1. INTRODUCTION
Mushrooms, higher fungi, or macrofungi are ubiquitous in nature. They are Basidiomycetous or Ascomycetous with a system of branching mycelia and distinct fruiting bodies that can be seen by the naked eye. In nature, mushrooms act as saprophytic, parasitic, and symbiotic (mycorrhiza), which play major role in the decomposition of massive forest litters, cycling of nutrients, and maintenance of soil fertility and ecological balance. They are indispensable partner of major timber species in the forest. Mushrooms have been traditionally until recently exploited for culinary and medicinal purposes. They are rich in carbohydrates, proteins, fibers, vitamins, minerals, and low-fat content [1,2]. Polysaccharides, β-glucan polymers whose main chains consist of β-(1→3) linkages with β-(1→6) branches [3], are one of the most important components of mushroom. Organic acids, alkaloids, terpenoids, steroids, phenolic compounds, and flavonoids have been reported for their promising application in a wide variety of industries, including food, agriculture, cosmetics, and pharmaceuticals [4]. Phenolic compounds (quercetin, catechin, myricetin, pyrogallol, and caffeic acid), carotenoids, ergosterols, tocopherols, ascorbic acid, terpenes, and polysaccharides present in edible mushrooms which showed antioxidant, anti-inflammatory, and anticancer activities [5]. Active compounds of Ganoderma lucidum such as polysaccharides, terpenoids, proteins, fatty acids, nucleotides, sterols, steroids, and vitamins showed antidiabetic, anti-oxidant, anticancer, anti-atherosclerotic, anti-inflammatory, antimicrobial, antiangiogenic, anti-arthritic, anti-herpetic, anti-nociceptive, anti-androgenic, antiaging, antiulcer, anti-fibrotic, anti-osteoporotic, hepatoprotective, hypolipidemic, chemopreventive, analgesic, immunomodulatory, and estrogenic activities [6].
The first mushroom that evolved on Earth between 715 and 810 million years ago from the Mbuji-Mayi Supergroup, Democratic Republic of Congo was discovered [7]. So far, the properly estimated total number of species in Kingdom Fungi is between 2.2 and 3.8 million [8], and currently, there are 150,238 recognized fungal species in Species Fungorum [9]. In Phylum Ascomycota, Wijayawardene et al. [10] provided notes on 6540 genera, 485 families, 115 orders and 17 classes, and updated the accepted genera by adding new 206 entries [11]. However, He et al. [12] published their notes and outline on 1928 genera with 1263 synonyms, 241 families, 68 orders, 18 classes, and four subphyla of Phylum Basidiomycota. In 2019, 1882 new fungal species, 214 new genera, 48 new families, 18 new orders, and three new classes have been recorded [13]. The top three fungal orders with the highest number of new species in 2019 include Hypocreales (199 species), Pleosporales (198 species) (both under Ascomycota), and Agaricales (141 species), which are under Basidiomycota [14]. According to the first global assessment on wild edible fungi by Food and Agriculture Organization in 2004, there were 2327 wild useful species recorded, 2166 edible species, 1069 species used as food, 470 species reported to have medicinal properties, and 181 species for other valuable uses [15]. Recently, there were more than 2000 edible and/or medicinal mushrooms that have been characterized [16].
The earliest records of fungi in the Philippines can be found in the writings of Spanish botanists and reports of various US and French expeditions, and most of these recorded fungi belong to Basidiomycetes, particularly Agaricales and Polyporales [17]. In 1920, Reinking [18] accounted the higher Basidiomycetes of the Philippines and their host. Teodoro [19], one of the Filipino pioneers in mycology, recorded the first listing of published and unpublished Philippine fungi up to 1935. After World War 2 in 1942, Filipino mycologists continued to survey and collect major groups of macrofungi such as Clavariaceae [20], Gasteromycetes [21], Discomycetes [22], Agaricales [23-29], and Auriculariales [30]. In 1986, Quimio [17] published the records of Philippine fungi, consisting of 672 species, based on the available reports in 1937–1977. Of which, only species of Pezizales (3), Hymenogasterales (6), Tremellales (1), Auriculariales (3), Aphyllophorales (33), Agaricales (55), Lycoperdales (22), Phallales (14), Nidulariales (2), and Sclerodermatales (11) were listed. Most of the above-mentioned groups of wild mushrooms were surveyed and collected from Mt. Makiling, Laguna, Philippines. Accordingly, in the past century, reports regarding wild mushroom diversity rarely exist due to very few mycologists, fungal taxonomists, and mushroomologists in the country. In 2002, Quimio [31] established the checklist and database of fungi in the Philippines based on published and unpublished records from 1806 to 2001. Since the last checklist of Quimio [31], there were no comprehensive and updated records of Philippine mushrooms available. At the turn of the 21st century, published works relating to ethnomycology and biodiversity of Philippine wild mushrooms continue to expand due to the increasing demand and interest for nutritious and medicinal foods.
In this review, we aimed to establish the most comprehensive checklist of naturally occurring mushrooms in the Philippines based on the available reports from 2001 to 2021. This review paper also aims to provide benchmark information about wild useful Philippine mushrooms for conservation and further exploration of their various applications.
2. NATURAL HABITAT OF PHILIPPINE WILD MUSHROOMS
The Philippines being an archipelagic country is composed of 7,107 islands, which is surrounded by main bodies of water in Southeast Asia including Philippine Sea, Luzon strait, South China Sea, Sulu Sea, and Celebes Sea. The largest island is Luzon Island in the north followed by Mindanao Island in the south. The country is generally mountainous with fertile plains, has numerous dormant and active volcanoes, has hills and valleys crossed by rivers, and miles of natural coastline.
The climate of the Philippines is tropical and maritime. It has relatively high humidity, temperature, and precipitation. The mean annual temperature of the entire country is 26°C, except Baguio City (with an altitude 1500 masl) with 18.3°C. The month January is the coolest, while May is the warmest. The relative humidity ranges from 71% (in March) to 85% (in September). The mean annual rainfall varies from 965 to 4064 milliliters annually. The rainfall distribution varies from one region to another depending on the direction of moisture-bearing winds and mountain systems. The climate of the archipelago can be divided into two major seasons: (1) rainy season, from June to November; and (2) dry season, from December to May [32].
The Philippines has four types of climates depending on the distribution of rainfall and the period of the dry season. Type I is characterized by having dry season from November to April and wet during the rest of the year. Type II has no dry season with a very pronounced maximum rain period from December to February. Type III has no very pronounced maximum rain period, with a short dry season lasting only from 1 to 3 months, either during the period from December to February or from March to May. Type IV has no dry season; rainfall is more or less evenly distributed throughout the year. The different regions of the country under the above-mentioned climate types are summarized by Lantican (2001), citing Kintanar (1984) [33,34].
Being one of the mega-biodiverse countries of the world, the Philippines is composed of two-thirds of the Earth’s biodiversity and it ranks fifth in the number of plant species and maintains 5% of the world’s flora [35]. Its unique tropical forest serves as a haven of different species of mammals, birds, reptiles, amphibians, and other organisms. The forest ecosystem also provides large benefits to increasing population of Filipinos. In some areas of the country, forest ecosystem is over-exploited due to commercial operations, conversion to agricultural land, and promotion of extractive industries such as mining, introduction of invasive alien species, and other activities. With these notable disturbances in the forest ecosystem, there is possibility that some of the wild genetic resources become extinct. Therefore, before extinction, it is indeed imperative to assess the Philippine biodiversity, particularly macrofungal species, and rescue their cell lines for possible conservation and utilization.
Herein, we listed the available reports on Philippine wild mushrooms from 2001 to 2021. The origin or source of wild mushrooms, where they were collected, the number of mushroom families and species identified, method of identification, and the corresponding references are provided in Table 1. Apparently, biodiversity survey of wild mushrooms in the Philippines has been mainly conducted and focused in many provinces of Luzon Island such as Nueva Ecija, Tarlac, Aurora, Bataan, Bulacan, Zambales, Pampanga, Nueva Vizcaya, Isabela, Cagayan, La Union, Ilocos Norte, Benguet, Ifugao, Mountain Province, Laguna, Quezon Province, Cavite, Rizal, Batangas, Bicol, Camarines Sur, and Palawan. However, very few works were found in the regions of Visayas (Municipalities of Northern Samar) and Mindanao (Davao Oriental, Dinagat Island, Agusan del Sur, Bukidnon, Surigao del Norte, Camiguin, Misamis Oriental, Cagayan de Oro).
Table 1: List of available reports on Philippine wild mushrooms from 2001 to 2021 with the place of collection, number of mushroom species identified, method of identification, and the corresponding references.
