1. INTRODUCTION
Lactic acid bacteria (LAB) are well-known probiotics. They are typically characterized as Gram-positive, non-spore-forming, non-motile, catalase-negative, and acid-producing bacteria growing under micro-aerobic or strictly anaerobic conditions [1]. They are known for their ability to survive in the gastrointestinal (GI) tract, production of lactic acid, anti-microbial substances such as bacteriocins, and other compounds such as hydrogen peroxide, diacyl, and many more. These metabolites are well studied for providing anti-tumor, anti-cancer, and toxin-reducing effects, and protection against antibiotic-associated inflammatory, pathogenic, and metabolic conditions [2-4]. Due to these properties, incorporation of the these probiotic microorganisms in foods has increased rapidly during the last two decades. Consumption of probiotic bacteria through food products and functional beverages is an ideal way to re-establish the balance of the intestinal microbiota [5]. However, with the changing lifestyles, several factors have been reported to affect the viability and probiotic activity of LAB cultures in food and the GI tract such as acidity, pH, dissolved oxygen content, redox potential, and hydrogen peroxide [6]. Therefore, efforts have been intensified to find efficient means to stimulate the growth and survival of probiotic microorganisms in bio-products.
One of the approaches utilized to achieve sufficiently high numbers of desirable microorganisms in the ecosystem of the human GI tract is through the use of prebiotics. Prebiotics are generally oligosaccharides substrates, usually non-viable and non-digestible, that selectively stimulate the beneficial microbiota and can be found naturally in fruits, vegetables, honey, and milk, or they can be produced industrially, by extracting and hydrolyzing hemicellulose derived from lignocellulosic materials [7]. Inulin is one of the most well-known soluble prebiotic, with the ability to reach the large intestine in an intact condition and later hydrolyze in the upper section of the intestine [8]. Earlier studies have demonstrated the effect of inulin, on the growth and viability of different strains of probiotics such as Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium lactis and hence increasingly used while preparing any functional foods [9]. However, the use of inulin at higher concentrations is quite expensive, even more than the use of a specially designed medium for Lactic acid bacteria – Mann-Rogosa-Sharpe (MRS) medium for the growth of these strains [10,11]. Honey includes both prebiotic oligosaccharides and antibacterial components, which can synergistically boost probiotic effectiveness against infections [12]. It not only increases the viable cell count but can also potentially enhance the survival of LAB in the GI tract and exhibit enhanced anti-microbial effect against pathogenic infections [13,14].
The present study is aimed to assess the effect of inulin and different varieties of honey on the shelf life, potential characteristics, and in vitro antibacterial activity of LAB. The study also evaluated the potential of prebiotic-enriched skimmed milk (PESM) as a substitute medium for growth of LAB and antagonistic activity of PESM grown LAB strains against methicillin-resistant Staphylococcus aureus (MRSA).
2. MATERIALS AND METHODS
2.1. Cultures
Three LAB strains comprising of Lactobacillus plantarum NCIM 2374 (NCIB 6376), L. acidophilus NCIM 2660 (ATCC 11975), and Lactobacillus casei var. rhamnosus NCIM 2364 (ATCC 7469) were used for this study. To evaluate their effect on the characteristics of the LAB, inulin (HiMedia, India) and three varieties of honey, namely, (D) Dabur, India, (P) Patanjali, India, and (R) raw honey (from local store at Pune, India), were used as prebiotics in this study. For evaluation of antimicrobial activity, a standard strain of S. aureus (NCIM 2127), and three clinically isolated strains of MRSA were procured from Bharati Hospital, Pune, India. All the LAB strains were maintained in de MRS medium (HiMedia, India), while the Staphylococci strains were enriched with Brain Heart Infusion (BHI) agar (HiMedia, India). All these cultures were incubated overnight at 37°C at still conditions.
2.2. The Growth of LAB
Effect of different varieties of honey and inulin on the growth of each strain of LAB was analyzed by supplementing them with undiluted honey samples and inulin individually, in the concentration of 0.5%, 1.0%, 1.5%, 2.0%, 2.5%, 5.0%, and 10%. All the prebiotic supplemented LAB strains were incubated overnight at 37°C. The flask without any supplementation served as control. Optical density (OD) at 620 nm was measured. Concentration of each of the variety of honey and inulin was recorded and the medium exhibiting the highest growth for each of the LAB strains was considered for further experiments.
