1. INTRODUCTION
Parkinson’s disease (PD) is a neurological disorder causing motor defects such as tremors, slow movement, postural instability, and muscle rigidity in which the substantia nigra pars compacta (SNpc) in the midbrain region is majorly affected, causing loss of dopaminergic neurons [1]. While people over 50 years are the victims of PD, young adults above 20 years are also affected [1,2]. Mutations in PD genes, viz. LRRK2, SNCA, PINK1, DJ1, and PARKIN also lead to major pathologies of PD such as an increase in oxidative stress, mitochondrial damage, protein misfolding, and failure of cellular energy metabolism [3]. 1-methyl,4-phenyl, 1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin, is widely used to induce the Parkinsonian phenotype in various animal models to study and elucidate the pathological mechanisms of PD development [4–7]. 1-methyl-4-phenylpyridinium (MPP+) is the main metabolite, which gets synthesized with the action of monoamine oxidase B, and MPP+ affects the dopaminergic neurons by entering through dopamine transporters causing increased reactive oxygen species, loss of adenosine triphosphate synthesis, DNA damage, and eventually neuroinflammation [8,9]. MPP+ is used to study PD as it provides a direct route to affect the dopaminergic neurons [10].
Even though there are numerous studies on the therapeutic strategies of PD, there is no complete cure for the disease apart from the drugs that provide relief from the symptoms [1]. Hence, it is essential to develop new drugs for PD. Plant flavonoids are great antioxidants that are now effective for various neurodegenerative diseases, such as PD, amyotrophic lateral sclerosis, Huntington’s disease, and Alzheimer’s disease [11]. Especially for PD, extracts and compounds derived from plant sources are under investigation to find a potential medicinal benefits against PD [12,13]. Quercetin is present in several fruits and vegetables and has demonstrated the potential to treat cancer, neurodegenerative diseases, etc. as it contains anti-allergic, antioxidant, and antiproliferative properties [14–17]. Quercetin at low concentrations has antioxidant property, whereas at higher concentrations, it exhibits prooxidant property [18]. Due to its antioxidant property, quercetin reduces the oxidative stress by scavenging the free radicals [19] and also used as a preclinical drug for the potential cure against PD [20–22].
Zebrafish is an established model organism to study neurodegenerative diseases due to its 70% genome homology to humans, easy maintenance, experimental manipulation of its embryos and larvae, and the translucent nature of its development [23]. In addition, zebrafish has emerged as a novel system for drug-screening studies [23]. Since quercetin serves as a potential PD drug, in this study, we have selected quercetin and demonstrated the rescue effects of quercetin on the motor defects in an MPP+-induced zebrafish PD model.
2. MATERIALS AND METHODS
2.1. Fish Maintenance
Male and female wild type adult zebrafish were purchased from a local aquarium and maintained in the laboratory aquarium as per standard conditions at 28.5°C temperature and 14 hours day/10 hours night cycle [24]. The zebrafish were fed regularly twice a day every day with nauplii-staged brine shrimp. The zebrafish embryos were harvested in the morning by setting up spawning in a pair-wise method the previous day evening as per standard procedures. The harvested embryos were maintained in Petri plates containing 1X E3 medium (10X E3 media: 0.065 g KCl, 0.405 g MgSO4, 1.46 g NaCl, and 0.24 g CaCl2). All the experiments were conducted as per the guidelines of the Institutional Animal Ethics Committee (IAEC), SRMIST, Kattankulathur, India. The ethical clearance certificate number is 16098/835re-S-04/IAEC 2016.
2.2. Drug Treatment
For our study, we divided the embryos equally into eight groups, namely control, DMSO1 (dimethyl sulfoxide), DMSO2, MPP+, Q1 (1-day exposure of quercetin alone), Q2 (2-day exposure of quercetin alone), MPP+/Q1, and MPP+/Q2. The treatment concentration of MPP+ (Sigma-Aldrich) of 1 mM was chosen according to Sallinen et al. [25]. The MPP+ stock (100 mg in 10 ml) solution was prepared by dissolving MPP+ in distilled H2O. The treatment concentration of quercetin (Sigma-Aldrich) was 12 μM according to Zhang et al. [26] and the stock solution was prepared by dissolving 1 mg of quercetin in 30 μl of 100% DMSO and then subsequently diluted to 12 μM in 1X E3 media. The 1-day postfertilization (dpf) embryos were dechorionated using two insulin syringes by observing them under the microscope and five dechorionated embryos were taken for each treatment group in a 24-well plate in triplicates. The control group embryos were raised in 1X E3 medium. The two DMSO groups of embryos were exposed to 0.04% DMSO at the 3 dpf stage for 24 hours in DMSO1 group of embryos and 48 hours in DMSO2 group. The embryos in the MPP+ group were given 2 days of exposure with 1 mM MPP+ commencing from 1 dpf stage. The Q1 group embryos were treated with 12 μM quercetin for 1 day at the 3 dpf stage. Similarly, the Q2 group embryos were treated with identical concentration of quercetin for 2 days commencing from the 3 dpf stage. For the MPP+/Q1 and MPP+/Q2 groups, the MPP+-exposed embryos were treated with 12 μM quercetin either for 1 day (MPP+/Q1 group) or 2 days (MPP+/Q2) commencing from the 3 dpf stage (Fig. 1).
