir H, Bouhfid R, Qaiss AK. Mechanical behavior of carbon/natural fiber-based hybrid composites. In:Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. United Kingdom:Woodhead Publishing;2019. 103-22. [CrossRef]
28. Dharmaratne PD, Galabada H, Jayasinghe R, Nilmini R, Halwatura RU. Characterization of physical, chemical and mechanical properties of Sri Lankan coir fibers. J Ecol Eng 2021;22:55-65. [CrossRef]
29. Hyness NR, Vignesh NJ, Senthamaraikannan P, Saravanakumar SS, Sanjay MR. Characterization of new natural cellulosic fiber from Heteropogon contortus plant. J Nat Fibers 2018;15:146-53. [CrossRef]