2.3. Preparation of LAB Sets
Based on the concentrations of inulin and honey sample showing the highest growth of LAB, the following sets were prepared for further experiments and labeled as follows:
I. L. plantarum
II. L. acidophilus
III. L. casei var. rhamnosus
IV. L. plantarum + 0.5% inulin
V. L. acidophilus + 0.5% inulin
VI. L. casei var. rhamnosus + 0.5% inulin
VII. L. plantarum + 5% honey (Dabur, India)
VIII. L. acidophilus + 5% honey (Dabur, India)
IX. L. casei var. rhamnosus + 5% honey (Dabur, India)
While preparing the sets, each of the medium was inoculated with 1% of LAB strains (6.25 × 104 cfu/mL), and incubated at 37°C for time period based on the experimentation.
2.4. Mean Doubling Time (Td)
The growth of LAB sets was analyzed by measuring the OD620nm of each set periodically after every 8 h. The growth rate was calculated using the equation μ = (ln X2 - ln X1)/(t2 - t1), where X2 and X1 are the initial and final cell concentrations at initial (t1) and final (t2) times. Later, the mean doubling time (Td) was calculated as Td = ln 2/m [15].
2.5. Characteristics of LAB
To understand the effect of inulin and honey on the LAB strains to be able to withstand osmotic stress and survive in the GI tract, its tolerance to change in pH, bile salt concentration, and NaCl concentration were assessed [16].
2.6. Tolerance to Low pH
The pH of the medium for all the sets was adjusted to 2.5 using 0.5 N HCl.
2.7. Tolerance to Bile Salt and NaCl
Tolerance to bile salt and NaCl was observed by the addition of 0.2%, 0.3%, and 0.5% w/v bile salt, and 5%, 8%, 10%, 12%, and 15% w/v NaCl separately to the medium for all the sets.
All the inoculated sets were incubated for 18–20 h at 37°C (still) and observed for growth by measuring OD at 620 nm.
2.8. Cell Surface Hydrophobicity
The effect of inulin and honey, on the adherence ability of LAB, was evaluated by testing the cell surface hydrophobicity of the LAB sets [17,18]. The cells from all the sets were harvested by centrifuging 5 mL of the medium at 4000 g at 4°C for 15 min. The cell pellets were washed twice with standard phosphate-buffered saline and resuspended in 12 mL potassium nitrate (KNO3) solution (0.1 mol/dm3; pH 6.2). The initial absorbance of the cell suspensions was measured at 620 nm (A0).
Each LAB set suspension was tested for its ability to adhere to the surface in presence of three solvents – xylene (non-polar solvent), ethyl acetate (polar basic solvent), and chloroform (polar acidic solvent). The solvent-suspension mixtures were prepared by adding 1ml of each of the solvent to 3 mL of each of the LAB sets individually. 1 ml of each solvent was mixed with 3 ml of MRS medium as a control. The solvent-suspension mixtures were incubated for 10 min at 37°C, mixed vigorously for 2 min, and again incubated for 20 min at 37°C. The aqueous phase was then removed from the mixtures, and the absorbance of the remainder was measured at 620 nm.
The percentage microbial adhesion to solvents (MATS) was calculated as MATS (%) = 1 − (A?/A?) × 100; where A? = Absorbance of the aqueous layer and A? = Absorbance of control.
2.9. The Growth of LAB in Skimmed Milk
The effect of inulin and honey on the growth of the LAB strains in skimmed milk was analyzed by inoculating 1% of LAB sets, I to IX (6.25 × 104 cfu/ml each) in pasteurized skimmed milk (separately), and incubating at 37°C overnight. Later, the viability of LAB was observed by storing these sets at 4°C for 5 weeks. Each set was diluted (1:10 v/v) with sterile 0.1% peptone water and serially diluted. After incubation for 18 h, the colonies were enumerated on MRS agar plates. Percent viability was calculated as follows [19]:
% viability = (Final cfu/ml after storage/initial cfu/ml) × 100
2.10. Antibacterial Activity of LAB
The antibacterial activity of the LAB strains grown in the presence of honey and inulin was evaluated. Each of the honey and inulin supplemented sets (I–IX) were prepared in skimmed milk individually and incubated overnight at 37°C. The antagonistic activity was determined using the agar well diffusion method as described by Bhola and Bhadekar (2019) [20]. The media plates were seeded with the clinically obtained MRSA isolates (A, B, and C), and S. aureus culture (D), individually. All the plates were kept for diffusion for 1 h at room temperature, post which they were incubated overnight at 37°C. Zone diameters (mm) were measured according to Barry et al. (1979) [21].
2.11. Statistical Analysis
All the experiments were performed in triplicates independently and the results were represented as an average with standard deviations and probability values to understand the level of significance.
