1. INTRODUCTION
Pandanus amaryllifolius Roxb. is a tropical plant in the Pandanaceae family which usually used as a source of natural coloring, flavoring, and bioactive compounds in food additives and the pharmaceutical industry [1,2]. Clemen-Pascual et al. [3] reported that pandan leaves contain alkaloids, flavonoids, glycosides, and tannins that can be used to reduce fever, antibacterial and anticancer. Moreover, the pandan leaves also contained polyphenols with antioxidant and antihyperglycemic activities [4,5]. However, information about the bioactivities and applications of pandan prop root is scarce since it is discarded as waste. Then it is interesting to study and utilize for value-added through the biotechnological process. Bhuyan and Sonowal [6] reported that pandan prop root contained various bioactive compounds such as phenolic compounds and flavonoids, which were able to scavenge the free superoxide radicals as anti-aging and anticancer properties. Peungvicha et al. [7] found that pandan prop root extracts prolonged the sleeping time and reduced the locomotor activity in mice, while Jimtaisong and Krisdaphong [8] reported on the antioxidant properties of pandan root and bioactivities for the possible development of novel healthy foods or functional products in the future.
Bacterial cellulose (BC) is composed of β-1,4 linkages of glucose polymer (C6H10O5)n and can be produced from various bacterial species, including Komagataeibacter [9]. BC is applied in foods, medicines, and cosmetic products [10], which were produced from various substrates such as coconut juice, fruit juice, rice syrup, and industrial waste [11,12]. A novel substrate for BC production will provide an alternative BC product that generates the unique functions and bioactivities which has not been found in the conventional substrate [13]. The pandan leave syrup was currently supplemented with the BC produced from low-grade rice as the flavoring [14]. But from our knowledge, the BC production from pandan prop root has not yet been reported, which is interesting to provide a novel BC from the pandan prop root in this study.
Here, the bioactivities of pandan prop root include antioxidant DPPH radical scavenging assay, total phenolic content (TPC), and antibacterial activities against Staphylococcus aureus DMST 2933 and Escherichia coli DMST 4212 were investigated, together with the development of a novel BC product from pandan prop root using enzymatic hydrolysis.
2. MATERIALS AND METHODS
2.1. Substrates, Enzyme and Bacterial Strain
Organic pandan prop root was obtained from Chachoengsao Province, Thailand. The prop root was washed twice in tap water, dried at 50°C in a hot air oven for 12 h, then powdered by an electric grinder and stored under dry condition. Commercial cellulase enzyme (iKnowZyme® cellulase) was purchased from Reach Biotechnology Co., Ltd. and stored at -20°C. Komagataeibacter xylinus AGR 60 was obtained from the Institute of Food Research and Product Development, Kasetsart University, Thailand. The AGR 60 strain was grown in a coconut juice inoculum medium reported by Noree et al. [13] and used as the starter culture for BC fermentation.
Pathogenic bacteria, including S. aureus DMST 2933 and E. coli DMST 4212, were obtained from the Department of Medical Sciences Culture Collection Center, Ministry of Public Health, Thailand, and grown on TSB medium at 37°C for 24 h and diluted to 106 CFU/mL for disc diffusion assay.
2.2. Chemical Composition Analysis of Pandan Prop Root
Proximate analysis of pandan prop root including protein, fat, moisture, and ash contents was analyzed by the Central Laboratory (Thailand) Co., Ltd. following the method of analysis for nutrition labeling (1993) p.106 and AOAC methods [15]. Hemicellulose, cellulose, and lignin were analyzed using AOAC methods [15]. All values were reported as g/100 g sample.
2.3. Methanol Extraction of Pandan Prop Root
Pandan prop root powder at 500 g was immersed in 1.0 L of 95% methanol at room temperature for 24 h. The extract solution was filtered using Whatman No.1 filter paper, and the solvent was removed using a rotary evaporator at boiling point [16]. The obtained pandan prop root was used to determine antioxidant (DPPH radical scavenging assay) and antibacterial activity on S. aureus DMST 2933 and E. coli DMST 4212 by the disc diffusion method.
