1. INTRODUCTION
Exopolysaccharides (EPSs) are polymers made up of monosaccharides that are linked through glycosidic bonds. They have high molecular mass and are secreted by plants, animals, and microorganisms. Fungi and bacteria have been prioritized over other microorganisms for their excellent exopolysaccharide production capabilities [1]. Chemically, EPSs are primarily composed of the carbohydrate moieties. In addition to carbohydrate molecules, inorganic and organic functional groups such as acetate, amine, phosphate, succinate, and sulfate have also been found in their chemical structures [2]. On the basis of their characteristics, these compounds can be employed as an alternative product of plants and algae being considered as non-hazardous and naturally biodegradable products [3].
Apart from bacteria, yeasts, lower filamentous fungi, and higher basidiomycetes are also known to produce EPS. Even though bacteria are regarded as the most common producers of EPS, the extraction procedure of EPS produced by yeasts and fungi is convenient and quick as compared to bacterial EPSs; and thus, it can be directed to a larger scale [4]. Fungi have been reported to be a promising source of pharmaceutically important compounds. The polysaccharides produced by fungi have been regularly investigated for their biological activities. The production of polysaccharides occurs in different phases of the fungal life cycle and can be found intracellular, attached to the cell, or secreted in the extracellular environment.
One of the members of basidiomycetes fungi, Fomitopsis pinicola, is commonly used as a medicinal mushroom in Asia [5]. Although there are a number of reports on properties EPS from Fomitopsis fruiting body, few studies have been carried out on the structural and morphological characteristics of EPS from Fomitopsis. Hsieh et al. [6] reported that there can be noteworthy differences in the structure or composition of polysaccharides from various sources. To the best of the author’s knowledge, no reports have been made to date on the compositional characteristics of EPS from Fomitopsis meliae.
Given the limited knowledge of the EPS synthesized by F. meliae, the present study was conducted to purify and characterize the EPS produced under submerged cultivation of Fomitopsis meliae AGDP-2. The characterization of EPS including monosaccharide composition (high-performance thin-layer chromatography [HPTLC] and HPLC), molecular weight (GPC), rheological behavior, surface morphology (SEM and AFM), as well as IR and nuclear magnetic resonance (NMR) spectra was thoroughly studied.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
The monosaccharide standards (glucose, mannose, arabinose, xylose, lactose, galactose, mannitol, sucrose, sorbitol, and xylitol) used for reference in HPTLC and HPLC as well as dextran standard used as reference in GPC were purchased from Sigma-Aldrich, St. Louis, USA. All other analytical grade chemicals were purchased from HiMedia, Mumbai, India.
2.2. Microorganisms and Growth Conditions
The native fungal isolate F. meliae AGDP-2 was maintained on PDA medium. For the production of EPS, six 8 mm diameter sized mycelial agar plugs were aseptically inoculated in 100 mL of statistically optimized Tien and Kirk medium (g/L: Glucose 37.5; peptone 6.0; yeast extract 2.0; CaCl2 0.4; KH2PO4 2.0; MgSO4 0.9; and pH 5.3) and were incubated at 28°C for 10 days.
2.3. Extraction of EPS and Biomass Determination
The dry biomass content was determined separating the biomass from fermentation broth using muslin cloth followed by drying at 60°C. EPS precipitation and extraction were achieved by adding ice-cold isopropyl alcohol to the filtrate in 1:1 v/v proportion. The crude extracted EPS was dried at 50°C to estimate the yield.
2.4. Purification of EPS
The precipitated EPS was dialyzed against distilled water overnight at 4°C. The EPS obtained was further subjected to Sephadex G-75 Gel Permeation Chromatography column. The fractions were collected at the flow rates of 0.2 mL/min. Total carbohydrate and protein contents of crude, dialyzed and purified EPS were measured.
2.5. Characterization of EPS
2.5.1. Total carbohydrate and protein content analysis
The total carbohydrate content of EPS was measured by phenol-sulfuric acid method using ?-glucose as a standard [7]. The total protein content of crude and partially purified EPS was measured by Folin-Lowry’s method. Bovine serum albumin was used as a standard [8].