3. RESULTS AND DISCUSSION
The unique health benefits of honey have made it a potential substitute for research, aiming to achieve a positive microbial balance in the GI tract and potentially contribute to the industry of functional foods. With this objective, the study aimed to evaluate the potential of honey as a prebiotic substitute for inulin.
The LAB strains were grown individually and measured for turbidity in the medium. It was observed that in the presence of 5% (D) honey, all three LAB strains exhibited an OD620 ranging from 0.97 to 0.99, post which it declined with an increase in the concentration. The OD620 for the LAB strains inoculated with (P) and (R) honey was observed to be in a range from 0.17 to 0.33, and 0.26 to 0.29, respectively. In the presence of 0.5% inulin, all three LAB strains showed the highest OD ranging from 0.83 to 0.96. The growth of all the prebiotic supplemented medium was observed to be more than the control [Figure 1]. Based on these observations, 5% (D) honey and 0.5% inulin were used for further experiments. Similar results were obtained by Chick et al. (2001), where Streptococcus thermophilus, Lactobacillus delbrueckii sub spp. bulgaricus, and L. acidophilus were able to grow in the presence of honey [22]. Inulin also showed a positive effect on the growth of LAB strains at the concentration of 0.5% w/v, which was significantly higher than the growth in control conditions [23,24].
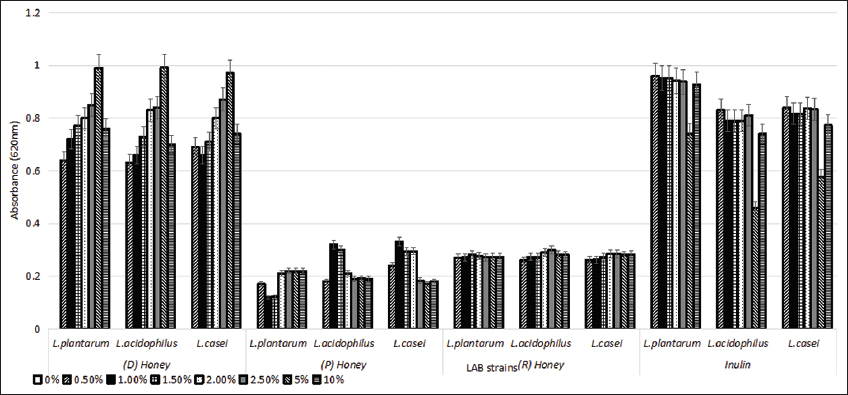 | Figure 1: Growth of lactic acid bacteria in presence of different concentrations of honey ([D]: Dabur, [P]: Patanjali, [R]: Raw), and inulin. Standard deviation bars derived from the mean of data from three independent experiments. [Click here to view] |
The calculated growth rate revealed that prebiotic-enriched LAB strains had a significantly lower mean doubling time (P < 0.05) control LAB strains, meaning that both, (D) honey and inulin, which exerted a prebiotic effect on the LAB strains. As represented in Figure 2, a decrease of 6.38%, 8.7%, and 18.37% was observed in the mean doubling time of inulin supplemented sets IV, V, and VI as compared to the Td of respective control sets I, II, and III. Decline in the mean doubling time of honey supplemented sets VII, VIII, and IX was 46.81%, 56.52%, and 57.75% as compared to the Td of respective control sets I, II, and III. The mean doubling time of the control sets was observed to be 4.7, 4,6, and 4.9 h respectively. Comparable results were observed in the past by many researchers where supplementation of prebiotics like inulin significantly reduced the mean doubling time of probiotic cultures [25,26]. However, very few studies were found on the effect of honey on the mean doubling time of lactobacilli strains. The decreased doubling time suggests that 5% (D) honey, similarly to inulin, can stimulate the growth of LAB in products during the manufacturing and storage of probiotic foods and beverages [27].