| Island | Origin/Source | No. of species | Method of identification | References |
|---|---|---|---|---|
| Luzon | Barangay Poblacion of Paracelis, Mountain Province | 29 | Morphological | [40] |
| Central Luzon State University Campus, Science City of Muñoz, Nueva Ecija | 1 | Molecular | [41] | |
| Mount Banahaw-San Cristobal Protected Landscape, Provinces of Laguna and Quezon; Mt. Makiling Forest Reserve in Laguna-Batangas; Municipalities of Pagbilao, Padre Burgos, and Atimonan, Quezon Province, Philippines | 72 | Morphological | [42] | |
| Bombongan–Lewin Subwatershed, Laguna | 163 | Morphological | [37] | |
| Northeastern Side of Quezon Protected Landscape, Southern Luzon | 53 | Morphological | [43] | |
| Municipalities of Bauko, Mt. Province, Buguias and Mankayan, Benguet | 6 | Morphological | [44] | |
| Paracelis, Mountain Province | 1 | Morphological | [45] | |
| Lingap Kalikasan Park, CLSU Campus, Science City of Muñoz, Nueva Ecija | 1 | Morphological | [46] | |
| Sitio Canding, Barangay Maasin, Municipality of San Clemente, Tarlac | 72 | Morphological (37) and Molecular (35) | [38] | |
| Lupao, Nueva Ecija | 1 | Molecular | [47] | |
| Central Luzon State University, Science City of Munoz, Nueva Ecija | 1 | Molecular | [48] | |
| Bicol University Kalikasan Forest Park, Legazpi City, Bicol | 39 | Morphological | [49] | |
| Consocep and Isarog Mountain, Camarines Sur | 36 | Morphological | [50] | |
| Mt. Umubi, Alfonso Castañeda, Nueva Vizcaya | 45 | Morphological | [51] | |
| Molave Forest, San Fernando City, La Union | 56 | Morphological | [52] | |
| Mt. Palemlem, Ilocos Norte | 133 | Morphological | [53] | |
| Northeastern slopes of Mt. Pao, Adams, Ilocos Norte | 120 | Morphological | [54] | |
| Municipalities of Banaue, Hunduan, Mayoyao, Province of Ifugao | 109 | Morphological (74) and Molecular (35) | [39] | |
| Cagayan State University Campus, Lallo, Cagayan | 34 | Morphological | [55] | |
| Lagawe, Ifugao, Cordillera Autonomous Region | 29 | Morphological | [56] | |
| Mt. Maculot, Cuenca, Batangas | 92 | Morphological | [57] | |
| Central Luzon State University Campus, Science City of Munoz, Nueva Ecija | 35 | Morphological | [58] | |
| Sitio Pastolan, Hermosa, Bataan | 7 | Morphological | [59] | |
| Mt. Mingan, Gabaldon, Nueva Ecija | 4 | Molecular | [60] | |
| Mt. Makiling, Laguna | 21 | Morphological | [61] | |
| Mt. Makiling, Laguna | 1 | Morphological | [62] | |
| Mt Makiling, Laguna | 3 | Morphological | [63] | |
| Sitio Pawac, and Sitio Binantag, Masoc, Bayombong, Nueva Vizcaya | 76 | Morphological (67) and Molecular (9) | [64] | |
| Mt. Palali, Quezon, Nueva Vizcaya | 1 | Morphological | [65] | |
| Sitio Pastolan, Barangay Payangan and Barangay Tubo-tubo, Bataan | 7 | Morphological | [66] | |
| Angat Watershed, Norzagaray, Bulacan | 21 | Morphological | [67] | |
| Isabela State University Campus, Echague, Isabela | 3 | Morphological | [68] | |
| Agro-ecosystem in Brgy. Bambanaba, Cuyapo, Nueva Ecija | 30 | Morphological | [69] | |
| Isabela State University Campus, Echague, Isabela | 31 | Morphological | [70] | |
| Cavite, Batangas, Quezon, Laguna, Rizal | 8 | Molecular | [71] | |
| Central Luzon State University Campus, Science City of Munoz, Nueva Ecija | 1 | Morphological | [72] | |
| Central Luzon State University Campus, Science City of Munoz, Nueva Ecija | 1 | Morphological | [73] | |
| Borders of Nasugbu, Batangas, and Ternate, Cavite in Southern Luzon | 41 | Morphological | [74] | |
| Mt. Makiling Forest Reserve, Los Baños, Laguna | 1 | Morphological | [75] | |
| Sitio Binbin, Brgy. General Luna, Carranglan, Nueva Ecija | 6 | Molecular | [76] | |
| Selected Areas in Central Luzon | 8 | Morphological | [77] | |
| Barangay Dampulan, Barangay Langla and Barangay Putlod, Jaen, Nueva Ecija | 5 | Molecular | [78] | |
| Central Luzon | 2 | Molecular | [79] | |
| Mt. Bangkay, Cuyapo, Nueva Ecija | 5 | Molecular | [80] | |
| Ternate and Maragondon, Cavite and Nasugbu, Batangas | 95 | Morphological | [81] | |
| Mt. Makiling Forest Reserve, Los Baños, Laguna | 20 | Morphological | [82] | |
| Solano, Bayombong and Bagabag in Nueva Vizcaya | 7 | Morphological | [83] | |
| Tayabas, Quezon and Majayjay, Laguna | 62 | Morphological | [84] | |
| Cavinti Underground River and Cave Complex, Cavinti, Laguna | 41 | Morphological | [85] | |
| City of San Fernando, La Union | 51 | Morphological | [86] | |
| Floridablanca, Pampanga; Capas, Tarlac and Botolan, Zambales | 76 | Morphological (69) and Molecular (7) | [87] | |
| Taal Volcano, Talisay area, Batangas | 75 | Morphological | [88] | |
| Provinces of Pampanga, Tarlac and Zambales | 14 | Morphological | [89] | |
| Mandaluyong and Tagaytay | 3 | Morphological | [90] | |
| Bazal-Baubo Watershed, Aurora | 107 | Morphological | [91] | |
| Puncan, Carranglan, Nueva Ecija | 7 | Morphological | [92] | |
| Mt. Makulot, Cuenca, Batangas | 97 | Morphological | [93] | |
| Mt. Makiling Forest Reserve, Laguna and Batangas | 27 | Morphological | [94] | |
| Central Luzon State University Campus, Science City of Munoz, Nueva Ecija | 4 | Morphological | [95] | |
| Mt. Nagpale, Abucay, Bataan | 6 | Morphological | [96] | |
| Visayas | Municipalities in Northern Samar | 18 | Morphological | [97] |
| Municipality of San Antonio, Northern Samar | 26 | Morphological | [98] | |
| Mindanao | Davao Oriental; Dinagat Island; Agusan del Sur; Bukidnon; Surigao del Norte; Camiguin; Misamis Oriental | 185 | Morphological | [36] |
| Dansolihon Slope, Cagayan de Oro City, Philippines | 39 | Morphological | [99] |
Among mushroom biodiversity reports, Tadiosa and Lubos [36] reported the greatest number of mushroom species (185) and families (76) from Mindanao, followed by the work of Soriano et al. [37], who reported 163 species under 35 families from Laguna. Most of the studied mushrooms (95%) were identified based on the micro- and macro-morphology of mushroom. In contrast, only 5% (119 species) of the surveyed mushrooms were molecularly identified using rDNA-ITS sequence analysis. Dulay et al. [38] and De Leon et al. [39] reported the most number of molecularly identified mushrooms from San Clemente, Tarlac, and three municipalities of Ifugao, respectively. Accordingly, mushroom identification using molecular technique in the Philippines is scarce.
3. CHECKLIST OF WILD MUSHROOMS IN THE PHILIPPINES
The 2371 identified mushrooms reported in 64 available Philippine wild mushroom biodiversity studies in 2001–2021 were taxonomically classified into 447 species, 193 genera, and 72 families [Table 2]. The checklist of wild ascomycetous and basidiomycetous mushroom species in the Philippine is presented in Table 3. Most of the reported mushroom species belong to Phylum Basidiomycota (92%). Mushrooms belonging to Basidiomycota were classified into 411 species, 172 genera, and 59 families while those belong to Ascomycota were classified into 36 species, 21 genera, and 13 families. In Basidiomycota, the largest family was represented by Polyporaceae (72 species), followed by Agaricaceae (33), Hymenochaetaceae (18), Ganodermataceae (16), Psathyrellaceae (16), Marasmiaceae (15), Rusullaceae (15), Mycenaceae (14), Meruliaceae (13), and Tricholomataceae (12) [Figure 1a]. At the genus level, Trametes had the highest number of species (18), followed by Polyporus (14), Ganoderma (13), Mycena (12), and Agaricus (11) [Figure 1b]. On the other hand, in Ascomycota, Xylariaceae had the highest number of species (12), followed by Pyronemataceae (6) and Sarcoscyphaceae (5). Xylaria represented the largest genus (11), followed by Cookeina (4). However, the top 10 most reported species of Philippine wild mushrooms in the 64 available studies are shown in Figure 1c.
Table 2: Summary of Philippine wild mushroom classification recorded in 2001–2021.
| Group | Families | Genera | Species |
|---|---|---|---|
| Ascomycota | 13 | 21 | 36 |
| Basidiomycota | 59 | 172 | 411 |
| Total | 72 | 193 | 447 |
Table 3: Checklist of wild ascomycetous and basidiomycetous mushroom species in the Philippines reported in 2001–2021.
| Phylum | Family | Irpex nitidus | References |
|---|---|---|---|
| Ascomycota | Cudoniaceae | Spathularia sp. | [37] |
| Helotiaceae | Bisporella sulfurina (Quél.) S.E. Carp. | [42] | |
| Helvellaceae | Helvella lacunosa Afzel. | [98] | |
| Hyaloscyphaceae | Dasyscyphus apalus (Berk. and Broome) Dennis | [91] | |
| Hypoxylaceae | Daldinia concentrica (Bolton ex Fries) Cesati and Notaris | [40] | |
| Hypoxylon fragiforme (Pers.) J. Kickx f. | [50] | ||
| Hysteriaceae | Hysterium angustatum Pers. | [85] | |
| Nectriaceae | Nectria cinnabarina (Tode) Fr. | [85] | |
| Pezizaceae | Peziza repanda Wahlenb. | [57] | |
| Plicariella scabrosa (Cooke) Spooner | [50] | ||
| Sarcosphaera coronaria (Jacq.) J. Schröt. | [36] | ||
| Pyronemataceae | Aleuria aurantia (Pers.) Fuckel. | [91] | |
| Octospora humosa (Fr.) Dennis | [84] | ||
| Otidea sp. | [91] | ||
| Scuttelinia scutellata (Linn.) Lamb. | [91] | ||
| Tarzetta sp. | [84] | ||
| Trichaleurina celebica (Henn.) M.Carbone, Agnello andP. Alvarado | [65] | ||
| Sarcoscyphaceae | Cookeina insititia (Berk. and M.A.Curtis) Kuntze | [64] | |
| Cookeina speciosa (Fr.) Dennis | [61] | ||
| Cookeina sulcipes (Berk.) Kuntze | [42] | ||
| Cookeina tricholoma (Mont.) Kuntze | [42] | ||
| Phillipsia domingensis Berk. | [64] | ||
| Sarcosomataceae | Galiella rufa (Shwein.) Nannf. and Korf. | [36] | |
| Tuberaceae | Tuber sp. | [91] | |
| Xylariaceae | Biscogniauxia sp. | [42] | |
| Xylaria allantodea (Berk.) Fr. | [91] | ||
| Xylaria cornu-damae (Shwein.) Berk. | [88] | ||
| Xylaria filiformis (Alb. and Schwein.) Fr. | [88] | ||
| Xylaria hypoxylon (Linn.) Grev. | [37] | ||
| Xylaria longiana Rehm, 1904 | [50] | ||
| Xylaria longipes Nitschke | [88] | ||
| Xylaria multiplex (Kunze) Fr. | [84] | ||
| Xylaria papulis Lloyd | [40] | ||
| Xylaria polymorpha (Pers.) Grev. | [38] | ||