2.4. Determination of Antioxidant Activity
2.4.1 DPPH radical scavenging assay
The antioxidant assay was reported as the amount of antioxidant with the ability to reduce DPPH absorption compared to the control (IC50), defined in units of μg/ml, following the modified procedure of Suwannakul et al. [17]. A reduction in DPPH concentration was recorded by the decrease in absorbance at 520 nm. Ascorbic acid was used as the positive control with ethanol as the negative control and extract without DPPH as the blank.
2.4.2 TPC
The TPC of pandan prop root extract was determined by the Folin-Ciocalteu method as described by Butsat and Siriamornpun [18]. Diluted Folin-Ciocalteu reagent (1:10) was added to 200 μL of the sample. Then, 800 μL of sodium carbonate (Na2CO3) was added, and the reaction volume was increased to 5.0 mL by distilled water. The reaction was incubated at room temperature for 2.0 h and measured at 760 nm. Gallic acid was used as the standard reagent, with results expressed as mg gallic acid equivalent per g of sample (mg GAE/g sample).
2.5. Antibacterial activity
To determine the in vitro activity of prop root crude extract against pathogenic bacteria, including S. aureus DMST 2933 and E. coli DMST 4212, prop root crude extracts at 12.5, 25, 50, and 100 mg/ml were incorporated in a 6.0 mm paper disc and tested using the paper disc diffusion method [19]. The testing plates were incubated at 35 ± 2°C for 18 h to determine the mean inhibition zone of each sample (mm). The extracted solvent was used as a control.
2.6. Hydrolysis of Pandan Prop Root by Commercial Hydrolytic Enzyme
Pandan prop root hydrolysis reactions were investigated in 250 mL Erlenmeyer flasks containing 50 mL of enzyme solution. Pandan prop root powder content was varied at 25–150 g/l in the reaction containing iKnowZyme® cellulase concentration at 4.0% (%v/v) dissolved in 0.1 M acetate buffer pH 5.5. The reactions were incubated at 50°C for 24 h, modified from Kobkam et al. [20] and determined for total soluble solids (TSS) content using a refractometer (RA-250WE, Kyoto Electronics, Kyoto, Japan). The optimum substrate concentration was used, with enzyme concentration varied at 1.0–6.0 (%v/v). The reactions were incubated at 50°C for 24 h and determined for TSS content as described above. The optimum substrate and enzyme concentrations were used to upscale the hydrolysis in a 5.0 L glass jar chamber for sugar syrup production.
2.7. Production of BC by K. xylinus AGR 60
Sugar syrup obtained from the hydrolysis of pandan prop root was used as the substrate for BC fermentation by K. xylinus AGR 60 in a plastic tray (15 × 15 × 6 cm) with the working volume at 500 mL. The fermentation medium was supplemented with 3.0 g of ammonium sulfate and 0.6 mL of glacial acetic acid following the methods of Jagannath et al. [21] and Noree et al. [13] with slight modifications and 10% (v/v) of K. xylinus AGR 60 was used as a starter culture. The fermentation was incubated at room temperature, and the characteristics of BC were determined after nine days of cultivation. To compare with the traditional BC production, coconut juice was used as a substrate. The thickness of each sample was recorded every day. At the end of fermentation, all the BC samples were dried at 50°C for 24 h to determine the dry weight. The structure of freeze-dried BC was determined by a scanning electron microscope (SEM) (model SU8020; Hitachi, Tokyo, Japan) at 10.0 kV, while functional groups and chemical bonds of BC produced from pandan prop root syrup were determined by Fourier-transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet is5, USA) in the range 4000–400 cm-1 following the methods of Saleh et al. [21] and Noree et al. [13].