2.3. Locomotor Behavior Assay
The experimental setup, data acquisition, and analysis for locomotor behavior assay were carried out as per Selvaraj and Santhakumar’s [27] study. The locomotion of larvae was captured by recording videos of individual larvae from respective treatment groups separately. A single larva was placed at the center of a Petri dish containing distilled H2O and allowed to acclimatize for 3 minutes. Then the Petri dish was placed on a white light source and the setup was covered using a box with a small opening to capture videos. A phone camera with 720 p resolution (Neffos Xlite) was used to capture 3-minute videos by placing it over the opening on top of the box. The acquired videos were converted into multiple image files with three frames per second (540 frames for 3 minutes) using Free studio v.6.7.0.712_r. Then the color images of the 540 frames were converted into gray scale images using light image resizer.4-7-0-0 with a width setting of 1024. Out of 540 files, the middle 180 files were further analyzed using ImageJ software with wrMTrck plugin. The trajectory path, movement speed, and total distance travelled were obtained from the image analysis which was further used for statistical validation.
 | Figure 1: Schematic diagram showing the treatment schedule for different experimental groups. [Click here to view] |
 | Figure 2: Trajectory paths of representative larva from different experimental groups. [Click here to view] |
2.4. Statistical Analysis
The statistical analysis of movement speed and distance travelled for all the experimental groups were performed using GraphPad Prism software version 8.0.2.263 (GraphPad Software, San Diego, CA; www.graphpad.com) by one-way analysis of variance (ANOVA) followed by Tukey’s test.
3. RESULTS AND DISCUSSION
Since PD is a movement disorder affecting dopaminergic neurons in the SNpc, it is important to quantify the locomotor phenotype of the animal PD models which will facilitate the analysis of rescuing potential of preclinical drugs of PD [28]. Numerous studies on MPP+ are there to study the pathologies of molecular mechanisms involved in PD using in vitro models [29–31], while less studies have utilized MPP+ to study PD in animal models [10,32]. A study reported that MPP+ might be better and more sensitive neurotoxin than MPTP to study PD in animal models as MPP+ directly reduced norepinephrine and dopamine levels but caused motor defects similar to MPTP [25]. In the present study, 1 mM MPP+ was used to induce motor defects in zebrafish larvae. After 2 days of 1 mM MPP+ exposure, the movement speed, distance travelled, and trajectory path of 5 dpf zebrafish larvae were evaluated using wrMTrck analysis. As shown in Figure 2, the trajectory path of MPP+-exposed larvae were significantly reduced compared to the control. The average distance travelled by a control larva was 54.51 mm, while it was significantly decreased to 7.80 mm after MPP+ exposure (Fig. 3). In addition, the average movement speed of MPP+-treated zebrafish larvae was also significantly reduced to 0.16 m/s when compared to the control 0.87 m/s (Fig. 3). These results (Table 1) indicated that MPP+ was able to induce motor defects in zebrafish larvae recapitulating a key aspect of PD phenotype. Due to its antioxidant property, quercetin is used in various studies to explore its therapeutic potential [20–22]. The treatment of quercetin in Sprague-Dawley rats was able to improve the motor defects induced using 6-hydroxydopamine that decreased the loss of dopaminergic neurons and oxidative stress [33]. A study by Mani et al. [34] in Alzheimer’s disease induced in zebrafish has shown that quercetin alleviated the behavioral defects induced by aluminum chloride. Also, quercetin was able to reduce the detrimental effects of lipopolysaccharide, which causes neuroinflammation, in an adult C57BL/6N mouse model [35].