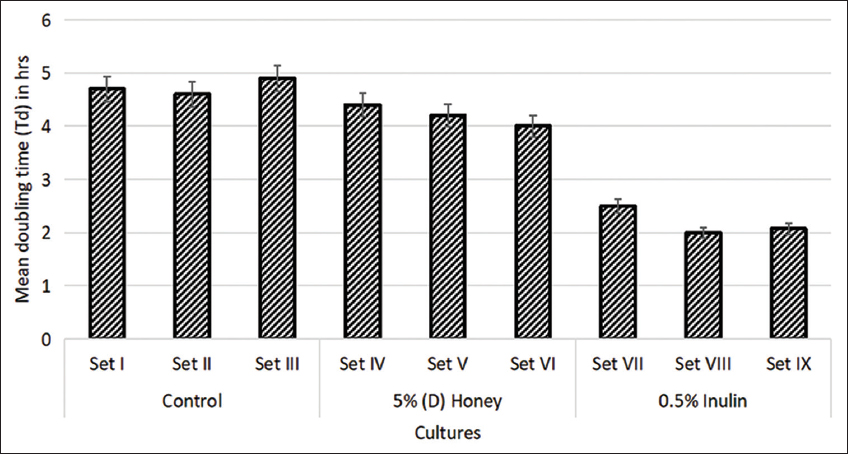 | Figure 2: Mean doubling time of lactic acid bacteria strains in present of 5% Dabur honey (d) and 0.5% inulin. Standard deviation bars derived from the mean of data from three independent experiments, with P value derived with significant difference with respective controls. [Click here to view] |
Probiotic bacteria are mostly delivered in food systems and survive in the human GI tract. However, conditions such as low pH and osmotic stress can hinder the growth, viability, and fermentation processes of Lactobacillus spp. [28,29]. The LAB sets were exposed to pH 2.5 for different time intervals and examined for growth. The TVC (×1013) of (D) honey-enriched sets IV, V, and VI increased to 1.282, 1.368, and 1.2 after 4 h, and inulin-enriched sets VII, VIII, and IX increased to 1.2, 1.297, and 1.272 after 4 h of incubation at 37°C. The TVC of all the prebiotic-enriched sets was higher than the control set (P < 0.05) and was observed to be persistent after 4 h of incubation time [Figure 3]. This showed an enhancement to the results obtained by Dixit et al. (2013), where the growth of L. acidophilus NCIM 2660, without any prebiotic, was observed to increase post 2 h of incubation and remained constant for 6 h [30]. Furthermore, past studies by Urdaneta and Casadesús (2017) and Guan et al. (2017) revealed that the growth rate of LAB strains increased in low pH for 2–3 h of incubation, post which no further growth was observed [31,32].
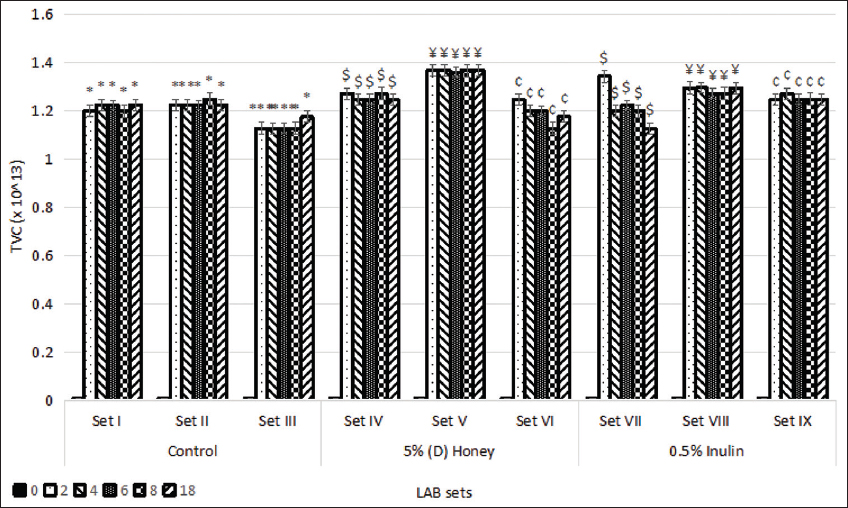 | Figure 3: Growth of lactic acid bacteria strains at pH 2.5 at different time intervals (h). Standard deviation bars derived from the mean of data from three independent experiments with * indicating P < 0.05, *** indicating P < 0.01, $ indicating P < 0.05 as compared to corresponding time intervals from Set I, ¥ indicating P < 0.05 as compared to corresponding time intervals from Set II, and ¢ indicating P < 0.05 as compared to corresponding time intervals from Set III. [Click here to view] |
As represented in Figure 4, the average absorbance of the control sets I, II, and III significantly dropped (P < 0.05) with the increase in bile salt concentration from 0.2% to 0.4%. The average TVC (×1013) of (D) honey-enriched sets was observed to be 0.722 which was higher (P < 0.05) than the control at 0.4% concentration of bile salt. Inulin-enriched sets also showed significantly higher tolerance to bile salts with an average TVC (−1013) of 0.87, 0.857, and 0.377 at 0.2%, 0.3%, and 0.4% bile salt concentration, respectively. Tolerance to bile salts has been viewed as a condition for colonization and metabolic action of probiotics in the host’s digestive system, affecting the intestinal microflora [33].