| Xylaria ridleyi Massee | [93] | ||
| Xylaria schweinitzii Berk and M.A Curtis | [84] | ||
| Basidiomycota | Agaricaceae | Agaricus arvensis Schaeff. | [70] |
| Agaricus augustus Fr. | [93] | ||
| Agaricus bisporus (J.E. Lange) Imbach | [90] | ||
| Agaricus campestris Linn. | [54] | ||
| Agaricus comtulus Fr. | [38] | ||
| Agaricus merrillii Copel. | [84] | ||
| Agaricus moelleri Wasser, 1976 | [50] | ||
| Agaricus perfuscus Copel. | [82] | ||
| Agaricus placomyces Peck | [58] | ||
| Agaricus trisulphuratus (Berk.) Singer | [87] | ||
| Agaricus xanthodermus Genev. | [38] | ||
| Calvatia cyathiformis (Bosc) Morgan | [70] | ||
| Calvatia gigantea (Batsch) Lloyd | [87] | ||
| Chlorophyllum molybdites (G. Mey.) Massee | [47] | ||
| Coprinus comatus (O.F.Müll.) Pers. | [37] | ||
| Cyathus rudis Pat. | [86] | ||
| Hymenagaricus sp. | [38] | ||
| Lepiota aspera (Pers.) Quel. | [91] | ||
| Lepiota cortinarius J.E.Lange | [53] | ||
| Lepiota cristata (Bolt.) Kumm. (1871) | [54] | ||
| Lepiota lilacea Bres. | [40] | ||
| Leucocoprinus birnbaumii R. Singer | [58] | ||
| Leucocoprinus cepistipes (Sowerby) Pat. | [58] | ||
| Leucocoprinus fragilissimus (Ravenel ex Berk. and M.A.Curtis) Pat. | [43] | ||
| Lycoperdon echinatum Pers. | [91] | ||
| Lycoperdon mammiforme Pers. | [98] | ||
| Lycoperdon perlatum Pers. | [84] | ||
| Lycoperdon pyriforme Schaeff. | [84] | ||
| Macrolepiota procera (Scop.ex Fr.) Sing. | [87] | ||
| Macrolepiota rhacodes (Vittad.) Singer. | [87] | ||
| Nidula sp. | [84] | ||
| Vascellum pratense (Pers.) Kreisel | [39] | ||
| Xanthagaricus flavosquamosus Li, Iqbal Hosen, and Song | [38] | ||
| Albatrellaceae | Albatrellus ellisii (Berk.) Pouzar | [44] | |
| Amanitaceae | Amanita alboflavescens Hongo | [39] | |
| Amanita cokeri (E.-J. Gilbert and Kühner) E.-J. Gilbert | [87] | ||
| Amanita fulva (Schaeff.) Fr. | [91] | ||
| Amanita onusta (Howe) Sacc. | [58] | ||
| Limacella illinita (Fr.) Murrill | [58] | ||
| Auriculariaceae | Auricularia auricula (Hook.) Underw. | [40] | |
| Auricularia auricula-judae (Bull.) Quél. | [51] | ||
| Auricularia cornea Ehrenb. | [39] | ||
| Auricularia delicata (Fr.) Henn. | [50] | ||
| Auricularia fuscosuccinea (Mont.) Henn. | [68] | ||
| Auricularia mesenterica (Dicks.) Pers. | [39] | ||
| Auricularia polytricha (Mont.) Sacc. | [51] | ||
| Auricularia tenuis (Lév.) Farl. | [87] | ||
| Bankeraceae | Phellodon niger (Fr.) P.Karst. | [87] | |
| Bolbitiaceae | Conocybe arrhenii (Fr.) Kits van Wav. | [40] | |
| Conocybe lactea (J.E.Lange) Métrod | [58] | ||
| Conocybe tenera (Schaeff.) Fayod | [43] | ||
| Panaeolus antillarum (Fr.) Dennis | [58] | ||
| Panaeolus campanulatus (L.) Quél. | [97] | ||
| Panaeolus cyanescens Sacc. | [40] | ||
| Panaeolus foenisecii (Pers.) J.Schröt. | [58] | ||
| Panaeolus papilionaceus (Bull.) Quél. | [43] | ||
| Panaeolus semiovatus (Sowerby) S.Lundell and Nannf. | [52] | ||
| Boletaceae | Boletus sp. | [42] | |
| Phylloporus bellus (Massee) Corner | [54] | ||
| Strobilomyces strobilaceus (Scop.) Berk. | [91] | ||
| Boletinellaceae | Boletinellus sp. | [82] | |
| Bondarzewiaceae | Heterobasidion annosum (Fr.) Bref. | [61] | |
| Cantharellaceae | Cantharellus aureus (Berk. and M.A. Curtis) Bres. | [88] | |
| Cantharellus cibarius Fr. | [57] | ||
| Cantharellus infundibuliformis (Scop.) Fr | [57] | ||
| Cantharellus minor Peck | [92] | ||
| Clavulina cristata (Holmsk.) J.Schröt. | [70] | ||
| Craterellus tubaeformis (Fr.) Quél. Homotypic synonym: Cantharellus tubaeformis | [53] | ||
| Mycena fibula (Fr.) Kuhner | [43] | ||
| Clavariaceae | Clavaria sp. | [49] | |
| Clavulinopsis sp. | [54] | ||
| Scytinopogon sp | [42] | ||
| Coniophoraceae | Coniophora puteana (Schum.) Karst. | [81] | |
| Meruliporia incrassata (Berk. and M.A. Curtis) Murrill | [52] | ||
| Corticiaceae | Corticium polygonoides P. Karst. | [52] | |
| Corticium roseum Pers. | [52] | ||
| Corticium salmonicolor Berk. and Broome | [57] | ||
| Cortinariaceae | Cortinarius callisteus (Fr.) Fr. | [57] | |
| Cortinarius corrugatus Peck | [98] | ||
| Gymnopilus lepidotus Hesler | [38] | ||
| Gymnopilus sapineus (Fr.) Maire | [85] | ||
| Hebeloma sp. | [54] | ||
| Crepidotaceae | Crepidotus herbarum Peck | [54] | |
| Crepidotus mollis (Schaeff.) Staude | [40] | ||
| Crepidotus variabilis (Pers.) P.Kumm. | [43] | ||
| Dacrymycetaceae | Calocera viscosa (Pers.) Fr. | [84] | |
| Dacrymyces chrysospermus Berk. and M.A. Curtis | [99] | ||
| Dacrymyces palmatus (Schwein.) Bres. | [84] | ||
| Dacryopinax spathularia (Schwein.) G.W.Martin | [38] | ||
| Guepinia fissa Berk. | [84] | ||
| Entolomataceae | Clitopilus prunulus (Scop.) P.Kumm. | [38] | |
| Entoloma cetratum (Fr.) M.M.Moser | [43] | ||
| Entoloma conferendum (Britzelm.) Noordel. | [39] | ||
| Entoloma jubatum (Fr.) P.Karst. | [39] | ||
| Entoloma lividum Quél. | [57] | ||
| Entoloma serrulatum (Fr.) Hesler | [91] | ||
| Exidiaceae | Exidia saccharina Fr. | [39] | |
| Exidia thuretiana (Lev.) Fr. | [85] | ||
| Fomitopsidaceae | Daedalea ambigua Berk. | [52] | |
| Daedalea dickinsii Yasuda | [70] | ||
| Daedalea quercina (L.) Pers. | [52] | ||
| Fomitopsis dochmia (Berk. and Broome) Ryvarden | [64] | ||
| Fomitopsis feei (Fr.) Kreisel | [40] | ||
| Fomitopsis pinicola (Sw.) P.Karst. | [97] | ||
| Fomitopsis rosea (Alb. and Schwein.) P.Karst. | [59] | ||
| Ischnoderma resinosum (Schrad.) P.Karst. | [50] | ||
| Postia fragilis (Fr.) Jülich | [44] | ||
| Ganodermataceae | Amauroderma auriscalpium (Pers.) Torrend | [86] | |
| Amauroderma rude (Berk.) Torrend | [53] | ||
| Amauroderma rugosum (Blume and T.Nees) Torrend | [42] | ||
| Ganoderma adspersum (Schulzer) Donk | [98] | ||
| Ganoderma applanatum (Pers.) Pat. | [40] | ||
| Ganoderma australe (Fr.) Pat. | [38] | ||
| Ganoderma fornicatum (Fr.) Pat., 1889 | [40] | ||
| Ganoderma gibbosum (Blume and T.Nees) Pat. | [38] | ||
| Ganoderma japonicum (Fr.) Sawada | [70] | ||
| Ganoderma lobatum (Cooke) G.F.Atk. | [91] | ||
| Ganoderma lucidum (Curtis) P. Karst | [40] | ||
| Ganoderma mangiferae (Lév.) Pat. | [86] | ||
| Ganoderma neo-japonicum Imazeki | [38] | ||
| Ganoderma pfeifferi Bres. | [55] | ||
| Ganoderma sinense J.D.Zhao, L.W.Hsu and X.Q.Zhang | [61] | ||
| Ganoderma tsugae Murrill | [51] | ||
| Geastraceae | Geastrum fimbriatum Fr. | [70] | |
| Geastrum saccatum Fr. | [37] | ||
| Geastrum schmidelii Vittad. | [38] | ||
| Geastrum triplex Jungh. | [57] | ||
| Sphaerobolus stellatus (Tode) Pers. | [87] | ||
| Gomphaceae | Ramaria myceliosa (Peck) Corner | [64] | |
| Hydnaceae | Hydnum sp. | [37] | |
| Hydnangiaceae | Laccaria ochropurpurea (Berk.) Peck | [54] | |
| Laccaria laccata (Scop) Cooke | [98] | ||
| Hygrophoraceae | Ampulloclitocybe clavipes (Pers) Redhead, Lutzoni, Moncalvo and Vilgalys | [98] | |
| Cantharocybe sp. | [38] | ||
| Hygrocybe coccinea (Schaeff.) P.Kumm. | [54] | ||
| Hygrocybe miniata (Fr.) P.Kumm. | [49] | ||
| Hygrocybe nitida (Berk. and M.A.Curtis) Murrill | [43] | ||
| Hygrophorus eburneus (Bull.) Fr. | [54] | ||
| Hygrophorus pratensis (Fr.) Fr. | [57] | ||
| Omphalina grossula (Pers.) Singer | [55] | ||
| Hygrophoropsidaceae | Hygrophoropsis aurantiaca (Wulfen) Maire | [81] | |
| Hymenochaetaceae | Coltricia perennis (L.) Murrill | [55] | |
| Fomes linteus (Berk. and M.A.Curtis) Cooke | [84] | ||
| Fomes pachyphloeus (Pat.) Bres. | [84] | ||
| Fomes senex (Nees and Mont.) Cooke | [57] | ||
| Fomitiporia punctata (Pilat) Murrill | [52] | ||
| Fuscoporia senex (Nees and Mont.) Ghobad-Nejhad | [86] | ||
| Fuscoporia torulosa (Pers.) T. Wagner and M. Fisch | [52] | ||
| Hymenochaete rubiginosa (Dicks.) Lév. | [53] | ||
| Hymenochaete tenuissima (Berk.) Berk. | [51] | ||
| Inonotus radiatus (Sowerby) P.Karst. | [97] | ||
| Phellinus caryophylli (Racib.) G.Cunn. Synonym: Fomes caryophylli (Racib.) Bres. | [36] | ||
| Phellinus gilvus (Schwein.) Pat. Synonym: Fomes gilvus (Schwein.) Speg. and Polyporus gilvus (Schwein.) Fr. | [52] | ||
| Phellinus igniarius (L.) Quél. | [43] | ||
| Phellinus linteus (Berk. and M.A.Curtis) Teng | [52] | ||
| Phellinus pini (Fr.) Ames | [92] | ||
| Phellinus punctatus (P.Karst.) Pilát | [43] | ||
| Phellinus rimosus (Berk.) Pilat | [36] | ||
| Polystictus connexus (Lév.) Cooke | [86] | ||
| Inocybaceae | Inocybe rimosa (Bull.) P.Kumm. | [98] | |
| Irpicaceae | Gloeoporus dichrous (Fr.) Bres. | [92] | |
| Laetiporaceae | Laetiporus sulphureus (Bull.) Murrill | [84] | |
| Phaeolus sp. | [37] | ||
| Lyophyllaceae | Lyophyllum sp. | [37] | |
| Termitomyces albuminosus (Beck.) Heim | [57] | ||
| Termitomyces bulborhizus T.Z.Wei, Y.J.Yao, Bo Wang and Pegler | [38] | ||
| Termitomyces clypeatus R.Heim | [58] | ||
| Termitomyces eurrhizus (Berk.) R.Heim | [44] | ||
| Termitomyces microcarpus (Berk. and Broome) R.Heim | [38] | ||
| Termitomyces robustus (Beeli) R.Heim | [87] | ||
| Termitomyces striatus (Beeli) R.Heim | [70] | ||
| Marasmiaceae | Campanella aff. eberhardtii (Pat.) Singer | [54] | |
| Chaetocalathus sp. | [38] | ||
| Crinipellis scabella (Alb. and Schwein.) Murrill | [43] | ||
| Gerronema keralense K.P.D.Latha and Manim. | [38] | ||
| Hydropus marginellus (Pers.) Singer | [38] | ||
| Marasmiellus palmivorus (Sharples) Desjardin | [38] | ||
| Marasmius epiphylloides (Rea) Sacc. and Trotter | [64] | ||
| Marasmius haematocephalus (Mont.) Fr. | [43] | ||
| Marasmius oreades (Bolton) Fr. | [49] | ||
| Marasmius plicatulus Peck | [49] | ||
| Marasmius rotula (Scop.) Fr. | [49] | ||
| Marasmius siccus (Schwein.) Fr. | [49] | ||
| Megacollybia platyphylla (Pers.) Kotl. and Pouzar | [39] | ||
| Pleurocybella porrigens (Pers.) Singer | [98] | ||
| Tetrapyrgos sp. | [54] | ||
| Meripilaceae | Meripilus giganteus (Pers.) P.Karst. | [80] | |
| Rigidoporus microporus (Sw.) Overeem | [51] | ||
| Aquascypha hydrophora (Berk.) D.A. Reid | [85] | ||
| Meruliaceae | Bjerkandera adusta (Willd.) P.Karst. | [98] | |
| Cymatoderma africanum Boidin | [91] | ||