2.5.2. Monosaccharide composition analysis
Primarily, the presence of sugar monomers in EPS was determined using HPTLC. In brief, EPS was hydrolyzed in 4 M trifluoroacetic acid (TFA) in a sealed tube at 100°C for 4 h. After hydrolysis, sample was cooled to room temperature and TFA was coevaporated with methanol to dryness under vacuum. The hydrolyzed EPS sample and standard sugars (glucose, maltose, xylose, and mannose) were run on silica gel 60 F254 plate (Merck, Germany). A sample of 10 ml was spotted on the TLC plate using micro syringe (HPTLC, CAMAG, Linomat 5). The mobile phase was composed of the ternary solvent system butanol/ethanol/H2O in the ratio of 5:3:2 v/v/v. After drying, the TLC plate was developed using methanol/H2SO4 developing solution (9:1 v/v). On heating the TLC plate above 100°C in hot air oven for about 5 min, the sugar spots were visually observed. Chromatogram was observed using Camag TLC scanner 3.
The hydrolyzed EPS was applied to HPLC with RI detector system (Shimadzu LC-2010C HT) having Aminex® HPX 87 H Ion Exclusion column (300 mm × 7.8 mm). The column temperature was maintained to 65°C temperature. A 5 mM sulfuric acid was used as a mobile phase to achieve separation of sugar monomers.
2.5.3. UV–visible spectroscopy
The homogeneity of EPS was checked using Thermo Scientific Multiskan Go spectrophotometer system. The EPS sample was scanned at the wavelength ranges of 200–1000 nm with the interval of 1 nm.
2.5.4. Fourier-transform infrared (FT-IR) analysis
To study the bonding patterns and identify the major functional groups, the EPS was subjected to FT-IR analysis using a PerkinElmer (Spectra GX) spectrometer. The solid EPS sample was pressed into KBr pellets. The IR spectrum was recorded between the frequency ranges of 4000-400 cm−1 at a resolution of 200 cm−1.
2.5.5. NMR spectroscopy
The detailed structure of EPS was studied using the NMR spectroscopy. The NMR spectroscopy of EPS was performed using JEOL ECZR series 600 MHz NMR spectrophotometer. The EPS sample (10 mg) was dissolved in D2O (0.5 mL, 99.9%) solvent and 1H, 13C, and 2D HSQC NMR studies were conducted. The operating frequency for 1H- and 13C-NMR was 600 MHz and 150 MHz, respectively. The chemical shift (δ) for proton NMR and 13C-NMR was recorded in the range of 1–10 ppm and 10–200 ppm, respectively. 1H-NMR spectra were acquired in total of 700 scan, whereas 13C-NMR spectra were obtained in total of 600 scans.
2.5.6. Determination of molecular weight
The weight-average molecular weight (Mw) and number average molecular weight (Mn) of EPS were analyzed by size exclusion chromatography. The GPC system was equipped with refractive index detector, PL aquagel-OH 40 column, and PLgel Mixed guard column. The filtered EPS sample was injected in the column and eluted at the flow rates of 1 mL/min at 35°C temperature. Dextran was used as a standard.
2.5.7. Field emission scanning electron microscopy and energy-dispersive X-ray spectroscopy (SEM-EDX)
The dried EPS sample was analyzed to study the surface morphology and microstructure using the field emission scanning electron microscope (FEG-SEM). The micrographs of the EPS surface were captured by operating the FE-SEM (JEOL-JAPAN; JSM-7600F) with an accelerated voltage of 10 kV. To study about the presence of various elements on the surface of the EPS, energy-dispersive X-ray spectroscopy was performed along with FE-SEM.
2.5.8. Atomic force microscopy (AFM)
Atomic force microscope (Asylum Research, USA, MFP-3D BIO) was used to study the molecular morphology of EPS. For the same, the polysaccharide was dissolved in distilled water at a concentration of 10 μg/mL and was filtered through 0.45 μm filter. Then, about 100 μL of the polysaccharide solution was placed on freshly cleaved mica surface and dried at room temperature. Micrographs of EPS were taken using AFM under tapping mode.
2.5.9. Rheological behavior of EPS
The viscosity of EPS solution was measured using a Brookfield viscometer. The EPS was dissolved in distilled water to make 1% solution. The effect of temperature (in range of 30–70°C) on the viscosity of the exopolysaccharide solution was measured at various shear rates (6–100 rpm). Moreover, the viscosity of EPS solution at various pH (1–13) was also measured. The viscosity of EPS solution was compared with guar gum.
3. RESULTS AND DISCUSSION
3.1. Purification of EPS
The dialyzed EPS was passed through a Sephadex-G75 column for further purification. As depicted in Figure 1, the EPS started eluting from the 5th fraction and the maximum elution was observed in the 20th fraction. The EPS purification profiled is displayed in Table 1.