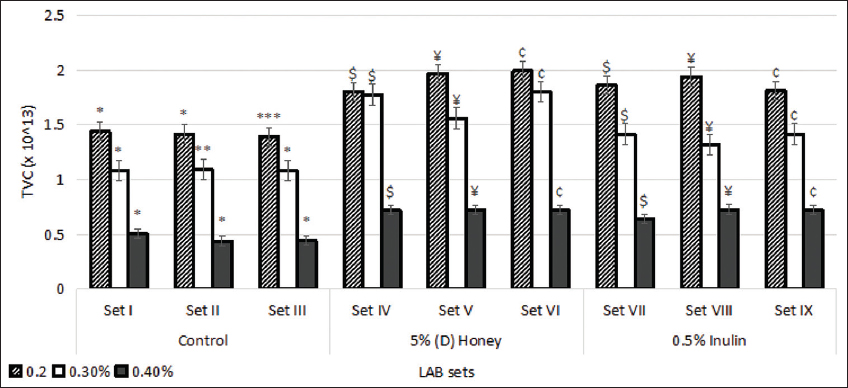 | Figure 4: Effect of different concentrations of bile salts on the growth of lactic acid bacteria strains. Standard deviation bars derived from the mean of data from three independent experiments with * indicating P < 0.05, *** indicating P < 0.01, $ indicating P < 0.05 as compared to corresponding concentration from Set I, ¥ indicating P < 0.05 as compared to corresponding concentration from Set II, and ¢ indicating P < 0.05 as compared to corresponding concentration from Set III. [Click here to view] |
The LAB sets were exposed to NaCl at the concentrations of 5%, 8%, 10%, 12%, and 15%. As represented in Figure 5, the average TVC (×1013) of the sets IV, V, and VI were observed to be 1.525, 1.531, and 1.411, respectively, compared to the average TVC (×1013) of control sets I, II, and III, of 0.696, 0.657, and 0.701, respectively. Similar results were observed for inulin-enriched sets VII, VIII, and IX with their average TVC (×1013) values of 1.432, 1.248, and 1.32, respectively. Furthermore, it was observed that the (D) honey-enriched sets were significantly more tolerant to NaCl at higher concentrations of 10%, 12%, and 15% than the inulin-enriched sets. NaCl is the most commonly used additive in fermented foods for the prevention of spoilage. Prebiotics are known to serve as a carbon source to the selected LAB strains, improving their tolerance to acids and salts, and indicating their ability to survive in the harsh GI environment and fermentation processes [34,35].
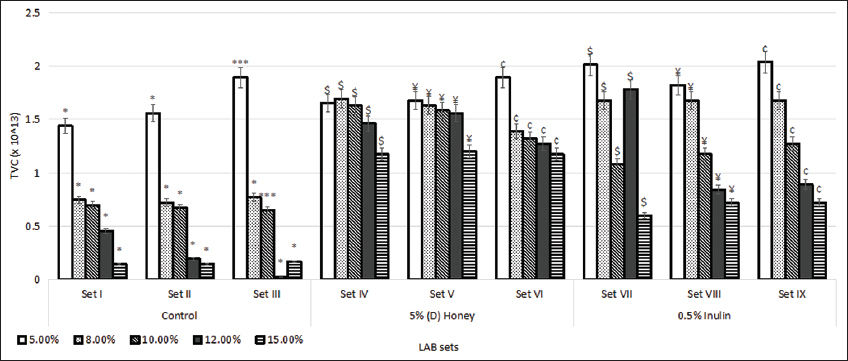 | Figure 5: Effect of different concentrations of NaCl on the growth of lactic acid bacteria strains. Standard deviation bars derived from the mean of data from three independent experiments with * indicating P < 0.05, *** indicating P < 0.01, $ indicating P < 0.05 as compared to corresponding concentration from Set I, ¥ indicating P < 0.05 as compared to corresponding concentration from Set II, and ¢ indicating P < 0.05 as compared to corresponding concentration from Set III. [Click here to view] |
The hydrophobic nature of lactobacilli sets was evaluated with xylene, ethyl acetate, and chloroform as solvents to assess the hydrophobic, electron donor (basic), and electron acceptor (acidic) characteristics of the LAB surface, which are attributed to carboxylic groups and Lewis acid-base interactions [36]. As presented in Figure 6, the sets had more than 50% hydrophobicity toward xylene. However, the average microbial adhesion to xylene was lowest for (D) honey-enriched LAB sets (66.83%) as compared to control LAB sets. Inulin-enriched sets exhibited 72.54% of adhesion in an average. The assay also revealed that all the sets had a higher affinity toward chloroform in the range of 78.96–90.46% and had the least affinity toward ethyl acetate in the range of 26.78–38.46%. According to Giarous et al. (2009), LAB strains should present a hydrophobic surface for a high capacity of adhesion to intestinal cells and solid materials [37]. Many previous studies have shown that the presence of (glyco-)proteinaceous material at the cell surface results in higher hydrophobicity, whereas hydrophilic surfaces are associated with the presence of polysaccharides [36,38]. The strains had a high affinity toward chloroform, indicating probiotic strains have the non-acidic and poor electron acceptor properties [39].
