1. INTRODUCTION
The global prevalence of diabetes mellitus (DM) is around 150 million and its rate of incidence may increase twofold by 2030. In diabetics, hyperglycemia and the expanded danger of intricacies from vascular illnesses are highly involved. Hyperglycemia is one of the complications which impacts male conceptive capacity at various levels, viz., reduced spermatogenesis, reduced amount of semen and viscosity, agonizing erection, sperm mortality, reduced motility of sperms, and fertility. Approximately 90% of diabetic patients have unsettling implications on sexual capacity, including diminished charisma, impotence, and fertility of libido [1]. Studies of Saumya and Basha [2] revealed that hyperglycemia induces oxidative injury in streptozotocin (STZ)-diabetic mice attributable to reduced reproductive ability and cellular dysfunction. Although there is a global prevalence of diabetes, few studies have been reported on diabetics who reside in temperate and continental zones. The effect of DM on the body’s physiological response to cold stress is a relatively new topic in research. Consequential exposure to cold stress activates the sympathetic nervous system leading to a surge in the level of plasma norepinephrine which could further exacerbate hyperglycemia [3,4]. Resultantly, a sizable number of diabetics residing in these zones, wherein the average annual temperature is 10°C or less, face additional challenges of cold-stress (CS), as the cold climate is associated with minimal sweat production and increased metabolic heat production. Besides, changes in HbA1C levels and improper glycemic management were reported in the diabetic population in these zones [5]. However, no studies have yet assessed the combined effects of cold stress on diabetics concerning reproductive potential.
The usage of herbal medicine is one of the promising prophylactic therapies for diabetes [6,7]. Moringa oleifera is an economically valuable tree, indigenous to the Himalayas and widely found in South India. The consumption of Moringa fruit and leaves is well known to offer nutritional as well as medicinal benefits as they are rich in phosphorous, calcium, potassium, iron, essential amino acids, as well as vitamins A and D [8]. Earlier studies have ascribed Moringa leaves having anti-fungal, anti-atherosclerotic, antioxidant, and anti-inflammatory properties [9,10]. We hypothesized that M. oleifera would improve the survival of diabetic subjects and exert a protective effect against free radical injury as its leaves possess several polyphenols like quercetin, rutin, and glycosides [9–12]. Therefore, this study intended to assess CS-caused exacerbated effects on oxidative stress indices. Additionally, this study appraises the efficacy of M. oleifera leaf extract in managing diabetic complications.
2. MATERIALS AND METHODS
2.1. Chemicals Used
Analytical grade chemicals obtained from Sigma-Aldrich and SRL India Pvt. Ltd. Mumbai.
2.2. Plant Sample Collection Authentication of Plant Material and Extraction
Moringa oleifera leaves gathered from the Jnana Bharathi Campus, Bangalore University, Bengaluru, Karnataka. The plant materials were authenticated by Dr. TG. Umesh, Professor and a consultant taxonomist. A voucher specimen was deposited with number BUB/ DB/PMB/MO/2018. They were dried at room temperature under the shade (25–30 days) and powdered. Initially, 1 kg of powdered leaves was taken for ethanol solvent extraction (70%) using the Soxhlet apparatus at 78°C. Later, the fraction was exposed under reduced pressure using a rotary evaporator to afford a concentrated extract. Furthermore, the Moringa oleifera leaf ethanolic extract (MOLE) was subjected to phytochemical screening and it was found to have potential of free radical scavenging ability and significant flavonoid as well as phenolic contents. The extract was further dissolved in tap water to obtain the required concentrations and the same was used for oral administration.
2.3. Animals
Three-month-old male Wistar rats weighing 200 ± 10 g were procured and they were acclimatized for a week to the housing conditions (12/12 hours light/dark cycle, temperature 25°C ± 2°C, and humidity 50% ± 5%) and segregated into groups, with six rats in each group.
2.4. Induction of Diabetes
To induce diabetes, rats were given intraperitoneal (i.p.) injection of STZ single dose of 45 mg/kg bw (in 0.1 mol/l of citrate buffer, pH 4.5). Blood glucose levels were monitored using Accu-Check glucometer. After 72 hours, diabetic rats (hyperglycemia < 200 mg/dl) were selected for the study, which is considered as the first day of the experiment (day-0).
2.5. Exposure of CS
Animals were exposed to cold stress in the Colton Biological Oxygen Demand (BOD) incubator for 6 hours/day for 60 days [13].
2.6. Experimental Design
All experimental procedures complied with the set of guidelines (Rules for the Care and Use of Laboratory Animals) laid down by the National Institution of Nutrition, Hyderabad, and the protocol of the study was approved by the Bioethics Committee of the Faculty of Zoology at Bangalore University, Bangalore (Protocol number: DOZ/BUB/2018–19 and 402/CPSCSEA 2009–12 and revival thereon). Care was taken to minimize animal usage and suffering. Control (C) animals served as Group-I and diabetics (D) as Group-II. Animals exposed to CS at 15°C were considered as Group-III. Diabetic animals exposed to CS (D + CS) at 15°C as Group-IV; and Group-V, VI and VII as prophylactic group supplemented with MOLE (D + CS+ MOLE at 100, 200, and 500 mg/kg bw by using oral gavage, respectively). The plant extract was given during the regimen process of CS exposure, neither before nor after CS exposure.
2.7. Dose Selection
We conducted a pilot trial by supplementing the following grades of MOLE at 100, 250, and 500 mg/kg bw/day doses to assess the feasibility of dose–response and the regimen continued for a period of 1, 7, 14, 30, 45, and 60 days with weekly and fortnightly intervals. Consequently, the dose of 250 and 500mg/kgbw/day offered better protection than 100 mg dose in 60-day treatment, thereby only 60-day regimen was continued in the study to assess oxidative stress indices.