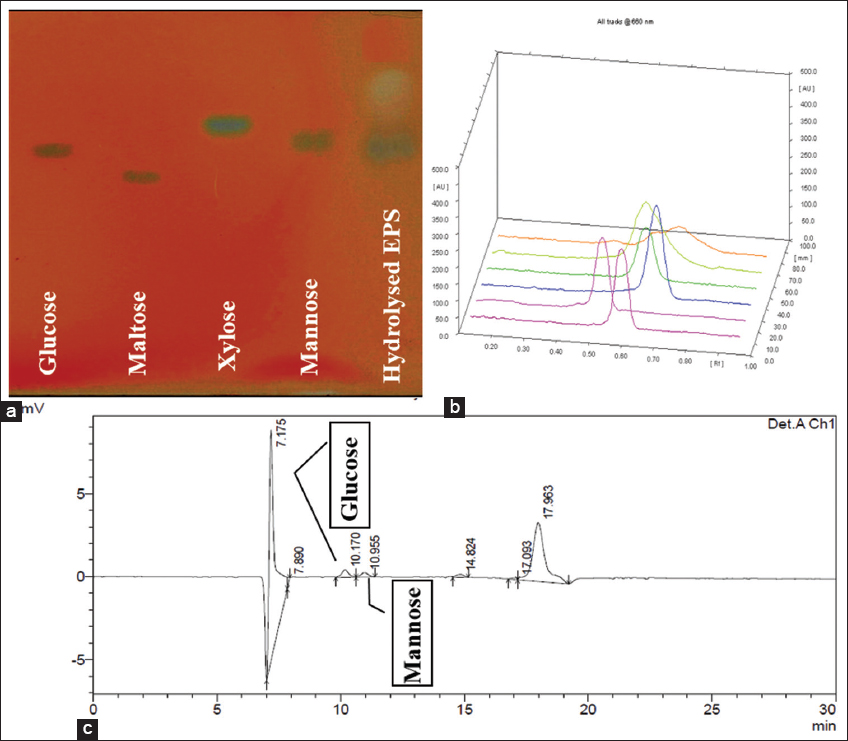 | Figure 1: (a) Separation of hydrolyzed EPS on TLC sheet, (b) HPTLC chromatogram, and (c) HPLC chromatogram. [Click here to view] |
Table 1: Purification profile of EPS.
| Samples | Volume (ml) | Total carbohydrate (mg) | Total protein (mg) | Carbohydrate/protein ratio | Yield (%) | Fold purification |
|---|---|---|---|---|---|---|
| Crude EPS | 30 | 259.2 | 4.5 | 57.55 | 100 | 1 |
| Dialyzed EPS | 33 | 248.7 | 3.96 | 62.80 | 95.84 | 1.09 |
| Sephadex G-75 | 33 | 28.95 | 0.062 | 466.93 | 11.16 | 8.11 |
The EPS was purified 8.11-fold with 11.16% yield. In contrast to our study, Su et al. [9] reported purification of EPS from Morchella aconica using DEAE-Cellulose 52 anion-exchange column chromatography with 5% purification yield. However, Sephadex G-100 column chromatography was used to purify the EPS obtained from Paecilomyces cicadae [10]. Limin et al. [11] reported purification schizophyllan from Schizophyllum commune using Sephacryl S-500 column chromatography.
3.2. Characterization of EPS
3.2.1. Monosaccharide composition analysis
The detection of sugar monomers in EPS was performed using HPTLC [Figure 1a and b]. The compositional monosaccharides present in EPS were glucose and mannose. This confirms that the EPS is a glucomannan with heteropolymeric nature. Similarly, using TLC monosaccharide composition of EPS from Lactobacillus fermentum TDS030603 was reported to have two monomers such as glucose and galactose [12].
The HPLC chromatogram of the hydrolyzed EPS from F. meliae AGDP-2 demonstrates and confirms that glucose and mannose are the major monomeric components present in varying concentrations [Figure 1c]. Herein, glucose and mannose were recorded as the predominant sugars showing peaks at retention times 10.170 and 10.995 min, respectively. EPS produced by a soil isolate Streptomyces sp. was also reported to be similarly composed of glucose and mannose monomers only [13]. Rani et al. [14] reported that probiotic Bacillus tequilensis FR9 isolated from the chicken gut was able to produce EPS which was composed of arabinose, glucose, galactose, mannose, and xylose as sugar monomers.