| Cymatoderma elegans Jungh. | [42] | ||
| Flavodon flavus (Klotzsch) Ryvarden | [39] | ||
| Irpex flavus Klotzsch | [84] | ||
| Irpex lacteus (Fr.) Fr. | [40] | ||
| Irpex nitidus (Pers.) Saaren. and Kotir. | [64] | ||
| Podoscypha bolleana (Mont.) Boidin | [91] | ||
| Podoscypha brasiliensis D.A.Reid | [76] | ||
| Podoscypha petalodes (Berk.) Boidin | [49] | ||
| Podoscypha subaffinis (Berk. and Curt.) Pat. | [91] | ||
| Poria straminea Bres. | [84] | ||
| Spongipellis pachyodon (Pers.) Kotl. and Pouzar | [98] | ||
| Mycenaceae | Favolaschia pustulosa (Jungh.) Kuntze | [43] | |
| Mycena acicula (Schaeff.) P.Kumm. | [43] | ||
| Mycena alcalina (Fr.) Quél. | [43] | ||
| Mycena cinerella (P.Karst.) P.Karst. | [43] | ||
| Mycena clavularis (Batsch) Sacc. | [43] | ||
| Mycena crocata (Schrad.) P. Kumm. | [54] | ||
| Mycena galericulata (Scop.) Gray | [43] | ||
| Mycena galopus (Pers.) P.Kumm. | [43] | ||
| Mycena inclinata (Fr.) Quél. | [53] | ||
| Mycena leptocephala (Pers.) Gillet | [97] | ||
| Mycena pura (Pers.) P.Kumm. | [43] | ||
| Mycena vulgaris (Pers.) P.Kumm. | [43] | ||
| Panellus mitis (Pers.) Singer | [40] | ||
| Panellus stipticus (Bull.) P.Karst. | [53] | ||
| Nidulariaceae | Cyathus striatus (Huds.) Willd. | [49] | |
| Omphalotaceae | Anthracophyllum melanophyllum (Fr.) Pegler and T.W.K.Young | [54] | |
| Collybia maculata (Alb. and Schwein.) P.Kumm. | [74] | ||
| Gymnopus androsaceus (L.) J.L. Mata and R.H. Petersen Homotypic synonym: Marasmius androsaceus (Linn.) Fr. | [54] | ||
| Lentinula edodes (Berk.) Pegler | [90] | ||
| Marasmiellus candidus (Fr.) Singer | [38] | ||
| Marasmiellus ramealis (Bull.) Singer Homotypic synonym: Marasmius ramealis (Bull.) Fr. 1838 | [40] | ||
| Marasmius foetidus (Sowerby) Fr. | [93] | ||
| Marasmius scorodonius (Fr.) Fr. | [43] | ||
| Omphalotus olearius (DC.) Singer | [53] | ||
| Peniophoraceae | Peniophora sp. | [49] | |
| Phallaceae | Aseroe rubra Labill. | [91] | |
| Dictyophora duplicata (Bosc) E.Fisch. | [57] | ||
| Dictyophora indusiata (Vent.) Desv. | [71] | ||
| Mutinus caninus (Huds.) Fr. | [87] | ||
| Phallus duplicatus Bosc | [87] | ||
| Phallus indusiatus Vent. | [38] | ||
| Phallus multicolor (Berk. and Broome) Cooke | [98] | ||
| Phanerochaetaceae | Hydnophlebia chrysorhiza (Torr.) Parmasto Homotypic synonym: Phanerochaete chrysorhiza (Eaton) Budington and Gilb. | [42] | |
| Merulius incarnatus Schwein. | [81] | ||
| Pulcherricium caeruleum (Lam.) Parmasto | [81] | ||
| Physalacriaceae | Armillaria sp. | [38] | |
| Oudemansiella canarii (Jungh.) Höhn. | [38] | ||
| Oudemansiella radicata (Relhan) Singer | [91] | ||
| Pleurotaceae | Hohenbuehelia petaloides (Bull.) Schulzer | [92] | |
| Pleurotus cornucopiae (Paulet) Rolland | [93] | ||
| Pleurotus cystidiosus O.K. Mill. | [48] | ||
| Pleurotus djamor (Rumph. ex Fr.) Boedijn | [38] | ||
| Pleurotus dryinus (Pers.) P.Kumm. | [51] | ||
| Pleurotus giganteus (Berk.) Karun. and K.D. Hyde | [64] | ||
| Pleurotus opuntiae (Durieu and Lév.) Sacc. | [86] | ||
| Pleurotus ostreatus (Jacq.) P.Kumm. | [54] | ||
| Pleurotus porrigens (Pers.) P.Kumm. | [70] | ||
| Pleurotus pulmonarius (Fr.) Quél. | [64] | ||
| Pleurotus tuber-regium (Fr.) Singer | [60] | ||
| Pluteaceae | Pluteus multiformis Justo, A.Caball. and G.Muñoz | [38] | |
| Pluteus salicinus (Pers.) P. Kumm. | [85] | ||
| Pluteus umbrosus (Pers.) P.Kumm. | [91] | ||
| Volvariella dunensis (Vila, Àngel and Llimona) Justo and M.L.Castro | [38] | ||
| Volvariella volvacea (Bull.) Singer | [56] | ||
| Polyporaceae | Coriolopsis polyzona (Pers.) Ryvarden | [36] | |
| Daedalea amanitoides P.Beauv. | [81] | ||
| Daedalea hobsoni Berk | [86] | ||
| Daedalea palisotii Fr. | [86] | ||
| Daedaleopsis confragosa (Bolton) J.Schröt. | [44] | ||
| Earliella scabrosa (Pers.) Gilb. and Ryvarden | [40] | ||
| Favolus acervatus (Lloyd) Sotome and T.Hatt. | [40] | ||
| Favolus albus Lloyd | [84] | ||
| Favolus alveolaris (DC.) Quel Homotypic synonyms: Neofavolus alveolaris (DC.) Sotome and T. Hatt Polyporus alveolaris (DC.) Bondartsev and Singer | [52] | ||
| Favolus emerici (Berk. ex Cooke) Imazeki | [38] | ||
| Favolus reniformis (Murrill) Sacc. and Trotter | [74] | ||
| Favolus tenuiculus P. Beauv | [85] | ||
| Fomes fomentarius (L.) Fr. | [42] | ||
| Hexagonia apiaria (Pers.) Fr. | [36] | ||
| Hexagonia glaber (P.Beauv.) Ryvarden | [43] | ||
| Hexagonia hydnoides (Sw.) M.Fidalgo | [53] | ||
| Hexagonia nitida Durieu and Mont. | [43] | ||
| Hexagonia tenuis (Fr.) Fr. | [51] | ||
| Lentinus cladopus Lév. | [87] | ||
| Lentinus crinipellis | [64] | ||
| Lentinus sajor-caju (Fr.) Fr. Homotypic synonym: Pleurotus sajor-caju (Fr.) Singer | [42] | ||
| Lentinus squarrosulus Mont. | [55] | ||
| Lentinus strigosus (Schwein.) Fr. | [40] | ||
| Lentinus swartzii Berk. | [41] | ||
| Lentinus tigrinus (Bull.) Fr. | [51] | ||
| Lentinus velutinus Fr. | [43] | ||
| Lenzites betulinus (L.) Fr. | [44] | ||
| Lenzites repanda (Mont.) Fr. | [74] | ||
| Lenzites striata (Swartz) Fr. | [57] | ||
| Microporus affinis (Blume and T.Nees) Kuntze Synonyms: Polystictus flabelliformins (Klotzsch) Fr. andPolystictus affinis (Blume and T.Nees) Fr. | [42] | ||
| Microporus subaffinis (Lloyd) Imazeki | [51] | ||
| Microporus vernicipes (Berk.) Kuntze | [53] | ||
| Microporus xanthopus (Fr.) Kuntze Synonym: Polystictus xanthopus (Fr.) Fr. | [40] | ||
| Panus conchatus (Bull.) Fr. | [56] | ||
| Panus rudis Fr. | [52] | ||
| Polyporus arcularius (Batsch) Fr. | [37] | ||
| Polyporus badius (Pers.) Schwein. | [42] | ||
| Polyporus brumalis Pers. | [39] | ||
| Polyporus cuticularis (Bull.) Fr. | [86] | ||
| Polyporus durus Jungh. | [81] | ||
| Polyporus grammocephalus Berk. | [36] | ||
| Polyporus leptocephalus (Jacq) Fr. | [55] | ||
| Polyporus picipes Fr. | [51] | ||
| Polyporus pinsitus Fr. | [59] | ||
| Polyporus roseus (Alb and Schwein.) Fr. | [81] | ||
| Polyporus semilaccatus (Berk.) Berk. | [94] | ||
| Polyporus squamosus Huds. | [36] | ||
| Polyporus tenuiculus (P.Beauv.) Fr. | [53] | ||
| Polyporus varius Pers. | [50] | ||
| Polystictus incomptus (Afzel. ex Fr.) Fr. | [86] | ||
| Polystictus occidentalis (Klotzsch) Fr. | [86] | ||
| Poria latemarginata (Fr.) Karst. | [86] | ||
| Trametes cinnabarina (Jacq.: Fr.) Fr. Homotypic synonym: Pycnoporus cinnabarinus (Jacq.) P.Karst. | [55] | ||
| Trametes aspera (Jungh.) Bres. | [81] | ||
| Trametes coccinea (Fr.) Hai J. Li and S.H. He Homotypic synonym: Pycnoporus coccineus (Fr.) Bondartsev and Singer | [39] | ||
| Trametes corrugata (Pers.) Bres. | [64] | ||
| Trametes elegans (Spreng.) Fr. Homotypic synonym: Lenzites elegans (Spreng.) Pat. | [40] | ||
| Trametes ellipsospora Ryvarden | [39] | ||
| Trametes flavida (Lév.) Zmitr., Wasser and Ezhov Basionym: Daedalea flavida Lév. | [52] | ||
| Trametes gibbosa (Pers.) Fr. | [40] | ||
| Trametes hirsuta (Wulfen) Lloyd Homotypic synonym: Polyporus hirsutus (Wulfen) Fr. | [40] | ||
| Trametes membranacea (Sw.) Kreisel | [49] | ||
| Trametes ochracea (Pers.) Gilb. and Ryvarden | [50] | ||
| Trametes pubescens (Schumach.) Pilát | [61] | ||
| Trametes polyzona (Pers.) Justo Homotypic synonym: Funalia polyzona (Pers.) Niemelä | [42] | ||
| Trametes sanguinea (L.) Lloyd Homotypic synonym: Pycnoporus sanguineus (L.) Murrill and Polyporus sanguineus Fr. | [40] | ||
| Trametes suaveolens (L.) Fr. | [39] | ||
| Trametes trogii Berk. | [98] | ||
| Trametes versicolor (L.) Lloyd Homotypic synonym: Coriolus versicolor (Lev.) Pat | [40] | ||
| Trametes villosa (Sw.) Kreisel | [52] | ||
| Trametopsis cervina (Schwein.) Tomšovský Homotypic synonym: Trametes cervina (Schwein.) Bres. | [87] | ||
| Tyromyces chioneus (Fr.) P.Karst. | [50] | ||
| Porotheleaceae | Trogia infundibuliformis Berk. and Broome | [43] | |
| Psathyrellaceae | Coprinellus aureogranulatus (Uljé and Aptroot) Redhead, Vilgalys and Moncalvo | [78] | |
| Coprinellus disseminatus (Pers.) J.E. Lange Homotypic synonym: Coprinus disseminatus (Pers.) Gray | [40] | ||
| Coprinellus micaceus (Bull.) Vilgalys, Hopple and Jacq.Johnson | [43] | ||
| Coprinellus pakistanicus Usman and Khalid | [38] | ||
| Coprinopsis atramentaria (Bull.) Redhead, Vilgalys and Moncalvo Homotypic synonym: Coprinus atramentarius (Bull.) Fr. | [51] | ||
| Coprinopsis cinerea (Schaeff.) Redhead, Vilgalys and Moncalvo Homotypic synonym: Coprinus cinereus (Schaeff.) Gray | [51] | ||
| Coprinopsis clastophylla (Maniotis) Redhead, Vilgalys and Moncalvo | [38] | ||
| Coprinopsis lagopus (Fr.) Redhead, Vilgalys and Moncalvo Homotypic synonym: Coprinus lagopus (Fr) Fr. | [51] | ||
| Coprinopsis musae Örstadius and E. Larss | [38] | ||
| Coprinopsis picacea (Bull.) Redhead, Vilgalys and Moncalvo | [53] | ||
| Coprinus niveus (Pers.) Fr. | [43] | ||
| Coprinus stercoreus Fr. Epicrisis | [43] | ||
| Parasola plicatilis (Curtis) Redhead, Vilgalys and Hopple Homotypic synonym: Coprinus plicatilis (Curtis) Fr. | [40] | ||
| Psathyrella candolleana (Fr.) Maire | [40] | ||
| Psathyrella multipedata (Peck) A.H. Sm. | [54] | ||
| Psathyrella typhae (Kalchbr.) A.Pearson and Dennis | [64] | ||
| Pterulaceae | Corticium confluens (Fr.) Fr. | [57] | |
| Radulomyces confluens (Fr.) M.P. Christ. | [52] | ||
| Russulaceae | Lactarius deliciosus (L.) Gray | [54] | |
| Lactarius plumbeus (Bull.) Gray | [43] | ||
| Lactarius pubescens Fr. | [54] | ||
| Lactarius trivialis (Fr.) Fr. | [88] | ||
| Lactarius piperatus (L.) Pers. Homotypic synonym: Lactifluus piperatus (L.) Kuntze | [43] | ||
| Russula aeruginea Lindblad | [54] | ||
| Russula cyanoxantha (Schaeff.) Fr. | [54] | ||
| Russula delica Fr. | [54] | ||
| Russula emetica (Schaeff.) Pers. | [54] | ||
| Russula fragilis Fr., 1838 | [54] | ||
| Russula mariae Peck | [42] | ||
| Russula rosea Pers. | [54] | ||
| Russula sanguinaria (Schumach.) Rauschert | [54] | ||
| Russula sanguinea Fr. | [84] | ||
| Russula virescens (Schaeff.) Fr. | [42] | ||