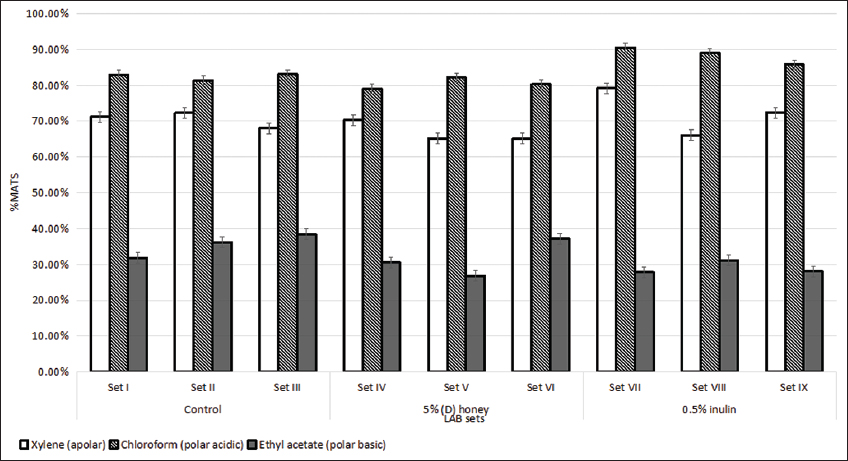 | Figure 6: Percent cell surface adhesion of lactic acid bacteria strains. Standard deviation bars derived from the mean of data from three independent experiments with P < 0.05. [Click here to view] |
With MRS medium being expensive, the growth and viability of LAB strain in skimmed milk as a cost-effective alternative was tested. The percentage viability of the sets in skimmed milk was evaluated after 5 weeks of incubation. As seen in Figure 7, the viability of the (D) honey-enriched sets IV, V, and VI, was 1.63, 2.31, and 2.51 times higher than the control sets showing 35%, 27.65%, and 24.34% viability, respectively. Inulin-enriched sets also showed 2.44 times higher viability on an average as compared to the control sets after 5 weeks of refrigerated storage. A study conducted earlier had observed a similar growth rate and viability of LAB strains in milk and milk-based products [40].
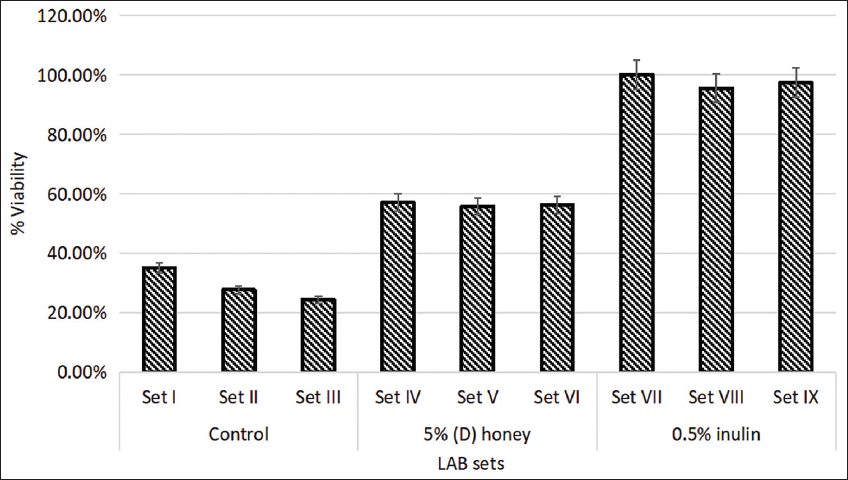 | Figure 7: Percent viability of lactic acid bacteria strains in prebiotic-enriched skimmed milk post 5 weeks. Standard deviation bars derived from the mean of data from three independent experiments with P < 0.05. [Click here to view] |
Agar well diffusion procedure was performed with all the LAB sets grown in PESM, and the zone diameters were measured against clinical isolates of MRSA. The zone diameters of these LAB sets were significantly higher than the control sets, showing an average zone diameter of 19.3 mm and 14.2 mm for standard S. aureus strain and MRSA strains, respectively. Honey-enriched LAB sets exhibited average zone diameters of 19.7 mm and 16 mm for standard S. aureus strain and MRSA strains, respectively. Inulin-enriched sets were able to show an average zone diameter of 19.3 mm and 17.7 mm for standard S. aureus strain and MRSA strains, respectively [Table 1]. The data were analyzed statistically using two-way ANOVA test and p value obtained was <5%. These results were compared to an earlier study observing an average zone diameter of 11.5 mm of LAB strains grown in MRS medium against S. aureus NCIM 2127 [20]. Skimmed milk serves as a potential growth environment for LAB strains, enabling them to produce antimicrobial bacteriocins and compounds at a higher concentration. Supplementation of (D) honey further enhanced the anti-MRSA activity of these LAB strains suggesting its potential to serve as a prebiotic and provide holistic metabolic benefits to gut microbiota.