3.2.2. UV–visible spectroscopy
The UV–visible spectra of EPS in the range of 200–1000 nm were studied. The EPS showed no absorptions at 260 nm and 280 nm, indicating the absence of contaminants such as nucleic acids and proteins. Similar results were also reported by Yu et al. [15] who showed the absence of absorption at 260 and 280 nm for the UV scans of AMP-1 and AMP-2 polysaccharides extracted from Armillaria mellea.
3.2.3. FT-IR spectroscopy
The IR spectrum of EPS from F. meliae AGDP-2 displayed in Figure 2 shows a range of absorption peaks from 3410 to 555 cm−1. The broad peak observed at 3410.99 cm−1 is a characteristic presence of the stretching vibration of hydroxyl ion presence or hydroxyl groups (O-H). This confirms the polymeric nature of the sample and also indicates the strong intra- and inter-molecular interactions in EPS chains [16]. The absorption in this region also represents the carbohydrate rings in the structure which are responsible for water solubility of EPS [17]. A weak C-H stretching vibration near 2932 cm–1 was also observed. The C-N and the amide (>C=O) stretching vibration observed as prominent absorption peak at 1641.48 cm−1, which indicated the characteristics of carbohydrates such as mannose [18]. The presence of carboxyl groups is indicated by a weak symmetrical stretching at 1373.45 cm−1. The absorption in the region of 1000–1200 cm−1 is observed due to the asymmetric stretch vibration of C-O-C and C-O-H in polysaccharides [19]. Finally, the absorption band at 828.30 cm−1 indicates the presence of b-glycosidic linkage between glycosyl residues [20]. The diverse hydroxyl, amino, and carboxyl functional groups may serve as the binding sites for divalent cations and could contribute to the emulsification potential of EPS [21]. FTIR spectroscopy is also being used by researchers to study about functional groups and bonding patterns of polysaccharides [15,22,23].
 | Figure 2: FT-IR spectra of EPS. [Click here to view] |
3.2.4. Molecular weight determination
One of the most convenient methods to determine the molecular weight of polysaccharides is GPC. The weight-average molecular weight (Mw) of EPS produced of F. meliae AGDP-2 under submerged conditions was calculated to be 2.48 x 106 Daltons and number average molecular weight (Mn) was estimated to be 2.19 × 106 Daltons. Molecular weight distribution, that is, polydispersity index of the EPS was 1.13. The similar method was used for estimating the molecular weight of polysaccharides produced by S. commune IBRC-M30213 and Neopestalotiopsis sp. strain SKE15 [23,24]. The weight-average molecular weights of these polysaccharides were found to be 37 × 104 Daltons and 1.5 × 106 Daltons, respectively. Molecular weights of these polysaccharides are quite less than that of EPS produced by F. meliae AGDP-2.
3.2.5. NMR analysis
The 1H, 13C, and 2D HSQC NMR of EPS from F. meliae AGDP-2 demonstrated the complex and heterogeneous nature of EPS [Figure 3]. The 1H-NMR spectrum of EPS contains numerous signals between δ 3.0 and 4.0 ppm that signifies ring proton region. Other signals between δ 5.5 and 5.0 ppm represent the presence of α-anomers in the EPS structure [25]. The anomeric proton of α-?-mannose was indicated by a resonance at δ 5.1 ppm [26]. The signals recorded in the ring proton region δ 3.0–4.0 ppm could not be resolved properly due to very high molecular weight and overlapping chemical shifts and that can be described as key region of sugar molecules [27].