Next, to rescue the MPP+-exposed zebrafish larvae, treatment with 12 μM quercetin was carried out at 3 dpf for 24 hours (MPP+/Q1) or 48 hours (MPP+/Q2) duration. Our analysis showed that treatment of quercetin for 2 days on MPP+-exposed zebrafish larvae recovered the motor behaviors such as movement speed and distance travelled. As shown in Figure 2, the trajectory path of MPP+/Q1 group larva was improved when compared to MPP+ but not similar to control. But the MPP+/Q2 group larvae showed complete recovery as evidenced by the trajectory paths, which were similar to control group. Similarly, for the locomotor parameters, such as distance travelled and movement speed, we could observe a progressive increase in recovery in the MPP+/Q1 and MPP+/Q2 larvae. The MPP+/Q1 larvae had values of 51.42 mm and 0.83 m/s (Fig. 3A and B) for distance travelled and movement speed parameters, respectively, compared to the control larvae displayed 54.51 mm and 0.87 m/s (Fig. 3C and D) for the respective locomotor parameters. But the MPP+/Q2 larvae exhibited significant recovery in the distance travelled and movement speed parameters with values 54.20 mm and 0.87 m/s (Fig. 3C and D), respectively, compared to MPP+/Q1. These results (Table 1) clearly suggest that quercetin is able to rescue MPP+-induced motor deficiency in zebrafish larvae and 2 days of quercetin treatment is required for complete recovery.
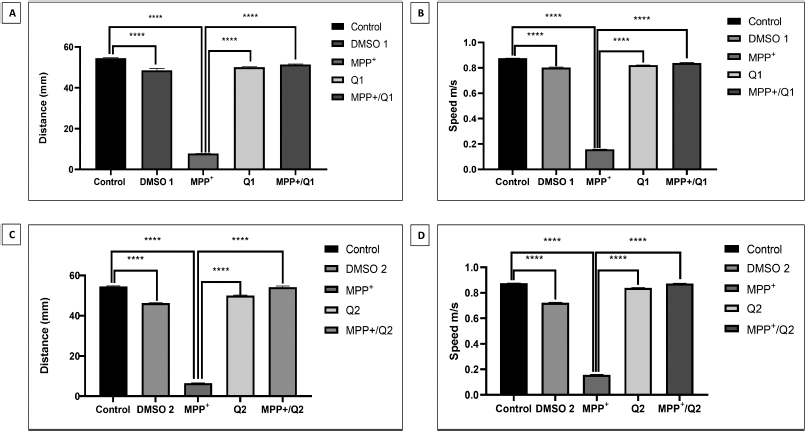 | Figure 3: Statistical analysis of key larval locomotor parameters from different experimental groups. The values were subjected to one-way ANOVA analysis followed by Tukey’s test. A and B: The values of distance travelled and movement speed from MPP+/Q1 group. C and D: The values of distance travelled and movement speed from MPP+/Q2 group. Data analyzed in mean ± SEM with ****p < 0.0001. [Click here to view] |
 | Table 1: Statistical analysis of distance travelled and movement speed of treatment control and treatment groups measured with mean ± standard error of the mean (SEM) using one-way ANOVA analysis followed by Tukey’s test. [Click here to view] |
We treated the zebrafish larvae with quercetin alone to check the effects of quercetin on motor functions. As per statistical analysis (Table 1),, 1-day (Q1) exposure of 12 μM quercetin alone at the 3 dpf stage displayed reduction in distance travelled by 50.02 mm and movement speed by 0.84 m/s (Fig. 3A and C) compared to control. Similarly, 2-day (Q2) exposure of quercetin at the 3 dpf stage decreased the distance travelled by 50.02 mm and movement speed by 0.83 m/s (Fig. 3B and D) compared to control. Studies have shown that quercetin inhibits monoamine oxidase-A (MAO-A) enzyme which modulates dopamine levels affecting the motor function [36–38]. The slight decrease in the motor movements in the Q1 and Q2 treatment groups compared to MPP+/Q1 and MPP+/Q2 might be due to the inhibition of MAO-A activity by quercetin in normal zebrafish larvae. Studies have reported that MPP+ caused oxidative stress leading to loss of dopaminergic neurons correlating to reduction in motor movements [39,40]. However, studies on quercetin demonstrated that it has the potential to attenuate the oxidative stress caused in neurodegenerative diseases [41,42] by activating nuclear factor (erythroid-derived 2)-like (Nrf2-ARE) pathway to regulate oxidative stress and cell death [43–45]. In the present study, quercetin treatment increased the motor functions of MPP+-induced larvae. The attenuation of motor defects by quercetin in MPP+-induced larvae might be due to the activation of Nrf2-ARE pathway in response to oxidative stress induced by MPP+. These claims need further investigation to confirm the physiological effects of quercetin as a neuroprotectant in MPP+-induced zebrafish larvae.