2.8. Statistical Analysis
The significance of the data was analyzed by one-way analysis of variance (SPSS 21.0, USA). Values were considered significant at P < 0.05 using Duncan’s multiple range tests.
3. RESULTS AND DISCUSSION
3.1. Chemical Composition of Pandan Prop Root
P. amaryllifolius Roxb. is a monocot that grows in tropical regions, commonly known as pandan. The prop roots of pandan are adventitious and used for aerial support, as shown in Figure 1. The leaves and stems have a unique and pleasant aroma. They are used as flavorings for various foods and herbal beverages and reduce fever, relieve indigestion and as a diuretic, cardio-tonic, and anti-diabetic [22-25]. Bhuyan and Sonowal [6] reported that pandan leaves and roots contained various bioactive compounds such as phenolic compounds and flavonoids, which showed the bioactivities of antioxidant, anticancer, and anti-aging bioactivities. The pandan prop root was traditionally used for diabetic patients treated with the antihyperglycemic property of 4-hydroxybenzoic acid [8,25]. However, the pandan prop root is usually discarded as waste, which is interesting to utilize as a novel substrate for food and beverage production.
The chemical composition of pandan prop root powder is shown in Table 1. The components were cellulose at 38.88 ± 0.11 g/100g and hemicellulose at 15.40 ± 0.16 g/100g, with small amounts of lignin (8.99 ± 0.03 g/100g), protein (4.67 ± 0.01 g/100g), carbohydrate (80.24 ± 0.11 g/100g), fat (1.11 ± 0.01 g/100g), moisture (7.56 ± 0.03 g/100g) and ash (6.31 ± 0.01 g/100g). Prop roots develop from the nodes to provide additional support for plants, and they contain cellulose as the major component. The high amount of cellulose showed the potential of the substrate for cellulase enzyme hydrolysis to produce fermentable sugar and bioactive compounds. The pandan prop root was investigated for bioactivities and used as a substrate for BC fermentation.
3.2. Antioxidant Activity of Pandan Prop Root Extract
Antioxidant assay of pandan prop root was expressed as the half-maximal inhibitory concentration (IC50) as described in the method. The IC50 value of DPPH scavenging activity from pandan prop root crude extract was 230.24 ± 10.69 μg/ml. TPC was 24.75 ± 0.74 mg GAE/g. Suwannakul et al. [17] reported the IC50 and TPC of pandan leaf extract at 110.57 ± 36.42 μg/ml and 57.25 ± 0.02 mg GAE/g, respectively. Results revealed that pandan prop root contained bioactive compounds and showed antioxidative activities for possible use as an ingredient in functional foods and cosmetic products in the future. At the same time, Jimtaisong and Krisdaphong [8] confirmed that pandan root extract exhibited antioxidant activity of DPPH as IC50 value at 2,340 μg/ml and TPC content at 28.8 mg GAE/g that could be used in topical pharmaceutical and cosmetic applications.
 | Figure 1: Pandanus amaryllifolius Roxb. (Pandanaceae) showing leaves and prop roots. [Click here to view] |
 | Table 1: Chemical composition of pandan prop root powder. [Click here to view] |
3.3. Antibacterial Activity
The crude extract of pandan prop root was evaluated for the antibacterial ability of S. aureus DMST 2933 and E. coli DMST 4212 using the disc diffusion assay method as described above. Results of crude extract at different concentrations on antibacterial activity are shown in Table 2. Crude extract of pandan prop root inhibited the growth of S. aureus DMST 2933 in all concentrations (12.5–100 mg/ml), with the highest mean zone of inhibition at 9.75 ± 0.35 mm using 100 mg/ml of crude extract. However, the pandan prop root crude extract showed no in vitro activity against E. coli DMST 4212, concurring with Dumaoal et al. [19], who reported that pandan leaf crude extract had activity against the growth of S. aureus ATCC 25923 but not against the growth of E. coli ATCC 25922. Pandan leaf and root contain phytochemicals as bioactive compounds and essential oil that inhibit the growth of some pathogenic bacteria [8,19]. Mar et al. [26] found 54 compounds in essential oil from pandan leaves that inhibited the growth of various Gram-positive and Gram-negative bacteria, including S. aureus, a common cause of minor skin infections, mainly when introduced into a wound or skin incision. From our knowledge, most of the previous findings focused on the antibacterial activity of pandan leaves. However, the study concurring with pandan prop root is scarce. Then, this study is the first report for evaluating the antibacterial activity of pandan prop root crude extract, which showed the potential for application as an ingredient for skin cosmetic and pharmaceutical products.