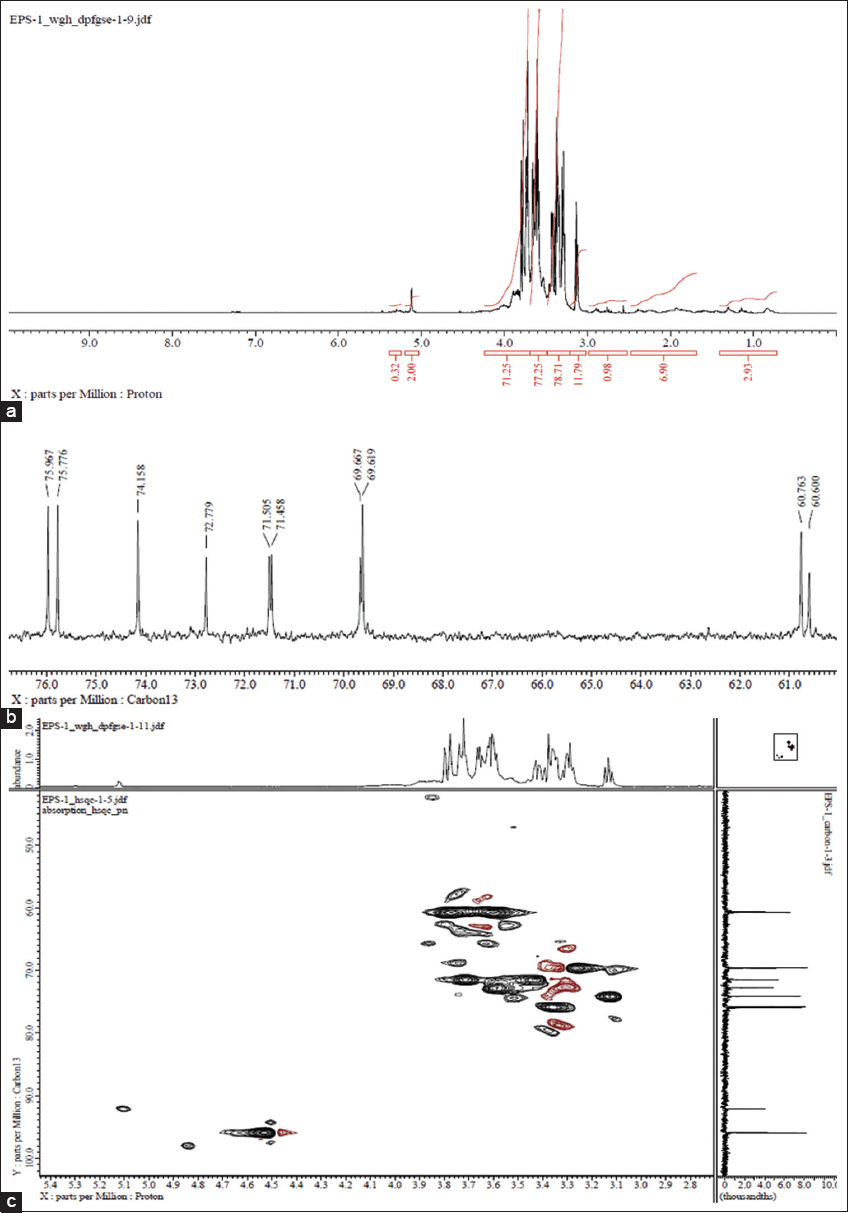 | Figure 3: NMR spectra of EPS; (a) 1H NMR (b) 13C NMR, and (c) 2D HSQC NMR. [Click here to view] |
The 13C-NMR spectrum of EPS from F. meliae AGDP-2 consists of two major regions: (i) anomeric region (δ 90–100 ppm) and (ii) ring carbons regions (δ 60–80 ppm). The chemical shifts at δ 72.77, 75.77, and 75.96 ppm represent the presence of β-?-glucose in EPS structure [26]. Moreover, the prominent signal at δ 69.61 and 69.66 ppm was attributed to the presence of α-?-mannose residues. The presence of glucose components was confirmed by the downfield shift at δ 60.60 and 60.76 ppm which were allotted to a C-6 carbon signal and corroborated that the two glucose units in the polymer chain backbone were linked through α-(1→6) linkages [28].
2D NMR analysis of EPS was also performed. The 2D HSQC NMR spectrum showed well-defined correlation in the ring proton regions. Goyzueta-Mamani et al. [29] also performed 2D HSQC NMR of chitin-like EPS produced by submerged fermentations of Mortierella alpine.
3.2.6. Surface morphology of EPS and elemental analysis
SEM is a powerful imaging technique to study and characterize the biological macromolecules and illustrating their common physical properties including surface morphology, microstructure, and texture. The SEM images [Figure 4a and b] revealed that the EPS from F. meliae AGDP-2 has a compact structure with crystalline structural moieties of an average size of 1.21 μm. Moreover, some microcracks were also observed in the structure of EPS.
 | Figure 4: (a and b) Scanning electron micrograph of EPS and (c) EDX analysis of EPS. [Click here to view] |
Similar methods were also employed by Vinothini et al. [22] for studying the surface of EPS from Streptomyces griseorubens GD5 where they observed compact and porous structure with flake-like structural units.