| Schizophyllaceae | Schizophyllum commune Fr. | [40] | |
| Sclerodermataceae | Pisolithus sp. | [42] | |
| Scleroderma citrinum Pers. | [44] | ||
| Scleroderma verrucosum (Bull) Pers. | [36] | ||
| Serpulaceae | Serpula similis (Berk. and Broome) Ginns | [38] | |
| Sparassidaceae | Sparassis radicata Weir | [97] | |
| Steccherinaceae | Junghuhnia collabens (Fr.) Ryvarden | [85] | |
| Nigroporus vinosus (Berk.) Murrill | [85] | ||
| Stereaceae | Aleurodiscus aurantius (Pers.) J. Schrot | [85] | |
| Aleurodiscus wakefieldiae Boidin and Beller | [85] | ||
| Stereum complicatum (Fr.) Fr. | [42] | ||
| Stereum hirsutum (Willd.) Pers. | [51] | ||
| Stereum insignatum Blume | [64] | ||
| Stereum lobatum (Kunze ex Fr.) Fr. | [51] | ||
| Stereum ostrea (Blume and T.Nees) Fr. | [51] | ||
| Stereum rugosum (Pers.) Fr. | [50] | ||
| Stereum sanguinolentum (Alb. and Schwein.) Fr. | [74] | ||
| Stereum subtomentosum Pouzar | [50] | ||
| Xylobolus sp. | [37] | ||
| Strophariaceae | Agrocybe sp. | [57] | |
| Deconica coprophila (Bull.) P.Karst. Homotypic synonym: Psilocybe coprophila (Bull.) P.Kumm. (1871) | [38] | ||
| Hypholoma fasciculare (Huds.) P.Kumm. | [64] | ||
| Naematoloma fasciculare (Huds.) P.Karst. | [58] | ||
| Pholiota highlandensis (Peck) A.H.Sm. and Hesler | [39] | ||
| Pholiota lignicola (Peck) Jacobsson | [42] | ||
| Psilocybe sp. | [37] | ||
| Stropharia rugosoannulata Farl. ex Murrill | [57] | ||
| Stropharia semiglobata (Batsch) Quél. | [43] | ||
| Stropharia squamosa (Pers.) Quél. | [58] | ||
| Suillaceae | Suillus granulatus (L.) Roussel | [36] | |
| Thelephoraceae | Thelephora anthocephala (Bull.) Fr. | [39] | |
| Thelephora terrestris Ehrh. | [52] | ||
| Tremellaceae | Tremella foliacea Pers. | [69] | |
| Tremella fuciformis Berk. | [37] | ||
| Tremella mesenterica Retz. | [39] | ||
| Tricholomataceae | Amparoina sp. | [53] | |
| Calyptella sp. | [37] | ||
| Clitocybe dealbata (Sowerby) P.Kumm. | [43] | ||
| Clitocybe geotropa (Bull.) Quél. | [53] | ||
| Clitocybe gibba (Pers.) P.Kumm. | [43] | ||
| Infundibulicybe gibba (Pers.) Harmaja | [98] | ||
| Micromphale sp. | [99] | ||
| Phyllotopsis nidulans (Pers.) Singer | [58] | ||
| Tricholoma flavovirens (Pers.) S.Lundell | [58] | ||
| Tricholoma lascivum (Fr.) Gillet | [50] | ||
| Tricholoma saponaceum (Fr.) P.Kumm. | [57] | ||
| Tricholomopsis rutilans (Schaeff.) Singer | [52] | ||
| Incertae sedis | Pseudohydnum gelatinosum (Scop.) P.Karst. | [58] | |
| Trichaptum abietinum (Dicks.) Ryvarden | [81] |
 | Figure 1: Top 10 families (a) and genera (b) with the most number of species, and the top 10 most reported species (c) of Philippine mushrooms in 2001–2021. [Click here to view] |
To the best of our knowledge, so far, this is the most comprehensive list of Philippine wild mushrooms available to date. In this review paper, we established the record of 447 wild mushroom species reported in the Philippines. This number is higher than the reported species in other countries such as Ethiopia with 66 [100], Cambodia with 302 [101], and Guatemala with 350 [102], but lower when compared to Columbia with 1239 [103] and Nepal with 1291 [104]. We believe that there are still numerous mushroom species in the Philippine wilderness waiting to be discovered and harnessed their full potentials, suggesting the need to assess the macrofungal diversity especially in Visayas and Mindanao to acquire vast number of mushroom species across the country.
4. ETHNOMYCOLOGICALLY IMPORTANT PHILIPPINE MUSHROOMS
Ethnomycology is the study that concerns the human’s cultural and traditional knowledge, belief, and practices on the utilization of useful fungi like mushrooms that are naturally occurring in their environment. The ethnomycological information has significant contribution in the conservation and exploration of these wild genetic mycoresources. The use of mushrooms such as Auricularia auricula, Auricularia polytricha, Calvatia sp., Lentinus tigrinus, Lentinus sajor-caju, Pleurotus sp., Schizophyllum commune, Termitomyces clypeatus, Trichobatrachus robustus, two other species of Termitomyces, and Volvariella volvacea as food, Mycena sp. as medicine, and G. lucidum as house decorations, and rituals performed prior collection of mushrooms including tribal dancing, praying, and kissing the ground by the Ayta communities in Central Luzon, Philippines were documented [89]. Another study with Ayta communities reported 15 species of mushrooms (e.g., V. volvacea, Termitomyces spp., A. polytricha, Auricularia auricula-judae, G. lucidum, Stereum sp., S. commune) utilized as food and alternative medicine for cough, weakness, common colds, and poor eyesight [66]. Mushrooms such as A. polytricha, Cantharellus cibarius, Inocybe rimosa, and S. commune were considered food by the people of Northern Samar [97]. The Gaddang communities in Nueva Vizcaya recognized ten species of mushrooms as food, but only seven were collected during the study including A. auricula, Auricularia fuscosuccinea, S. commune, V. volvacea, Lentinus sp., Pleurotus sp., and Polyporus sp. and revealed beliefs such as spontaneous lightning induces growth of mushroom and asking permission of spirits before collecting mushrooms [83].
Moreover, Kalanguya tribe in Carranglan, Nueva Ecija claimed 36 mushroom species used as food and one as insect repellant; however, only ten species were obtained during collection, namely, bang-ugan (Meripilus giganteus), bugatan, buo (Scleroderma citrinum), but-taytay (Microporus sp.), gum-gumot (Leucoagaricus cepaestipes), kuyupan (Podocypha brasiliensis), lingtan, uongusa, upot (Russula virscens), and wek-wek [76]. In addition, 13 useful mushrooms, namely, Agaricus sp., A. auricula, Coprinellus disseminatus, L. sajor-caju, Lenzites elegans, Mycena sp., Oudemansiella canarii, Phellinus sp., Pleurotusostreatus, S. commune, Trametes elegans, Vascellum pratense, and V. volvacea were collected, and the utilization of Trametes sp. as remedy for stomach ache and headache, and human body cleansing by the indigenous people in the three municipalities of Ifugao Province were documented [105]. The Bugkalot indigenous community in Alfonso Castaneda, Nueva Vizcaya, recognized 17 mushroom species used as food (A. auricularia-judae, A. polytricha, Boletus sp., Clitocybe sp., Coprinopsis atramentaria, Coprinopsis lagopus, Coprinus cinereus, L. tigrinus, two other species of Lentinus, Mycena sp., Panaeolus sp., Pleurotus dryinus, two species of Polyporus, S. commune, and Stereum lobatum) and five mushroom species utilized as medicine (Fomitopsis sp., Ganoderma applanatum, G. lucidum, Polyporus picipes, and Polyporus sp.) [51].
These above-cited ethnomycological studies are strong evidence of the importance of wild useful mushrooms to the indigenous communities and ethnic groups in the Philippines. Accordingly, wild mushrooms are generally considered as food and alternative medicines. The information on the reported edibility of Philippine wild mushrooms warrants investigation on their nutritional compositions for the development of innovative and high value mushroom-based food products. However, the claimed medicinal properties of some wild mushrooms also ignite high interest on the evaluation of their biological properties and further elucidation of their bioactive components to validate the indigenous claims. More ethnomycological documentation across the country, highlighting other ethnic and indigenous groups, is highly recommended.
5. SUCCESSFULLY CULTIVATED PHILIPPINE MUSHROOMS
Mushroom cultivation can provide nutritious and healthy food for human consumption, ensuring food security, and at the same time, generate livelihood and promote environmental protection in the countryside. With the increasing attention to the values of mushrooms, mushroom production is also gradually increased worldwide, with China recorded as the top mushroom-producing country globally [106]. The most widely cultivated edible mushrooms around the world include Lentinus edodes, Pleurotus spp. [107], and Agaricus bisporus [108]. In the Philippines, some exotic mushrooms such as Pleurotus species, Agaricus sp., Calocybe indica, L. edodes, and Cyclocybe cylindracea are introduced and cultivated in small to medium scale of production.
To date, the Center for Tropical Mushroom Research and Development (CTMRD) in Central Luzon State University, Philippines has successfully rescued the cell lines of various wild useful mushrooms and generated their production technologies. These include Collybia reinakeana [109,110], Coprinus comatus [111], Mycena sp. [112], S. commune [112,113], L. sajor-caju [114], L. tigrinus [115], Lentinus squarrosulus [116], Polyporus grammocephalus [73,116], Lentinus swartzii [41], Panaeolus antillarium [117], Panaeolus cyanescens [117], Pleurotus cystidiosus [118], Oudemansiella canarii [119], Ganoderma curtissii [120], Fomitopsis feei [45], Pycnoporus sanguineus [46], Lentinus strigosus [72], G. lucidum [121], A. polytricha [96,122,123], and V. volvacea [124,125]. The optimal culture conditions for mycelia growth and fruiting body production of these Philippine wild mushrooms have been established. Pure culture of mushroom mycelia is maintained in semi-solid indigenous culture media from natural sources such as coconut water, potato, rice bran, and corn grit, and commercially available dehydrated culture media like potato dextrose agar. The fruiting bodies of mushroom are propagated in polypropylene bag using formulated substrates such as rice straw and sawdust. Moreover, the CTMRD developed the zero-rice waste technology that demonstrate the efficient utilization of agro-industrial wastes from rice production as substrates for mushroom production, feed for livestock production and fertilizer for vegetable production, and other technologies such as tilapia and mushroom growth chamber production, and aseptic cultivation.