3.4. Hydrolysis of the Pandan Prop Root by Commercial Hydrolytic Enzyme
For sugar syrup production, pandan prop root powder was hydrolyzed by commercial cellulase as described above. Maximum TSS was found at 2.67 ± 0.29 Brix using prop root powder at 100 g/l with 4.0% (v/v) of the commercial enzyme after incubated at 50°C for 24 h, as shown in Figure 2. At low substrate concentrations (25, 50, and 75 g/l), the obtained fermentable sugar was lower than the optimum substrate concentration (100 g/l). TSS increased at higher substrate concentration until beyond the point of optimum concentration [Figure 2a] when TSS slightly decreased due to limits of mixing of enzyme-substrate suspension as reported by Sangngern et al. [27] and Lomthong et al. [28]. In the case of enzyme concentration, which directly affects the hydrolysis efficiency due to increasing the degradation rate when increasing the enzyme concentration until the optimum point. Lomthong et al. [29] reported that substrate and enzyme concentrations play an important role in sugar syrup production from broken rice. This is similar to Thongpoem et al. [30] finding that substrate concentration affected the sugar syrup production from unripe banana flour when using enzymatic hydrolysis. Therefore, the optimum conditions at 100 g/l with 4.0% (v/v) of the commercial enzyme were upscaled in a 5.0 L glass jar chamber to produce pandan prop root syrup and used as a substrate for BC fermentation.
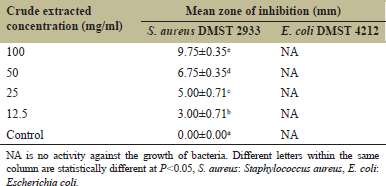 | Table 2: Average antibacterial area diameter for S. aureus DMST 2933 and E. coli DMST 4212 of crude pandan prop root extract. [Click here to view] |
 | Figure 2: Effects of substrate (a) and enzyme (b) concentrations on total soluble solids (TSS) content from hydrolysis of pandan prop root powder by iKnowZyme® cellulase. [Click here to view] |
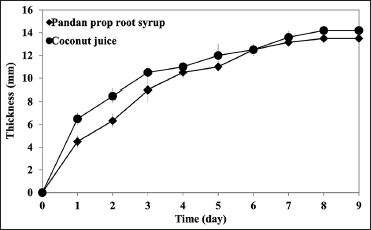 | Figure 3: Time course of bacterial cellulose fermentation by Komagataeibacter xylinus AGR 60 at room temperature for 9 days. [Click here to view] |
3.5. Production of BC by K. xylinus AGR 60
BC was fermented from pandan prop root syrup using K. xylinus AGR 60 at room temperature for nine days. K. xylinus AGR 60 was reported as a potent commercial strain for BC production, which coconut juice and other syrups could be used as a substrate for fermentation [13]. Time courses of BC fermentation by pandan prop root syrup and coconut juice are shown in Figure 3. Results indicated that BC produced from pandan prop root had the highest thickness at 13.5 ± 0.05 mm with 7.90 ± 0.10 g of dry weight, and slightly lower than BC produced from coconut juice (14.2 ± 0.60 mm with 8.35 ± 0.22 g). The pH of coconut juice decreased from 4.40 ± 0.10 to 3.43 ± 0.25, while the pH of pandan prop root syrup decreased from 4.37 ± 0.12 to 3.78 ± 0.14. The dry weight matter was used to calculate BC production (P, g/l) and BC production rate (Rp, g/l/day), as shown in Table 3. BC production (P, g/l) values from pandan prop root syrup and coconut juice were 15.80 ± 0.20 and 16.70 ± 0.44 g/l, respectively, while BC production rates were recorded