In our study, quercetin rescued MPP+-induced motor defects which might correlate with the alleviation of oxidative stress in the dopaminergic neurons and recovery of dopaminergic functions by quercetin. Our study showed that 2 days of 12 μM quercetin treatment is sufficient to completely rescue zebrafish larvae from MPP+-induced motor defects and this model will be extremely useful to study mechanistic functions of quercetin in neuroprotection.
4. CONCLUSION
In this study, we induced Parkinsonian features in zebrafish larvae using MPP+ which was confirmed by performing locomotor analysis using ImageJ and wrMTrck plugin. Quercetin rescued the motor defects induced by MPP+ in zebrafish larvae. This study concludes that the MPP+-induced zebrafish larval model could be used to screen preclinical PD drugs. In addition, quercetin has the rescue effect in the zebrafish PD model.
5. ACKNOWLEDGMENT
The authors thankfully acknowledge the financial assistance received from the DST-SERB, New Delhi (EMR/2017/000465). The facilities extended by SRMIST are gratefully acknowledged. T.T. and S.M. thank SRMIST for the postgraduate studentships.
6. CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
7. FUNDING
The funding for this study was provided by the DST-SERB, New Delhi (EMR/2017/000465).
8. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Radhakrishnan D, Goyal V. Parkinson’s disease: a review. Neurol India 2018;66(7):26. CrossRef
2. Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 2010;39(2):156–61. CrossRef
3. Antony PMA, Diederich NJ, Krüger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J 2013;280(23):5981–93. CrossRef
4. Langston JW. The MPTP story. J Parkinsons Dis 2017;7(s1):S11–9. CrossRef
5. Song LK, Ma KL, Yuan YH, Mu Z, Song XY, Niu F, et al. Targeted overexpression of α-synuclein by rAAV2/1 vectors induces progressive nigrostriatal degeneration and increases vulnerability to MPTP in mouse. PLoS One 2015;10(6):e0131281. CrossRef
6. Seo J, Lee Y, Kim BS, Park J, Yang S, Yoon HJ, et al. A non-human primate model for stable chronic Parkinson’s disease induced by MPTP administration based on individual behavioral quantification. J Neurosci Methods 2019;311:277–87. CrossRef
7. Sarath Babu N, Murthy CLN, Kakara S, Sharma R, Brahmendra Swamy CV, Idris MM. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease in zebrafish. Proteomics 2016;16(9):1407–20. CrossRef
8. Pasquali L, Caldarazzo-Ienco E, Fornai F. MPTP neurotoxicity: actions, mechanisms, and animal modeling of parkinson’s disease. In: Handbook of neurotoxicity, Springer New York, New York, NY, p 237–75, 2014. CrossRef
9. Hare DJ, Adlard PA, Doble PA, Finkelstein DI. Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 2013;5(2):91. CrossRef
10. Kalyn M, Hua K, Noor SM, Wong CED, Ekker M. Comprehensive analysis of neurotoxin-induced ablation of dopaminergic neurons in zebrafish larvae. Biomedicines 2020;8(1):1. CrossRef
11. Maher P. The potential of flavonoids for the treatment of neurodegenerative diseases. Int J Mol Sci 2019;20(12):3056. CrossRef
12. Zhang ZJ, Cheang LCV, Wang MW, Li GH, Chu IK, Lin ZX, et al. Ethanolic extract of fructus alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol 2012;32(1):27–40. CrossRef
13. Sandoval-Avila S, Diaz NF, Gómez-Pinedo U, Canales-Aguirre AA, Gutiérrez-Mercado YK, Padilla-Camberos E, et al. Neuroprotective effects of phytochemicals on dopaminergic neuron cultures. Neurologia 2019;34(2):114–24. CrossRef
14. Singh A, Tripathi P, Yadawa AK, Singh S. Promising polyphenols in Parkinson’s disease therapeutics. Neurochem Res 2020;45(8):1731–45. CrossRef
15. Luo X, Bao X, Weng X, Bai X, Feng Y, Huang J, et al. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci 2021:120064. CrossRef
16. Hisaka T, Sakai H, Sato T, Goto Y, Nomura Y, Fukutomi S, et al. Quercetin suppresses proliferation of liver cancer cell lines in vitro. Anticancer Res 2020;40(8):4695–700. CrossRef
17. Okumo T, Furuta A, Kimura T, Yusa K, Asano K, Sunagawa M. Inhibition of angiogenic factor productions by quercetin in vitro and in vivo. Medicines 2021;8(5):22. CrossRef
18. Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci 2019;20(13):3177. CrossRef
19. D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 2015;106:256–71. CrossRef