Mycelial biomass production of C. comatus [111], P. cyanescens [126], G. lucidum, S. commune, P. cystidiosus, V. volvacea [127], L. tigrinus, L. sajor-caju [128], S. commune [129,130], V. volvacea [124], Coprinopsis cinerea [131], L. tigrinus [132], G. lucidum [133], Chlorophyllum molybdites [47], P. cystidiosus [48], L. tigrinus [134], P. sanguineus [135], and Lentinus species in liquid or submerged culture has also been demonstrated [136]. Studies on the nutritional and physical requirements for spore germination of V. volvacea [124], S. commune [137], L. tigrinus [115], G. lucidum [121], L. swartzii, and L. strigosus [138] have been established and their morphogenesis from spore germination up to fruiting body maturation were documented.
In addition, the effects of different supplements or additives such as rice bran in pulp and paper waste [139], in Pleurotus substrate spent [140], ruminant’s dung [141], agricultural wastes [142], vitamin A [143], and Moringa oleifera leaf extract [144], and physical factor such as light-emitting diode [145] have also been evaluated for the improvement of mushroom production as well as their composition and bioactivity.
6. NUTRITIONAL AND MEDICINAL PROPERTIES OF PHILIPPINE MUSHROOMS
Edible mushrooms are excellent source of nutritious and unique umami-taste food. They are rich in carbohydrates, proteins, fibers, vitamins, minerals, and low-fat content [1,2]. Chemical compositions of Philippine mushrooms have also been elucidated. Mushrooms, including L. tigrinus, C. comatus, P. cyanescens, P. antillarium, L. sajor-caju, L. strigosus, P. grammocephalus, T. elegans, Trichaleurina celebica, P. cystidiosus, P. sanguineus, and Xylaria papulis, have been shown to contain carbohydrates, proteins, crude fibers, crude fat, vitamins, and minerals and important bioactive metabolites such as alkaloids, flavonoids, phenols, coumarins, triterpenes, tannins, saponins, anthraquinones, athrones, and steroids [48,65,118,126,135,140,141,146-153]. Other mushrooms, namely, C. reinakeana, C. comatus, V. volvacea, G. lucidum, P. cystidiosus, L. tigrinus, L. sajor-caju, Geastrum triplex, T. clypeatus, and S. commune have also been reported as source of amino acids, fatty acids, acyglycerols, triacylglycerols, ergosterol, and minerals [111,127,154-161].
Edible mushrooms have also been exploited for a very long time as natural alternative remedy for various diseases. The therapeutic values and medicinal properties of edible mushrooms have found to stem from numerous biologically active compounds or metabolites [162-164]. Besides nutrients and active metabolites, Philippine mushrooms have been revealed to exhibit important bioactivities, including antioxidants [48,126,128,133-135,147,150-153,165,167] antibacterial [59,118,135,146,150,152,167-169] hypoglycemic [146,170] antihypertensive [154,155,171] anticancer [160,172-174] anticoagulative, anti-inflammatory [154-156,175] teratogenic [65,148,176-182] aphrodisiac, diuretic [182] thrombolytic [44] and potential anti-obesity activities [183]. Noticeably, among bioactivities, antioxidant, antibacterial, and teratogenic were the most evaluated properties of mushrooms. However, the most studied Philippine mushrooms are species under Ganoderma, Lentinus, Volvariella, Collybia, Coprinus, Panaeolus, Termitomyces, Schizophyllum, Auricularia, and Pleurotus.
7. CURRENT STATUS, CHALLENGES, PERSPECTIVES, AND CONCLUDING REMARKS
Mushrooms have great nutritional, medicinal, and economic values and are of high interest for many researchers worldwide. In the Philippines, the most studied aspect of mushroom research according to published works from 2001 to 2021 is on mushroom bioactivity with 29.19%, followed by the optimization/cultivation (22.36%), ethnomycology/biodiversity (19.88%), and chemical composition (15.53%) [Figure 2]. In contrast, developmental biology is the least studied aspect with 2.48%, followed by bioremediation (4.97%) and molecular identification (5.59%). Looking at the global status, Philippine mushroom research is limited and could be considered as an emerging research undertaking [Table 4]. These important data that investigation of the following areas must be considered: (a) ethnomycological and biodiversity studies must be conducted particularly in the Visayas and Mindanao areas; (b) detailed taxonomic and phylogenetic analyses using advanced molecular approaches to delineate the unique molecular profile and discover new species of mushrooms; (c) breeding and selection of strains to achieve superior strains for commercial cultivation; (d) development of cultivation technologies and/or conditions for the improvement of biomass and metabolites production efficiency; (e) isolation and characterization of biologically active chemical compounds responsible to the biological properties of mushrooms such as antioxidant, antibacterial, anticancer, anti-diabetic, anti-hypertensive, and anti-inflammatory; and (f) screening of more Philippine wild mushrooms for their biological properties, especially anticancer, and establish their mechanism of action using advanced approaches (genomic, proteomic, and transcriptomic) at different model systems (cellular and organism level) to establish their molecular targets, which is necessary for drug development. Moreover, the slow progress in Philippine mushroom research could be attributed to the limited number of competent researchers in this field and the lack of advanced research facilities dedicated to mushroom research. It is therefore of urgent need to increase the number of mycologist and experts in this field by developing and mentoring young minds and by improving the science and agriculture curricular program, advance the mushroom research and production facilities, create a national mushroom research center, and establish international research linkages and collaborations.
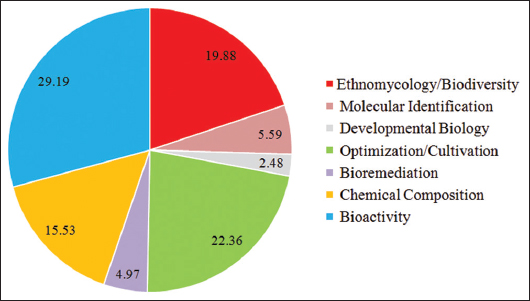 | Figure 2: Percentage of Philippine mushroom research aspects. [Click here to view] |
Table 4: Status of global and Philippine mushroom research.
| Research aspects | Global status | Philippines status |
|---|---|---|
| Biodiversity | New mushroom species have been discovered and recorded | No new species discovered |
| Molecular identification | Advanced molecular techniques have been introduced and effectively employed | Very basic molecular techniques and more on morphological approach |
| Developmental biology | New high-yield mushroom strains were generated and the transcriptional landscape of the different developmental stages of mushrooms has recently been studied | Optimal conditions for spore germination and morphogenesis of few mushrooms were established |
| Biomass production | Mushrooms are cultivated in chemically-defined medium or substrates in large scale production, and innovative cultivation techniques have been developed | Mushroom culture conditions were optimized, and practical production technologies have been generated |
| Chemical composition | Chemical components of mushrooms have been well-elucidated and the bioactive compounds have been isolated and characterized. New active compounds have been discovered | Proximate composition analysis was done, and some chemical components were characterized |
| Bioactivity | The different underlying mechanisms that linked to the bioactivity of the bioactive compounds have been studied | Preliminary bioactivity screening assay of the mushroom crude extracts have been employed |
In conclusion, this review highlights the existence, diversity, and distribution of wild mushrooms in the Philippines. Most importantly, this establishes the most comprehensive checklist of Philippine wild mushrooms, highlighting the ethnomycologically important, successfully cultivated, and pharmacologically significant mushroom species, which is very essential for the conservation and exploration of their numerous advantages. This review also shows the position of Philippine mushroom research in the global scenario, which provides direction toward the major mushroom research areas that require urgent and special attention.
8. ACKNOWLEDGMENT
This work was supported by the Center for Tropical Mushroom Research and Development, College of Science, Central Luzon State University, Philippines.
9. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
10. FUNDING
Science Education Institute, Department of Science and Technology, Philippines.
11. CONFLICTS OF INTEREST
All authors declare no conflicts of interest in this work.
12. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
14. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Martinez-Medina GA, Chavez-Gonzalez ML, Verma DK, Prado-Barragan LA, Martínez-Hernandez JL, Flores-Gallegos AC,
2. Beelman RB, Kalaras MD, Richie JP Jr. Micronutrients and bioactive compounds in mushrooms:A recipe for healthy aging?Nutr Today 2019;54:16-22. [CrossRef]
3. Friedman M. Mushroom polysaccharides:Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016;5:40. [CrossRef]
4. Singh R, Kumar M, Mittal A, Mehta PK. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotechnology 2017;7:15. [CrossRef]
5. Sánchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth Syst Biotechnol 2017;2:13-22. [CrossRef]
6. Ahmad MF.
7. Bonneville S, Delpomdor F, Preat A, Chevalier C, Araki T, Kazemian M,
8. Hawksworth DL, Lucking R. Fungal diversity revisited:2.2 to 3.8 million species. Microbiol Spectr 2017;5:5-4. [CrossRef]
9. Species Fungorum;2020. Available from:http://www.speciesfungorum.org [Last accessed on 2020 Dec 08].
10. Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL. Notes for genera:Ascomycota. Fungal Divers 2017;86:1-594. [CrossRef]
11. Wijayawardene NN, Hyde KD, Divakar PK, Rajeshkumar KC, Weerahewa D, Delgado G,
12. He MQ, Zhao RL, Hyde KD, Begerow D, Kemler M, Yurkov A,
13. Cheek M, Lughadha EN, Kirk P, Lindon H, Carretero J, Looney B,
14. Index Fungorum;2020. Available from:http://www.indexfungorum.org [Last accessed o 2020 Dec 08].
15. Martins A. The numbers behind mushroom biodiversity. In:Isabel CF, Ferreira IC, Morales P, Barros L, editors. Wild Plants, Mushrooms and Nuts:Functional Food Properties and Applications. 1st ed. New York:John Wiley and Sons, Ltd.;2017. [CrossRef]
16. Meenu M, Xu B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom:A concise review. Food Chem 2019;289:545-57. [CrossRef]
17. Quimio TH. Records of Philippine fungi. Proc Indian Natl Sci Acad 1986;96:359-62. [CrossRef]
18. Reinking OA. Higher basidiomycetes from the Philippines and their hosts, II. Philippine J Sci 1920;16:167-79.
19. Teodoro NG. An enumeration of Philippine fungi. Techn Bull Dept Agric Comm Manila 1937;4:1-585.
20. Dogma IJ. Philippine
21. Reynolds DR. A key to known Philippine Gasteromycetes. Philipp Agric 1967;50:268-78.
22. Quimio TH. Some discomycetes from Mt. makiling (Philippines). Nova Hedwigia 1978;28:515-26.
23. Quimio TH. Agaricales of Mt. Makiling. Proceeding 44th Annual and Scientific Congress;(National Research Council of the Philippines);1977. 35-42.
24. Quimio TH. Some unreported agaricales of Mt. makiling. Nova Hedwigia 1982;37:421-32.
25. Quimio TH. Some unreported agaricales of Mt. makiling (Philippines). Nova Hedwigia 1983;38:421-32.
26. Quimio TH. Agaricales of Mt. Makiling, Laguna, Philippines. Vol. 47. Proceedings of the Asian International Mycological Congress, Chiba, Japan;1996.
27. Quimio TH, Opina NL, Lantican MT. Agaricales of Mt. makiling III. genus
28. Quimio TH, Opina NL. Agaricales of Mt. makiling II. genus
29. Quimio TH. Suayan ZA. Agaricales of Mt. makiling I. genus
30. Quimio TH. Philippine Auricularias:Taxonomy, nutrition and cultivation. Mushroom Sci 1981;11:685-96.
31. Quimio TH. Checklist and database of Philippine Fungi (1806-2001). Laguna:ASEAN Regional Center for Biodiversity Conservation;2002.
32. PAGASA. Climate of the Philippines. Available from https://bagong.pagasa.dost.gov.ph/information/climate-philippines [Last accessed on 2020 Feb 22].
33. Lantican RM. The Science and Practice of Crop Production. College, Los Banos, Laguna, Philippines;SAMEO SEARCA and UPLB;2001. 330.
34. Kintanar RL. Climate of the Philippines. Philippines:PAGASA;1984.
35. Convention on Biological Diversity n.d. Philippines Main Details Biodiversity Facts. Available from:https://www.cbd.int/countries/profile/?country=ph [Last accessed on 2022 Feb 21].