at 1.76 ± 0.02 and 1.85 ± 0.05 g/l/day from pandan prop root syrup and coconut juice, respectively. Moukamnerd et al. [12] reported BC production from banana peel and passion fruit peel at 0.89 ± 0.04 and 0.31 ± 0.07 g/l, respectively, and lower than BC production from pandan prop root syrup in this study, while production of BC from pandan prop root syrup was slightly lower than from coconut juice. This study’s lower BC production from a novel substrate resulted from some coconut juice nutrients that promoted BC fermentation, such as minerals, vitamins, and amino acids [31]. Noree et al. [13] reported lower production of BC from rice syrup than coconut juice by K. xylinus AGR 60 that had a similar structure with higher bioactive compound content. Photographs and electron micrographs of BC produced from coconut juice and pandan prop root syrup are shown in Figure 4. The BC sample produced from pandan prop root syrup [Figure 4c] was slightly brown compared to the BC produced from coconut juice [Figure 4a]. The color faded after boiling at 100°C for 30 min. The BC structure investigated by SEM was similar in both samples [Figure 4b and d]. FTIR spectra of BC produced from pandan prop root syrup are shown in Figure 5. FTIR spectra patterns were similar to spectra of BC produced from coconut juice, as reported previously [13]. Bands assigned to –OH and –CH stretching were found at wavenumbers 3200–3400 cm-1, similar to BC produced from banana peel and passion fruit peel reported by Moukamnerd et al. [10] and rice syrup reported by Noree et al. [13]. The absorption band at 1600–1650 cm-1 was recorded as carboxyl (C=O) functional group [32], while peaks at 950–1100 cm-1 were recorded as the C–O–C functional group by Noree et al. [13]. This study is the first report showing the possibility of using pandan prop root as the substrate to produce novel BC as an alternative application in future functional food and pharmaceutical products.
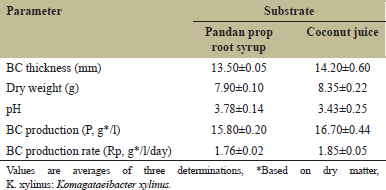 | Table 3: Bacterial cellulose (BC) production parameters from pandan prop root syrup and coconut juice by K. xylinus AGR 60. [Click here to view] |
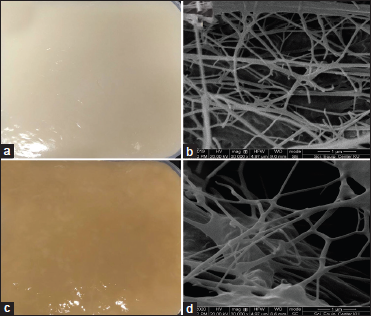 | Figure 4: Photographs and scanning electron micrographs of bacterial cellulose produced from coconut juice (a and b) and pandan prop root syrup (c and d) by fermentation of Komagataeibacter xylinus AGR 60 at room temperature for 9 days. [Click here to view] |
 | Figure 5: Fourier-transform infrared spectra of the pandan prop root bacterial cellulose at wavenumbers 4000–400 cm-1. [Click here to view] |
4. CONCLUSION
P. amaryllifolius Roxb. (Pandanaceae) prop root was shown to be a source of bioactive compounds, with interesting applications for novel healthy foods or functional drink products. Pandan prop root was elucidated for its antioxidant and antibacterial bioactivities to inhibit the growth of S. aureus. Application of pandan prop root in BC fermentation using enzymatic hydrolysis was also achieved. Results revealed a novel process and progression in bio-cellulose production technology using enzymatic hydrolysis.