Energy-dispersive X-ray analysis technique has been widely utilized for quantitative elemental of a sample. In this study, seven elements including O, K, Na, Mg, S, Cl, and Ca were detected in the elemental analysis of the EPS from F. meliae AGDP-2 and their levels in terms of weight% are also displayed [Figure 4c]. Such elements present on the EPS surface may have role in binding with carboxyl and hydroxyl groups of monosaccharides [14]. Similarly, during EDX analysis of EPS from S. griseorubens GD5, eight elements were detected [22].
3.2.7. AFM
AFM is a valuable technique to for topographical characterization. It can be employed to study the random linear or spherical structures of polysaccharides. EPS is found to be primarily comprised with spherical and irregular shaped lumps [Figure 5a and b]. The EPS formed large lumps which revealed about molecular aggregation, and the structures of polysaccharide molecules were branched and entangled, which can be attributed to their hydroxyl and carboxyl groups that can form strong intermolecular and intramolecular interactions with each other or with water molecules [30]. The average height of EPS ranged from 2 to 20 nm. According to Shen et al. [31] if mean height is more than 1 nm, then the sample may be branched and interwoven with one another.
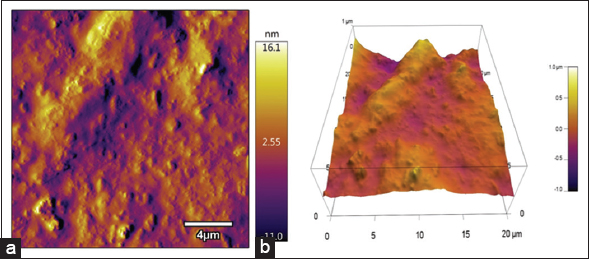 | Figure 5: (a) Atomic force micrograph of EPS and (b) 3D visualization of AFM. [Click here to view] |
The similar phenomenon is observed with the AFM analysis of the present study. Moreover, as the molecules are tightly packed, it can be said that EPS may have high affinity toward water and may possess pseudoplastic behavior [32]. Gu et al. [33] reported that polysaccharide SPU70-W1 from Sagittaria sagittifolia L. was mainly consisting of large spherical lumps and mean height was about 1.58 nm, which is very less than that is observed in our studies. Exopolysaccharide produced by Streptococcus thermophilus CC30 was also found to have lumps with mean height ranging from 10 to 30 nm [34].
3.2.8. Rheological behavior of EPS
The viscosity of EPS and guar gum at different shear rates (6–100 rpm) and temperatures (30–70°C) was measured. It was observed that the viscosity remarkably decreased by increasing temperature and shear rate as depicted in Figure 6a and b. The viscosity of EPS at 30°C and 6 rpm was about 139 cP which decreased to 68.8 cP at the shear rate of 100 rpm. However, viscosity of EPS was measured to be 58.5 cP at 70°C and 6 rpm that decreased to 44.57 cP at the shear rate of 100 rpm. The similar observations were also reported for guar gum. The results clearly indicated that increasing temperature and shear rate resulted in declining the viscosity of the EPS solution. This behavior indicates pseudoplastic property of the biopolymer. It is possible that the long chains of the EPS make an alignment in the direction of flow when the spindle speed increases. Therefore, fewer interactions among relative polymer chains are created. These findings were in agreement with those reported by Qiao et al. [35] for the biopolymer viscosity extracted from Enteromorpha prolifera.
 | Figure 6: (a) Viscosity of EPS at various temperatures and shear rates, (b) VISCOSITY of guar gum at various temperatures and shear rates, and (c) viscosity of EPS and guar gum at different pH values. [Click here to view] |
The viscosity of 1% w/v aqueous solution of the EPS was assessed. As shown in Figure 6c, the viscosity of the EPS is dependent to changes in pH values. At the slightly acidic pH of 5, the viscosity of the EPS was 148 cP, while the viscosity declined in the alkaline environments. In contrast, the viscosity of guar gum was found to be maximum of about 294.4 cP at pH 9, and the same remained quiet low under acidic conditions. It is may be possible that shifting the pH value to either alkaline side may disturb the molecular conformation of EPS chains due to affecting by a net electric charge of the molecules [36].