Table 1: Diameters of growth inhibition zone of MRSA isolates by LAB strains grown in PESM
| MRSA | Control | 5% (D) Honey | 0.5% Inulin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| isolates | Set I | Set II | Set III | Set IV | Set V | Set VI | Set VII | Set VIII | Set IX | |
| A | 10 ± 0.5 | 12 ± 0.34 | 15 ± 0.46 | 17 ± 0.12 | 15 ± 0.17 | 16 ± 0.17 | 18 ± 0.5 | 16 ± 0.47 | 19 ± 0.5 | |
| B | 13 ± 0.46 | 15 ± 0.5 | 17 ± 0.34 | 15 ± 0.46 | 14 ± 0.3 | 18 ± 0.5 | 16 ± 0.42 | 17 ± 0.16 | 17 ± 0.27 | |
| C | 15 ± 0.27 | 15 ± 0.41 | 16 ± 0.27 | 15 ± 0.5 | 19 ± 0.5 | 15 ± 0.3 | 19 ± 0.56 | 15 ± 0.34 | 22 ± 0.5 | |
| D | 17 ± 0.24 | 19 ± 0.16 | 22 ± 0.18 | 18± 0.24 | 17 ± 0.24 | 24 ± 0.5 | 21± 0.5 | 19 ± 0.5 | 21± 0.27 | |
± : Standard deviation values derived from the mean of data from three independent experiments (F value: 16.34)
4. CONCLUSION
Dietary applications of probiotic strains and non-digestible oligosaccharides aim to improve gut microflora and promote a more beneficial bacterial community. Honey can be identified as a potential prebiotic due to the presence of oligosaccharides that can promote the growth of lactobacilli, as well as antimicrobial components that can work in tandem with probiotics to combat certain pathogens. This synbiotic combination has the potential to improve the distinct health benefits of probiotic microorganisms, while also presenting opportunities for the development of novel functional foods.
5. ACKNOWLEDGMENT
We gratefully acknowledge the support from Bharati Vidyapeeth (deemed to be) University to undertake this work. We are also thankful to Microbiology Department of Bharati Hospital for providing clinical isolates.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
No funding was available for the study.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Mokoena MP. Lactic acid bacteria and their bacteriocins:Classification, biosynthesis and applications against uropathogens:A mini-review. Molecules 2017;22:1255. [CrossRef]
2. Ren D, Zhu J, Gong S, Liu H, Yu H. Antimicrobial characteristics of lactic acid bacteria isolated from homemade fermented foods. Biomed Res Int 2018;2018:5416725. [CrossRef]
3. Nazir Y, Hussain SA, Hamid AA, Song Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. Biomed Res Int 2018;2018:3428437. [CrossRef]
4. Zommiti M, Feuilloley MG, Connil N. Update of probiotics in human world:A nonstop source of benefactions till the end of time. Microorganisms 2020;8:1907. [CrossRef]
5. Dahiya D, Nigam PS. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms 2022;10:665. [CrossRef]
6. Terpou A, Papadaki A, Lappa I, Kachrimanidou V, Bosnea L, Kopsahelis N. Probiotics in food systems:Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019;11:1591. [CrossRef]
7. De Figueiredo FC, De Oliva-Neto P. Advances and new perspectives in prebiotic, probiotic and symbiotic products for food nutrition and feed. In:Brienzo M, editors. Hemicellulose Biorefinery:A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy. Clean Energy Production Technologies. Singapore:Springer;2022. 311-36. [CrossRef]
8. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi S,
9. Palanivelu J, Thanigaivel S, Vickram S, Dey N, Mihaylova D, Desseva I. Probiotics in functional foods:Survival assessment and approaches for improved viability. Appl Sci 2022;12:455. [CrossRef]
10. De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol 1960;23:130-5. [CrossRef]
11. Hayek SA, Gyawali R, Aljaloud SO, Krastanov A, Ibrahim SA. Cultivation media for lactic acid bacteria used in dairy products. J Dairy Res 2019;86:490-502. [CrossRef]
12. Mohan A, Quek SY, Gutierrez-Maddox N, Gao Y, Shu Q. Effect of honey in improving the gut microbial balance. Food Qual Saf 2017;1:107-15. [CrossRef]
13. Rastall RA, Diez-Municio M, Forssten SD, Hamaker B, Meynier A, Moreno FJ,
14. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr 2019;10:S49-66. [CrossRef]
15. Charioui I, Chikhaoui M, Filali FE, Abbassi M, Banaoui A, Kaaya A. Effect of the medium culture on cells growth and accumulation of carotenoids in microalgae hypersaline
16. Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S,
17. Xu H, Jeong HS, Lee HY, Ahn J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett Appl Microbiol 2009;49:434-42. [CrossRef]
18. Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons:A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 1980;9:29-33. [CrossRef]
19. Mani-López E, Palou E, López-Malo A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J Dairy Sci 2014;97:2578-90. [CrossRef]