5. ACKNOWLEDGMENTS
This research was supported by Institute of Research and Development Rajamangala University of Technology, Thanyaburi (DRF63D0606).
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
7. FUNDING
The RMUTT Research Foundation Scholarship supported this research (Grant No. DRF65D0601).
8. CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All relevant data are provided within the manuscript.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ghasemzadeh A, Jaafar HZ. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations ofMalaysia. BMC Complement Altern Med 2013;13:1-9. CrossRef
2. Minh NP, Vo TT, Phong TD, Van Toan N, Nam VH. Green pigment extraction from pandan (Pandanus Amaryllifolius) and its application in food industry. J Pharm Sci 2019;11:925-9.
3. Clemen-Pascual LM, Macahig RA, Rojas NR. Comparative toxicity, phytochemistry, and use of 53 Philippine medicinal plants. Toxicol Rep 2022;9:22-35. CrossRef
4. Aini R, Mardiyaningsih A. Pandan leaves extract (Pandanus amaryllifolius Roxb) as a food preservative. J Kedokteran Indones 2016;7:166-73. CrossRef
5. Chiabchalard A, Nooron N. Antihyperglycemic effects of Pandanus amaryllifolius Roxb. leaf extract. Pharmacogn Mag 2015;11:117. CrossRef
6. Bhuyan B, Sonowal R. An overview of Pandanus Amaryllifolius Roxb. Exlindl. and its potential impact on health. Curr Trends Pharm Res 2021;8:138-57.
7. Peungvicha P, Wongkrajang Y, Kongsaktrakoon B, Temsiririrkul R, Watanabe H. Pandanus amaryllifolius root extract prolongs sleeping time and reduces locomotor activity in mice. J Pharm Sci 2014;41:6-12.
8. Jimtaisong A, Krisdaphong P. Antioxidant activity of Pandanus amaryllifolius leaf and root extract and its application in topical emulsion. Trop J Pharm Res 2013;12:425-31. CrossRef
9. Naloka K, Matsushita K, Theeragool G. Enhanced ultrafine nanofibril biosynthesis of bacterial nanocellulose using a low-cost material by the adapted strain of Komagataeibacter xylinus MSKU12. Int J Biol Macromol 2020;150:1113-20. CrossRef
10. Azeredo H, Barud H, Farinas CS, Vasconcellos VM, Claro AM. Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst 2019;3:7. CrossRef
11. Hussain Z, Sajjad W, Khan T, Wahid F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019;26:2895-911. CrossRef
12. Moukamnerd C, Ounmuang K, Konboa N, Insomphun C. Bacterial cellulose production by Komagataeibacter nataicola TISTR 2661 by agro-waste as a carbon source. Chiang Mai J Sci 2020;47:16-27.
13. Noree S, Tongdang C, Sujarit K, Chamdit S, Thongpool V, Trakarnpaiboon S, et al. Application of raw starch degrading enzyme from Laceyella sacchari LP175 for development of bacterial cellulose fermentation using colored rice as substrate. 3 Biotech 2021;11:1-11. CrossRef
14. Photphisutthiphong Y, Vatanyoopaisarn S. The production of bacterial cellulose from organic low-grade rice. Curr Res Nutr Food Sci 2020;8:206-16. CrossRef
15. Helrich K, Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists. Virginia: Association of Official Analytical Chemists; 1990.
16. Ngadi N, Yahya NY. Extraction of 2-acetyl-1-pyrroline (2AP) in pandan leaves (Pandanus amaryllifolius Roxb.) via solvent extraction method: Effect of solvent. J Teknol 2014;67:51-4. CrossRef
17. Suwannakul S, Chaibenjawong P, Suwannakul S. Antioxidant anticancer and antimicrobial activities of ethanol Pandanus amaryllifolius Roxb. leaf extract (In Vitro)-a potential medical application. J Int Dent 2018;11:383-9.