4. CONCLUSION
The present study is focused on purification, characterization, and application of EPS produced by F. meliae AGDP-2. Using Sephadex-G75 column chromatography, EPS was purified by 8.11-fold. Furthermore, the chemical and structural analysis of F. meliae EPS was done by HPLC, HPTLC, UV–visible spectroscopy, FT-IR, GPC, NMR, SEM-EDX, and AFM. The monosaccharide compositional analysis revealed that EPS is glucomannan which is mainly composed of glucose and mannose monomers. The weight-average molecular weight was calculated to be 2.48 × 106 Da. FTIR spectroscopy showed the occurrence of various functional groups and glycosidic linkage. NMR studies confirmed the presence of alpha-anomers of glucose and mannose residues. SEM analysis displayed micrometer sized crystal-like structures on EPS surface with some microcracks. Elemental analysis of EPS surface was done by EDX techniques which confirmed the presence of various elements including O, C, Na, Mg, S, Ca, and Cl. AFM studied revealed that EPS is composed of several spherical or irregular shaped lumps and is highly dense and branched. Moreover, studies on rheological behavior explained pseudoplastic behavior of EPS. Looking at the physicochemical properties, the purified EPS can be further investigated for the applications in various fields such as food industries, medical and pharmaceutical industries, as well as in white biotechnology.
5. ACKNOWLEDGMENT
Authors wish to acknowledge Sophisticated Instrumentation Centre for Applied Research and Testing – SICART, Bio-AFM Facility, IIT Bombay, and SAIF, IIT Bombay, for providing instrumentation facility.
6. Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
7. FINANCIAL SOURCE OF SUPPORT
This study was financially supported by the University Grants Commission, New Delhi, by providing NFOBC fellowship (Student ID: 201819-NFO-2018-19-OBC-GUJ-69853).
8. CONFLICTS OF INTEREST
The authors declare that they have no known competing interest.
9. ETHICAL APPROVALS
this study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
No additional or supplementary data files associated with the manuscript.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Andhare P, Delattre C, Pierre G, Michaud P, Pathak H. Characterization and rheological behaviour analysis of the succinoglycan produced by Rhizobium radiobacter strain CAS from curd sample. Food Hydrocol 2017;64:1-8. [CrossRef]
2. Mishra A, Jha B. Microbial exopolysaccharides. Prokaryotes 2013;4:179-92. [CrossRef]
3. Mahapatra S, Banerjee D. Fungal exopolysaccharide:Production, composition and applications. Microbiol Insights 2013;6:1-16. [CrossRef]
4. Ramirez MA. Characterization and safety evaluation of exopolysaccharide produced by Rhodotorula minuta BIOTECH 2178. Int J Food Eng 2016;2:31-5. [CrossRef]
5. Cheng JJ, Lin CY, Lur HS, Chen HP, Lu MK. Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus, Fomitopsis pinicola. Process Biochem 2008;43:829-34. [CrossRef]
6. Hsieh C, Hsu TH, Yang FC. Production of polysaccharides of Ganoderma lucidum (CCRC36021) by reusing thin stillage. Process Biochem 2005;40:909-16. [CrossRef]
7. Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956;28:350-6. [CrossRef]
8. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [CrossRef]
9. Su CA, Xu XY, Liu DY, Wu M, Zeng FQ, Zeng MY, et al. Isolation and characterization of exopolysaccharide with immunomodulatory activity from fermentation broth of Morchella conica. DARU J Pharma Sci 2013;21:1-6. [CrossRef]
10. He L, Wu X, Cheng J, Li H, Lu X. Purification, composition analysis, and antioxidant activity of exopolysaccharides from mycelial culture of Paecilomyces cicadae (Miq.) Samson (Ascomycetes). Int J Med Mushrooms 2010;12:51-62. [CrossRef]
11. Limin H, Jianchun Z, Tianyi W, Jian Z, Jike L, Qizhi W, et al. Purification and characterization of schizophyllan from Schizophyllum commune. Eng Sci 2013;2:88-92.