36. Tadiosa ER, Lubos LC. Common macroscopic fungi and mosses of North Central Mindanao. Asian J Biodivers 2019;10:26-129. [CrossRef]
37. Soriano JK, Pampolina N, Carandang V. Macrofungal taxa and diversity for monitoring of productivity and sustainability of Bombongan-Lewin Subwatershed in Laguna, Philippines. Ecosyst Dev J 2021;11:82-92.
38. Dulay RM, Carandang VI, Kalaw SP, Reyes RG. Distribution and species listing of wild macrofungi in sitio Canding, Barangay Maasin, San Clemente, Tarlac Province, Philippines. J Appl Biol Biotechnol 2020;8:7-15.
39. De Leon AM, Cruz AS, Evangelista AB, Miguel CM, Pagoso EJ, Dela Cruz TE,
40. De Leon AM, Pagaduan MA, Panto B, Kalaw SP. Species listing of macrofungi found in Paracelis Mountain Province, Philippines. CLSU Int J Sci Technol 2021;5:21-39. [CrossRef]
41. Dulay RM, Cabrera EC, Kalaw SP, Reyes RG. Optimization of culture conditions for mycelial growth and fruiting body production of naturally-occurring Philippine mushroom
42. Parlucha JA, Soriano JK, Yabes MD, Pampolina NM, Tadiosa ER. Species and functional diversity of macrofungi from protected areas in mountain forest ecosystems of Southern Luzon, Philippines. Trop Ecol 2021;62:359-67. [CrossRef]
43. Brazas FP Jr., Taglinao LP, Revilla AG, Javier RF, Tadiosa ER. Diversity and taxonomy of basidiomycetous fungi at the northeastern side of Quezon protected landscape, Southern Luzon, Philippines. J Agric Sci Technol A 2020;10:1-11. [CrossRef]
44. Culliao AG, Lumang-Ay RS, Kingat GM, Colallad TP, Canaria MV. Preliminary bioactivity screening of crude extracts of six wild macrofungi from pine forests in Benguet and Mt. Province, Philippines. Manila J Sci 2020;13:74-88.
45. De Leon AM, Dulay AR, Villanueva AL, Kalaw SP. Optimal culture conditions and toxicity assessment of
46. Dulay RM, Damaso EJ Jr. The successful cultivation of Philippine wild mushroom
47. Garcia BL, Undan JR, Dulay RM, Kalaw SP, Reyes RG. Molecular identification and optimization of cultural conditions for mycelial biomass production of wild strain of
48. Garcia K, Garcia CJ, Bustillos R, Dulay RM. Mycelial biomass, antioxidant, and myco-actives of mycelia of abalone mushroom
49. Guerrero JJ, Banares EN, General MA, Imperial JT. Rapid survey of macro-fungi within an urban forest fragment in Bicol, Eastern Philippines. Österr Z Pilzk 2020;28:37-43.
50. Paguirigan JA, David BA, Elsisura RN, Gamboa AJ, Gardaya RF, Ilagan JP,
51. Torres ML, Tadiosa ER, Reyes RG. Species listing of macrofungi on the Bugkalot Tribal community in Alfonso Castañeda, Nueva Vizcaya, Philippines. Curr Res Environ Appl Mycol 2020;10:475-93. [CrossRef]
52. Alcantara MK, Calupitan JR, Lazo AM, Salazar LK, Mancera JP, Tadiosa ER,
53. Clutario MJ, Cuizon JE. Diversity Survey of Agarics and Polypores on the Eastern Slopes of Mt. Palemlem, Ilocos Norte, Philippines. Doctoral Dissertation;2019.
54. Aquino HLC, Silvestre JR. Diversity and Ethnomycological Studies of the Agaricales on the Northeastern Slopes of Mt. Pao, Adams, Ilocos Norte, Philippines. Doctoral Dissertation. 2019.
55. Laggui ZK. Taxonomic classification, population density and distribution of macro-basidiomycetes at CSU-Lallo. J Biodivers Environ Sci 2019;14:142-52.
56. Ramirez RB Jr., Capili JT. High altitude deep forest mushrooms in Lagawe, Ifugao, Cordillera Autonomous Region, Northern Philippines. J Biodivers Environ Sci 2019;14:25-30.
57. Arenas MC, Tadiosa ER, Reyes RG. Taxonomic inventory based on physical distribution of macrofungi in Mt. Maculot, Cuenca, Batangas, Philippines. Int J Biol Pharm Allied Sci 2018;7:672-87. [CrossRef]
58. Culala JM, Dulay RM. Species listing of naturally occurring mushrooms in Central Luzon State University, Science City of Munoz, Nueva Ecija, Philippines. Int J Biol Pharm Allied Sci 2018;7:1890-9. [CrossRef]
59. Gaylan CM, Estebal JC, Tantengco OA, Ragragio EM. Anti-staphylococcal and antioxidant properties of crude ethanolic extracts of macrofungi collected from the Philippines. Pharmacogn J 2018;10:106-9. [CrossRef]
60. Guzman CD, Baltazar MM, Sanchez AJ, Linsangan MG, Dulay RM. Molecular identification of four wild higher basidiomycetes collected in Mt. Mingan, Gabaldon, Nueva Ecija, Philippines. J Biodivers Environ Sci 2018;13:46-51.
61. Nacua AE, Pacis HY, Manalo JR, Soriano CJ, Tosoc NR, Padirogao R,
62. Ragasa CY, Tan MC, De Castro ME, Mariquit M, Oyong GG, Shen CC. Sterols from
63. Ragasa CY. Anticancer compounds from nine commercially grown and wild Philippine mushrooms. Manila J Sci 2018;11:42-57.
64. Ramel DM. Species listing and mycophagy of macrofungi found in Barobbob Watershed, Bayombong, Nueva Vizacya, Cagayan Valley Region. Master of Science Thesis. Central Luzon State University, Science City of Munoz, Philippines;2018.
65. Sogan MM, Maslang JA, Dulay RM. Myco-chemicals and teratogenic activity of wild mushroom
66. Tantengco OA, Ragragio EM. Ethnomycological survey of macrofungi utilized by Ayta communities in Bataan, Philippines. Curr Res Environ Appl Mycol 2018;8:104-8. [CrossRef]
67. Liwanag JM, Santos EE, Flores FR, Clemente RF, Dulay RM. Species listing of macrofungi in Angat watershed reservation, Bulacan province, Luzon Island, Philippines. Int J Biol Pharm Allied Sci 2017;6:1060-8.
68. Romorosa ES, De Guzman CT, Martin JR, Jacob JK. Preliminary investigation on the pharmacological properties of wood-rotting mushrooms collected from Isabela State University, Echague, Isabela, Philippines. Int J Agric Technol 2017;13:2591-6.
69. Dulay RM, Maglasang CC. Species listing of naturally occurring mushrooms in agroecosystem of Barangay Bambanaba, Cuyapo, Nueva Ecija, Philippines. Int J Biol Pharm Allied Sci 2017;6:1459-72.
70. Jacob JK, Romorosa ES, Kalaw SP. Species listing of macroscopic fungi in Isabela State University, Isabela as baseline information. Int J Agric Technol 2017;13:1199-203.
71. Arenas MC, Tadiosa ER, Grecebio JD, Renato GR. Evaluation of its and Lsu Loci in DNA barcoding of selected medicinal macrofungi from Calabarzon region, Philippines. Int J Biol Pharm Allied Sci 2017;6:2087-100.
72. Dulay RM, Rivera AG, Garcia EJ. Mycelial growth and basidiocarp production of wild hairy sawgill
73. Dulay RM, Rivera AG. Mycelial growth and fruiting body production of Philippine (CLSU) strain of
74. Angeles LP, Arma EJ, Basaca CW, Biscocho HE, Castro AE, Cruzate SM,
75. De Castro ME, Dulay RM, Enriquez ML. Toxic and teratogenic effects of medicinal and culinary mushroom,
76. De Leon AM, Kalaw SP, Dulay RM, Undan JR, Alfonzo DO, Undan JQ,
77. Kalaw SP, Alfonso DO, Dulay RM, De Leon AM, Undan JQ, Undan JR,
78. Lopez AV, Aquino JD, Undan JQ, Waing KG, Undan JR. Molecular identification and phylogeny of some wild microscopic fungi from selected areas of Jaen, Nueva Ecija, Philippines. Adv Environ Biol 2016;10:153-8.
79. Reyes RG, Undan JQ, Dulay RM, Kalaw SP, Undan JR. The first report on the molecular identification of Termitomyces of Central Luzon, Philippines. Int J Pharm Res Allied Sci 2016;5:140-5.
80. Undan JQ, Alfonso DO, Dulay RM, De Leon AM, Kalaw SP, Undan JR,
81. Arenas MC, Tadiosa ER, Alejandro GJ, Reyes RG. Macroscopic fungal flora of mts. Palaypalay-Mataas na Gulod Protected Landscape, Southern Luzon, Philippines. Asian J Biodivers 2015;6:693. [CrossRef]
82. De Castro ME, Dulay RM. Macrofungi in multistorey agroforestry systems in Mt. Makiling Forest Reserve, Los Banos, Laguna, Philippines. J Chem Biol Phys Sci 2015;5:1646.
83. Lazo CR, Kalaw SP, De Leon AM. Ethnomycological survey of macrofungi utilized by Gaddang communities in Nueva Vizcaya, Philippines. Curr Res Environ Appl Mycol 2015;5:256-62. [CrossRef]
84. Tadiosa ER, Arenas MC, Reyes RG. Macroscopic Fungi of Mts. Banahaw-San Cristobal Protected Landscape Northwestern side, with a description of
85. Niem JM, Baldovino MM. Initial checklist of macrofungi in the karst area of Cavinti, Laguna. Museum Publ Nat Hist 2015;4:11-25.
86. Tadiosa ER, Arsenio JS. A taxonomic study of wood-rotting basidiomycetes at the molave forest of San Fernando City, La Union Province, Philippines. Asian J Biodivers 2014;5:483. [CrossRef]
87. De Leon AM, Luangsa-Ard JJ, Karunarathna SC, Hyde KD, Reyes RG, Dela Cruz TE. Species listing, distribution, and molecular identification of macrofungi in six Aeta tribal communities in Central Luzon, Philippines. Mycosphere 2013;4:478-94. [CrossRef]
88. Tadiosa ER, Briones RU. Fungi of Taal volcano protected landscape, Southern Luzon, Philippines. Asian J Biodivers 2013;4:296. [CrossRef]
89. De Leon AM, Reyes RG, Dela Cruz TE. An ethnomycological survey of macrofungi utilized by Aeta communities in Central Luzon, Philippines. Mycosphere 2012;3:251-9. [CrossRef]
90. Llarena ZM, Solidum JN. Mycoremediation of toxicants from chosen sites in the Philippine setting. Int J 2012;3:340-2.
91. Tadiosa ER, Agbayani EA, Agustin NT. Preliminary study on the Macrofungi of Bazal-Baubo Watershed, Aurora Province, Central Luzon, Philippines. Asian J Biodivers 2011;2. [CrossRef]
92. Sibounnavong P, Cynthia CD, Kalaw SP, Reyes RG, Soytong K. Some species of macrofungi at Puncan, Carranglan, Nueva Ecija in the Philippines. J Agric Technol 2008;4:105-15.
93. Tadiosa ER, Arsenio JS, Marasigan MC. Macroscopic fungal diversity of Mount Makulot, Cuenca, Batangas, Philippines. J Nat Stud 2007;6:111-24.
94. Tadiosa ER, Militante EP. Identification of important wood-decaying fungi associated with some Philippine dipterocarps at the Makiling Forest. Sylvatrop 2006;16:17-37.
95. Musngi RB, Abella EA, Lalap AL, Reyes RG. Four species of wild
96. Tayamen MJ, Reyes RG, Floresca EJ, Abella EA. Domestication of wild edible mushrooms as non-timber forest products resources among the Aetas of Mt. Nagpale, Abucay, Bataan:
97. Flores AA Jr., Alvarez ML, Cortez FE, Perez BE, Sanico FL, Somoray MJ,
98. Jusayan RR, Vicencio MC. The macrofungi in the island of San Antonio, Northern Samar, Philippines. Int J Trend Sci Res Dev 2019;3:968-75. [CrossRef]
99. Perpetua NO, Almaquer MT, Dragon JM. Biodiversity of Mushrooms at Dansolihon, Cagayan de Oro City, Philippines. Adv Biol Res 2013;2.