20. Bhola J, Bhadekar R.
21. Barry AL, Coyle MB, Thornsberry C, Gerlach EH, Hawkinson RW. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J Clin Microbiol 1979;10:885-9. [CrossRef]
22. Chick H, Shin HS, Ustunol Z. Growth and acid production by lactic acid bacteria and bifidobacteria grown in skim milk containing honey. J Food Sci 2001;66:478-81. [CrossRef]
23. Darilmaz DO, Sönmez ?, Beyatli Y. The effects of inulin as a prebiotic supplement and the synbiotic interactions of probiotics to improve oxalate degrading activity. Int J Food Sci Technol 2019;54:121-31. [CrossRef]
24. Nagpal R, Kaur A. Synbiotic effect of various prebiotics on
25. Massot-Cladera M, Azagra-Boronat I, Franch À, Castell M, Rodríguez-Lagunas MJ, Pérez-Cano FJ. Gut health-promoting benefits of a dietary supplement of vitamins with inulin and acacia fibers in rats. Nutrients 2020;12:2196. [CrossRef]
26. Chungada AS, Deshpande KG, Thakre RA. Pursuance of skim milk containing prebiotics on survival and activity of probiotics lactobacilli bacteria. Int J Curr Microbiol App Sci 2018;7:1311-21. [CrossRef]
27. Desai AR, Powell IB, Shah NP. Survival and activity of probiotic lactobacilli in skim milk containing prebiotics. J Food Sci 2006;69:FMS57-60. [CrossRef]
28. Ilha EC, Da Silva T, Lorenz JG, Rocha GO, Sant'Anna ES. Lactobacillus paracasei isolated from grape sourdough:Acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur Food Res Technol 2015;240:977-84. [CrossRef]
29. Kandola S. Investigation of bile tolerance and deconjugation ability of various
30. Dixit G, Samarth D, Tale V, Bhadekar R. Comparative studies on potential probiotic characteristics of
31. Guan X, Xu Q, Zheng Y, Qian L, Lin B. Screening and characterization of lactic acid bacterial strains that produce fermented milk and reduce cholesterol levels. Braz J Microbiol 2017;48:730-9. [CrossRef]
32. Urdaneta V, Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med (Lausanne)2017;4:163. [CrossRef]
33. Yokota A. Comprehensive studies on the interactions between bile acid and lactic acid bacteria/bifidobactria/gut microbes. Jpn J Lactic Acid Bact 2019;30:143-52. [CrossRef]
34. Bao Y, Zhang Y, Li H, Liu Y, Wang S, Dong X,
35. Berrada N, Lemeland JF, Laroche G, Thouvenot P, Piaia M.
36. Kos B, Šuškovi?J, Vukovi?S, Šimpraga M, Frece J, Matoši?S. Adhesion and aggregation ability of probiotic strain
37. Giaouris E, Chapot-Chartier MP, Briandet R. Surface physicochemical analysis of natural
38. Hanczakowska E, Swiatkiewicz M. Effect of herbal extracts on piglet performance and small intestinal epithelial villi. Czech J Anim Sci 2012;57:420-9. [CrossRef]
39. Bhagat D, Raina N, Kumar A, Katoch M, Khajuria Y, Slathia PS,
40. Bovo F, Franco LT, Rosim RE, De CA. Ability of a