12. Fukuda K, Shi T, Nagami K, Leo F, Nakamura T, Yasuda K, et al. Effects of carbohydrate source on physicochemical properties of the exopolysaccharide produced by Lactobacillus fermentum TDS030603 in a chemically defined medium. Carbohydr Polym 2010;79:1040-5. [CrossRef]
13. Elnahas MO, Amin MA, Hussein MM, Shanbhag VC, Ali AE, Wall JD. Isolation, characterization and bioactivities of an extracellular polysaccharide produced from Streptomyces sp. MOE6. Molecules 2017;22:1396. [CrossRef]
14. Rani RP, Anandharaj M, Sabhapathy P, Ravindran AD. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int J Biol Macromol 2017;96:1-10. [CrossRef]
15. Yu G, Yue C, Zang X, Chen C, Dong L, Liu Y. Purification, characterization and in vitro bile salt-binding capacity of polysaccharides from Armillaria mellea mushroom. Czech J Food Sci 2019;37:51-6. [CrossRef]
16. Li W, Ji J, Rui X, Yu J, Tang W, Chen X, et al. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT Food Sci Technol 2014;59:732-9. [CrossRef]
17. Imran MY, Reehana N, Jayaraj KA, Ahamed AA, Dhanasekaran D, Thajuddin N, et al. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int J Biol Macromol 2016;93:731-45. [CrossRef]
18. Kavita K, Singh VK, Mishra A, Jha B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr Polym 2014;101:29-35. [CrossRef]
19. Mahapatra S, Banerjee D. Production and structural elucidation of exopolysaccharide from endophytic Pestalotiopsis sp. BC55. Int J Biol Macromol 2016;82:182-91. [CrossRef]
20. Yu L, Xu S, Deng C, Li H, Yang Q, Xu Z, et al. Preparation and partial structural characterization of the exopolysaccharide from Bacillus mucilaginosus SM-01. Carbohydr Polym 2016;146:217-23. [CrossRef]
21. Singh RP, Shukla MK, Mishra A, Kumari P, Reddy CR, Jha B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr Polym 2011;84:1019-26. [CrossRef]
22. Vinothini G, Latha S, Arulmozhi M, Dhanasekaran D. Statistical optimization, physio-chemical and bio-functional attributes of a novel exopolysaccharide from probiotic Streptomyces griseorubens GD5. Int J Biol Macromol 2019;134:575-87. [CrossRef]
23. Fooladi T, Soudi MR, Alimadadi N, Savedoroudi P, Heravi MM. Bioactive exopolysaccharide from Neopestalotiopsis sp. strain SKE15:Production, characterization and optimization. Int J Biol Macromol 2019;129:127-39. [CrossRef]
24. Mohammadi A, Shojaosadati SA, Tehrani HJ, Mousavi SM, Saleh T, Khorasani AC. Schizophyllan production by newly isolated fungus Schizophyllum commune IBRC-M 30213:Optimization of culture medium using response surface methodology. Ann Microbiol 2018;68:47-62. [CrossRef]
25. Sasikumar K, Kozhummal Vaikkath D, Devendra L, Nampoothiri KM. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour Technol 2017;241:1152-6. [CrossRef]
26. Ismail B, Nampoothiri KM. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 2010;192:1049-57. [CrossRef]
27. Karuppiah P, Venkatasamy V, Viswaprakash N, Ramasamy T. A statistical approach on optimization of exopolymeric substance production by Halomonas sp. S19 and its emulsification activity. Bioresour Bioprocess 2015;2:1-10. [CrossRef]
28. Du R, Xing H, Yang Y, Jiang H, Zhou Z, Han Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr Polym 2017;174:409-16. [CrossRef]
29. Goyzueta-Mamani LD, de Carvalho JC, Magalhães AI Jr., Soccol CR. Production of arachidonic acid by Mortierella alpina using wastes from potato chips industry. J Appl Microbiol 2021;130:1592-601. [CrossRef]
30. Ji X, Zhang F, Zhang R, Liu F, Peng Q, Wang M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao:Purification and structural characterization. Food Chem 2019;274:494-9. [CrossRef]
31. Shen J, Zhang D, Zhang FH, Gan Y. AFM characterization of patterned sapphire substrate with dense cone arrays:Image artifacts and tip-cone convolution effect. Appl Surface Sci 2018;433:358-66. [CrossRef]
32. Ahmed Z, Wang Y, Anjum N, Ahmad A, Khan ST. Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir-Part II. Food Hydrocol 2013;30:343-50. [CrossRef]
33. Gu J, Zhang H, Zhang J, Wen C, Zhou J, Yao H, et al. Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L. Carbohydr Polym 2020;246:116595. [CrossRef]
34. Kanamarlapudi SL, Muddada S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed Res Int 2017;2017:4201809. [CrossRef]
35. Qiao L, Li Y, Chi Y, Ji Y, Gao Y, Hwang H, et al. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohydr Polym 2016;136:1307-14. [CrossRef]
36. Li L, Liao BY, Thakur K, Zhang JG, Wei ZJ. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int J Biol Macromol 2018;109:761-71. [CrossRef]