18. Butsat S, Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem 2010;119:606-13. CrossRef
19. Dumaoal OS, Alaras LB, Dahilan KG, Depadua SA, Pulmones CJ. In vitro activity of pandan (Pandanus amaryllifolius) leaves crude extract against selected bacterial isolates. J Philipp Associat Insti Res 2010;4:101-24. CrossRef
20. Kobkam C, Tinoi J, Kittiwachana S. Alkali pretreatment and enzyme hydrolysis to enhance the digestibility of rice straw cellulose for microbial oil production. Appl Sci Eng Prog 2018;11:247-56. CrossRef
21. Jagannath A, Kalaiselvan A, Manjunatha SS, Raju PS, Bawa AS. The effect of pH, sucrose and ammonium sulphate concentrations on the production of bacterial cellulose (Nata-de-coco) by Acetobacter xylinum. World J Microbiol Biotechnol 2008;24:2593-9. CrossRef
22. Cheeptham N, Towers GH. Light-mediated activities of some Thai medicinal plant teas. Fitoterapia 2002;73:651-62. CrossRef
23. Ghasemzadeh A, Jaafar HZ. Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in pandan (Pandanus amaryllifolius Roxb.) using response surface methodology. Sci World J 2014;2014:523120. CrossRef
24. Wakte KV, Thengane RJ, Jawali N, Nadaf AB. Optimization of HS-SPME conditions for quantification of 2-acetyl-1-pyrroline and study of other volatiles in Pandanus amaryllifoliusRoxb. Food Chem 2010;121:595-600. CrossRef
25. Peungvicha P, Temsiririrkkul R, Prasain JK, Tezuka Y, Kadota S, Thirawarapan SS, Watanabe H. 4-Hydroxybenzoic acid: A hypoglycemic constituent of aqueous extract of Pandanus odorus root. J Ethnopharmacol 1998;62:79-84. CrossRef
26. Mar A, Mar AA, Thin PP, Zin MM. Study on the phytochemical constituents in essential oil of Pandanus amaryllifolious Roxb. Leaves and their antibacterial efficacy. Yadanabon Univ Res J 2019;10:1-9.
27. Sangngern N, Puangnark T, Nguansangiam W, Saithong P, Kitpreechavanich V, Lomthong T. Production and development of vinegar fermentation from broken Riceberry rice using raw starch-degrading enzyme hydrolysis. 3 Biotech 2020;10:1-9. CrossRef
28. Lomthong T, Netprasom P, Kancharu N, Jitmala K, Areesirisuk A, Trakarnpaiboon S, et al. Very high gravity (VHG) bioethanol production using modified simultaneous saccharification and fermentation of raw cassava chips with molasses by Kluyveromyces marxianus DMKU-KS07. Waste Biomass Valori 2021;12:3683-93. CrossRef
29. Lomthong T, Saelee K, Trakarnpaiboon S, Siripornvisal S, Kitpreechavanich V. Potential of recombinant raw starch?degrading enzyme from Escherichia coli for sugar syrup and bioethanol productions using broken rice powder as substrate. Starch Stärke 2021;2021:2100201. CrossRef
30. Thongpoem P, Chorum M, Rittisorn S, Saithong P, Permpool J, Kitpreechavanich V, et al. Saccharification of unripe banana flour using microwave assisted starch degrading enzyme hydrolysis for development of wine and vinegar fermentations. Int Food Res J 2021;28:969-75.
31. Prades A, Dornier M, Diop N, Pain JP. Coconut water uses, composition and properties: A review. Fruits 2012;67:87-107. CrossRef
32. Saleh AK, Soliman NA, Farrag AA, Ibrahim MM, El-Shinnawy NA, Abdel-Fattah YR. Statistical optimization and characterization of a biocellulose produced by local Egyptian isolate Komagataeibacter hansenii AS. 5. Int J Biol Macromol 2020;144:198-207. CrossRef