100. Dejene T, Oria-de-Rueda JA, Martín-Pinto P. Wild mushrooms in Ethiopia:A review and synthesis for future perspective. For Syst 2017;26:eR02. [CrossRef]
101. Kim NK, Lee JH, Jo JW, Lee JK. A checklist of mushrooms of Cambodia. J For Environ Sci 2017;33:49-65. [CrossRef]
102. ArzúRF, Comandini O, Rinaldi AC. A preliminary checklist of macrofungi of Guatemala, with notes on edibility and traditional knowledge. Mycosphere 2012;3:1-21. [CrossRef]
103. Vasco-Palacios AM, Franco-Molano AE. Diversity of Colombian macrofungi (
104. Devkota S, Aryal HP. Wild mushrooms of Nepal. In:Plant Diversity in Nepal. Kathmandu:Botanical Society of Nepal;2020. 41-54.
105. De Leon AM, Fermin SM, Rigor RP, Kalaw SP, Dela Cruz TE, Stephenson SL. Ethnomycological report on the macrofungi utilized by the indigenous community in Ifugao Province, Philippines. Philipp Agric Sci 2018;101:194-205.
106. Food and Agriculture Organization. Harvested Area and Production Quantity of Mushroom around Word from 2009 to 2018. Rome, Italy:Food and Agriculture Organization;2018. Available from:http://www.fao.org/faostat/en/#data/QC [Last accessed on 2020 Dec 04].
107. Bellettini MB, Fiorda FA, Maieves HA, Teixeira GL, Avila S, Hornung PS,
108. Kabel MA, Jurak E, MäkeläMR, de Vries RP. Occurrence and function of enzymes for lignocellulose degradation in commercial
109. Reyes RG, Eguchi F, Iijima T, Higaki M. Influence of medium composition and plant growth regulators on the mycelial growth of
110. Reyes RG, Abella EA, Eguchi F, Iijima T, Higaki M. Physiology of
111. Reyes RG, Lopez LL, Kumakura K, Kalaw SP, Kikukawa T, Eguchi F.
112. Garcia B, Reyes R, Santos J, Abella E. Domestication of wild edible mushrooms as non-timber forest products resources among the Aetas of Mt. Nagpale, Abucay, Bataan:
113. Gisala KJ, Reyes R, Abella E. Development of production technology for kudit (
114. Cuevas MJ, Reyes R, Kalaw S. Biophysiology of
115. Dulay RM, Cabrera EC, Kalaw SP, Reyes RG. Optimal growth conditions for basidiospore germination and morphogenesis of Philippine wild strain of
116. De Leon AM, Reyes RG, Cruz TE.
117. Bustillos RG, Dulay RM, Kalaw SP, Reyes RG. Optimization of culture conditions for mycelial growth and basidiocarp production of Philippine strains of
118. Kalaw SP, Albinto RF. Functional activities of Philippine wild strain of
119. Dulay RM, Damaso EJ Jr. The first report on the successful rescue and domestication of Philippine wild mushroom
120. Bellere AD. Mycelial growth of
121. Magday JC Jr., Dulay RM, Bungihan ME. Optimization of mycelial growth and cultivation of fruiting body of Philippine wild strain of
122. Ramos R, Reyes R, Abella E. Mycelial production technology for
123. Zurbano LY. Mycelial growth and fructification of earwood mushroom (
124. Reyes RG, Eguchi F, Iijima T, Higaki M. Physiological considerations for the efficient colonization of fukurotake,
125. Reyes RG. Indoor cultivation of paddy straw mushroom,
126. Bustillos RG, Dulay RM, Bauto JJ, Pascual F, Baltazar K, Bunag HW,
127. Dulay RM, Ray K, Hou CT. Optimization of liquid culture conditions of Philippine wild edible mushrooms as potential source of bioactive lipids. Biocatal Agric Biotechnol 2015;4:409-15. [CrossRef]
128. Dulay RM, Flores KS, Tiniola RC, Marquez DH, Cruz AG, Kalaw SP,
129. Reyes RG, Grassel W, Rau U. Coconut water as a novel culture medium for the biotechnological production of
130. Dulay RM, De Castro ME. Heavy metal resistance and mycoremediation potential of split-gill basidiomycetes,
131. Dulay RM, Cardona EM, Kalaw SP, Reyes RG. Optimization of liquid culture conditions of
132. Dulay RM, Andres SM, Asuncion AF, Calalang AS, Cumbe AP. Mycelial biomass production and radical scavenging activity of
133. Bustillos RG, Francisco CS, Dulay RM. Liquid culture and antioxidant properties of
134. Liwanag EJ, Dulay RM, Kalaw S. Mycelial growth of Philippine mushroom
135. Mendoza WC, Dulay RM, Valentino MJ, Reyes RG. Mycelial biomass and biological activities of Philippine mushroom
136. Dulay RM, Cabrera EC, Kalaw SP, Reyes RG. Optimization of submerged culture conditions for mycelial biomass production of fourteen
137. Bulseco MG, Abella EA, Reyes RG. Morphogenesis of
138. Dulay RM, Cabrera EC, Kalaw SP, Reyes RG, Hou CT. Cultural conditions for basidiospore germination of
139. Dulay RM, Parungao AG, Kalaw SP, Reyes RG. Aseptic cultivation of
140. Dulay RM, Gagarin WS, Abella EA, Kalaw SP, Reyes RG. Aseptic cultivation and nutrient compositions of
141. Dulay RM, Sanguesa KB, Ablaza JL, Joson AJ, Peria JN, Quejada CS,
142. De Leon AM, Reyes RG. Enriched cultivation of three wild strains of
143. Dulay RM, Harada HL, Santos MM, Miguel CM, De Castro ME. Mycelial growth and fruiting body performance of three Philippine edible mushrooms on Vitamin A-supplemented media. Int J Biol Pharm Allied Sci 2017;6:308-15.
144. Mimis BR, Martin AM, Manalo AN, Dulay RM.
145. Damaso EJ Jr., Dulay RM, Kalaw SP, Reyes RG. Effects of color light emitting diode (LED) on the mycelial growth, fruiting body production, and antioxidant activity of
146. Dulay RM, Arenas MC, Kalaw SP, Reyes RG, Cabrera EC. Proximate composition and functionality of the culinary-medicinal tiger sawgill mushroom,
147. Dulay RM, Cabalar AC, De Roxas MJ, Concepcion JM, Cruz NE, Esmeralda M,
148. Raneses MA, Dulay RM, De Leon AM. Proximate nutritive composition and teratogenic effect of
149. De Leon AM, Orpilla JO, Cruz KV, Dulay RM, Kalaw SP, Dela Cruz TE. Optimization of mycelial growth and mycochemical screening of
150. Dulay RM, Pamiloza DG. Proximate composition and bioactivities of hairy sawgill mushroom,
151. Aquino YK, Vega LD, Medrano NR, Dulay RM. Mycochemicals, antioxidant and cytotoxic activities of
152. Nanglihan KE, Dulay RM, Kalaw SP. Myko-actives and functional activities of Philippine wild mushroom
153. De Leon AM, Diego EO, Domingo LK, Kalaw SP. Mycochemical screening, antioxidant evaluation and assessment of bioactivities of
154. Reyes RG, Kalaw SP, Dulay RM, Gonzaga R, Yoshimoto H, Kikukawa T,
155. Eguchi F, Kalaw SP, Dulay RM, Miyasawa N, Yoshimoto H, Seyama T,
156. Reyes RG, Kalaw SP, Dulay RM, Yoshimoto H, Miyazawa N, Eguchi F. Philippine native and exotic species of edible mushrooms grown on rice straw-based formulation exhibit nutraceutical properties. Philipp Agric Sci 2013;96:198-204.
157. Hou CT, Lin JT, Dulay RM, Ray K. Identification of molecular species of acylglycerols of Philippine wild edible mushroom,
158. Hou CT, Lin JT, Dulay RM, Ray K. Identification of the molecular species of acylglycerols containing hydroxy fatty acids in wild edible mushroom
159. Lin JT, Hou CT, Dulay RM, Ray K, Chen GQ. Structures of hydroxy fatty acids as the constituents of triacylglycerols in Philippine wild edible mushroom,
160. Ragasa CY, Oyong GG, Tan MC, De Los Reyes, MM, De Castro ME. Cytotoxic sterols from Philippine mushrooms. Asian J Chem 2020;32:1197-202. [CrossRef]
161. Umagat MR, Dulay RM, Olivo JC, Abon MD, Francisco BE, Kalaw SP,
162. Painuli S, Semwal P, Egbuna C. Mushroom:Nutraceutical, mineral, proximate constituents and bioactive component. In:Functional Foods and Nutraceuticals. Berlin:Springer;2020. 307-36. [CrossRef]
163. Ho LH, Zulkifli NA, Tan TC. Edible mushroom:Nutritional properties, potential nutraceutical values, and its utilisation in food product development. In:An Introduction to Mushroom. London:IntechOpen;2020. 19-38.
164. Lu J, He R, Sun P, Zhang F, Linhardt RJ, Zhang A. Molecular mechanisms of bioactive polysaccharides from
165. Dulay RM, Vicente JJ, Dela Cruz AG, Gagarin JM, Fernando W, Kalaw SP,
166. Reyes RG, Nair MG. Ligninolytic and leaf litter degrading mushrooms from the Philippines with antioxidant activities. Int J Pharm Res Allied Sci 2016;5:67-74.
167. Dulay RM, Miranda LA, Malasaga JS, Kalaw SP, Reyes RG, Hou CT. Antioxidant and antibacterial activities of acetonitrile and hexane extracts of
168. Reyes RG, del Rosario MA, Padua JP, Malonzo MA, Barza AJ, Sumi R,
169. Chang AK, Frias RR Jr., Alvarez LV, Bigol UG, Guzman JP. Comparative antibacterial activity of commercial chitosan and chitosan extracted from
170. Hussin FR, Vitor RJ, Joaquin JO, Clerigo MM, Paano AM. Anti-hyperglycemic effects of aqueous
171. Eguchi F, Dulay RM, Kalaw SP, Yoshimoto H, Miyazawa N, Seyama T,
172. Arnante ME, Clerigo MM, Paano AM, Enriquez ML. Cytotoxic and Genotoxic Activity of an Extract from the Mushroom
173. Buniel PA, Scheewe HW, Sanico CG Jr., Alima ZD, Demayo CG. Assessing the genotoxic and cytotoxic responses of the H-29 cancer cell lines on the ethanolic extracts of the oyster mushroom,
174. Dulay RM, Valdez BC, Li Y, Chakrabarti S, Dhillon B, Kalaw SP,
175. Dulay RM, De Guzman ML, Magisa RR, Mariano JC, San Pedro MC, Mangansat NJ,
176. Dulay RM, Kalaw SP, Reyes RG, Alfonso NF, Eguchi F. Teratogenic and toxic effects of lingzhi or reishi medicinal mushroom,
177. Dulay RM, Kalaw SP, Reyes RG, Cabrera EC. Embryo-toxic and teratogenic effects of Philippine strain of
178. Dulay RM, Rivera AG, De Castro ME. Wild paddy straw mushroom (
179. Dulay RM, Pamiloza DG, Ramirez RL. Toxic and teratogenic effects of mycelia and fruiting body extracts of
180. De Castro ME, Dulay RM. Toxic and teratogenic effects of
181. Bustillos RG, Paguio ZK, Hermosa DP, Dulay RM. Philippine coprophilous mushrooms (
182. Dulay RM, Apolinar AA, Tiniola RC, Kalaw SP, Reyes RG. Aphrodisiac and diuretic activity of Philippine wild higher basidiomycetes,
183. Aquino YK, Dulay RM, Kalaw SP. Effect of