20. Ay M, Luo J, Langley M, Jin H, Anantharam V, Kanthasamy A, et al. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s disease. J Neurochem 2017;141(5):766–82. CrossRef
21. Ghaffari F, Hajizadeh Moghaddam A, Zare M. Neuroprotective effect of quercetin nanocrystal in a 6-hydroxydopamine model of Parkinson disease: biochemical and behavioral evidence. Basic Clin Neurosci J 2018;9(5):317–24. CrossRef
22. Singh S, Jamwal S, Kumar P. Neuroprotective potential of quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regen Res 2017;12(7):1137. CrossRef
23. Razali K, Othman N, Mohd Nasir MH, Doolaanea AA, Kumar J, Ibrahim WN, et al. The Promise of the zebrafish model for Parkinson’s disease: today’s science and tomorrow’s treatment. Front Genet 2021;12:655550. CrossRef
24. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203(3):253–310. CrossRef
25. Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J, Panula P. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem 2009;108(3):719–31. CrossRef
26. Zhang ZJ, Cheang LCV, Wang MW, Lee SMY. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med 2011;27(2):195–203. CrossRef
27. Selvaraj V, Santhakumar K. Analyzing locomotor activity in zebrafish larvae using wrMTrck. Zebrafish 2017;14(3):287–91. CrossRef
28. Opara J, Ma?ecki A, Ma?ecka E, Socha T. Motor assessment in Parkinson`s disease. Ann Agric Environ Med 2017;24(3):411–5. CrossRef
29. Zeng R, Luo DX, Li HP, Zhang QS, Lei SS, Chen JH. MicroRNA-135b alleviates MPP+-mediated Parkinson’s disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 2019;65:125–33. CrossRef
30. Jiao FJ, Wang QZ, Zhang P, Yan JG, Zhang Z, He F, et al. CDK5-mediated phosphorylation of XBP1s contributes to its nuclear translocation and activation in MPP+-induced Parkinson’s disease model. Sci Rep 2017;7(1):5622. CrossRef
31. Zhang Z, Lai D, Wang L, Yu P, Zhu L, Guo B, et al. Neuroprotective effects of the andrographolide analogue AL-1 in the MPP+/MPTP-induced Parkinson’s disease model in vitro and in mice. Pharmacol Biochem Behav 2014;122:191–202. CrossRef
32. Yazdani U, German DC, Liang CL, Manzino L, Sonsalla PK, Zeevalk GD. Rat model of Parkinson’s disease: chronic central delivery of 1-methyl-4-phenylpyridinium (MPP+). Exp Neurol 2006;200(1):172–83. CrossRef
33. Haleagrahara N, Siew CJ, Mitra NK, Kumari M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci Lett 2011;500(2):139–43. CrossRef
34. Mani RJ, Mittal K, Katare DP. Protective effects of quercetin in zebrafish model of Alzheimer’s disease. Asian J Pharm 2018;12(2):660–6.
35. Khan A, Ali T, Rehman SU, Khan MS, Alam SI, Ikram M, et al. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol 2018;9:1–16. CrossRef
36. Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s disease. CNS Neurosci Ther 2014;20(1):10–9. CrossRef
37. Yoshino S, Hara A, Sakakibara H, Kawabata K, Tokumura A, Ishisaka A, et al. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 2011;27(7–8):847–52. CrossRef
38. Alzghoul L, Bortolato M, Delis F, Thanos PK, Darling RD, Godar SC, et al. Altered cerebellar organization and function in monoamine oxidase A hypomorphic mice. Neuropharmacology 2012;63(7):1208–17. CrossRef
39. Wiemerslage L, Schultz BJ, Ganguly A, Lee D. Selective degeneration of dopaminergic neurons by MPP+ and its rescue by D2 autoreceptors in Drosophila primary culture. J Neurochem 2013;126(4):529–40. CrossRef
40. Song Q, Peng S, Zhu X. Baicalein protects against MPP+/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson’s disease. Neurotoxicology 2021;87:188–94. CrossRef
41. Bao D, Wang J, Pang X, Liu H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules 2017;22:1122. CrossRef
42. Bournival J, Quessy P, Martinoli M-G. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol 2009;29:1169–80. CrossRef
43. Sharma A, Parikh M, Shah H, Gandhi T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020;6(4):e03803. CrossRef
44. Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antúnez K, Jones DP, et al. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 2010;49(5):738–47. CrossRef
45. Saw CLL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, et al. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol 2014;72:303–11. CrossRef