2.8. Monitoring Blood Glucose Levels
Blood was collected by tail vein puncture from all rats (control and experimental) and blood glucose levels were recorded using a glucometer (Accu-Check; Roche Diagnostics, Indianapolis, IN) on day-0, day-7, day-14, day-30, day-45, and day-60.
2.9. Biochemical Parameters Studied
On the 60th day, all animals were sacrificed for isolation of testis and epididymis and tissue homogenates (w/v) were prepared in ice-cold phosphate buffer (0.1 M; pH 7.4). Upon centrifugation (11,000 rpm for 10 minutes at 4°C), the supernatant was used for following biochemical assays.
2.9.1. Malondialdehyde (MDA) assay
In the assay as described by Niehaus and Samuelsson [14], peroxidation of tissue lipids lead to the formation of MDA which reacts with Thiobarbituric acid (TBA) to form a stable chromophoric product (pink in color) and its intensity was recorded spectrophotometrically at 535 nm.
2.9.2. Antioxidant enzyme assays
The activity of superoxide dismutase (SOD) was recorded as per the method of Misra and Fridovich [15]. In the assay, the inhibition of epinephrine auto-oxidation was measured at 480 nm. Catalase activity (CAT) was measured by the method of Aebi [16] using hydrogen peroxide as a substrate and activity recorded at 240 nm. The activity of glutathione-peroxidase (GSH-Px) was assayed at 340 nm according to Lawrence and Burk [17] using Nicotinamide adenine dinucleotide phosphate (NADPH) and hydrogen peroxide (H2O2) as substrates. Likewise, Glutathione-s transferase (GST) activity was determined using the method of Habig et al. [18]. In the assay 1-chloro-2,4-dinitrobenzene (CDNB) reacted with GSH in producing a colored complex of 2, 4-dinitrophenyl-S-glutathione and its intensity was recorded at 340 nm.
2.9.3. Reduced glutathione (GSH) assay
Tissue GSH levels were measured by the method of Ellman [19]. In the assay, the intensity of yellow-colored chromogen formed was measured spectrometrically at 420 nm.
2.9.4. Total protein assessment
The total protein content of the tissue samples was measured by Lowry et al.’s [20] method using bovine serum albumin (BSA) as standard. In the assay, the intensity of the color complex formed was measured by UV/Vis spectrophotometer at 660 nm.
2.10. Statistical Analysis
Biochemical data were screened by one-way analysis of variance (ANOVA) with the Bonferroni post-hoc test at p < 0.05, which was used to compare control and positive control by using Statistical Package for the Social Sciences software (version 20.0). Comparisons among MOLE-supplemented groups were carried out by one-way ANOVA at p < 0.01 with Duncan’s post-hoc multiple range test (DMRT).
3. RESULTS
3.1. Changes in Blood Glucose Levels
The blood glucose levels monitored in experimental rats displayed a hyperglycemic state. On day-60, the diabetic rats showed higher glucose levels while cold-stressed groups at 15°C had significantly moderate glucose levels from that of control (Table 1). Co-exposure to diabetes, as well as cold stress, revealed a higher impact on glucose levels. The MOLE-supplemented groups showed a remarkable recovery of blood glucose levels from day14 onwards (Table 1). On day-60th, among MOLE-supplemented groups, doses at 250 and 500 mg/kg bw/day showed significantly low levels of glucose when compared to a positive control (Group-IV).
3.2. Effect of CS and Diabetes on Oxidative Stress Indices in the Testis and Epididymis
In testis and epididymis, the lipid peroxidation (LPO) levels (Fig. 1a) were found to be significantly increased (p < 0.05) due to a persistent hyperglycemic state, while cold-stressed animals exhibited a moderate increase in LPO activity. Contrarily, phytoextract supplementation exhibited considerable amelioration by suppressing MDA levels in both testes and epididymis studied. In comparison, the dose of 500 mg/kg bw/day was found to be beneficial than the other doses tested. A similar trend was observed in SOD activity levels (Fig. 1b).
Catalase measurements (Fig. 1c) showed significant (p < 0.05) increments in diabetic and cold-stressed groups. Among the MOLE-supplemented groups, 250 mg/kg bw/day was found to decrease CAT levels significantly (p < 0.05) when compared to other doses. Likewise, significant (p < 0.05) increments were observed concerning glutathione peroxidase activity (Fig. 1d) in diabetic and cold-stressed groups, while MOLE supplementation at 500 mg/kg bw/day dose was favorable in reversing GPx levels comparatively. A similar trend was observed regarding GST activity levels (Fig. 2a) in experimental rats, wherein 500 mg/kg bw/day dose was favorable (p < 0.05) in reversing GST activity comparatively.
 | Table 1: Dose-dependent effect of MOLE on blood glucose levels (mg/dl) of cold stress-exposed diabetic rats at different intervals. [Click here to view] |
 | Figure 1: Dose-dependent effect of MOLE on oxidative stress indices in testis and epididymis of cold-exposed diabetic rats. (a) Changes in LPO levels; (b) changes in SOD activity; (c) changes in CAT activity; and (d) changes in GPx activity. *p < 0.05 significantly different from diabetic control; $p < 0.05 significantly different from diabetic control (D) by using Bonferroni post-hoc. Different superscripts (a, b, c, and d) indicate significant (p < 0.01) differences among antioxidant treatments compared to positive control (D + CS) using DMRT post-hoc. [Click here to view] |
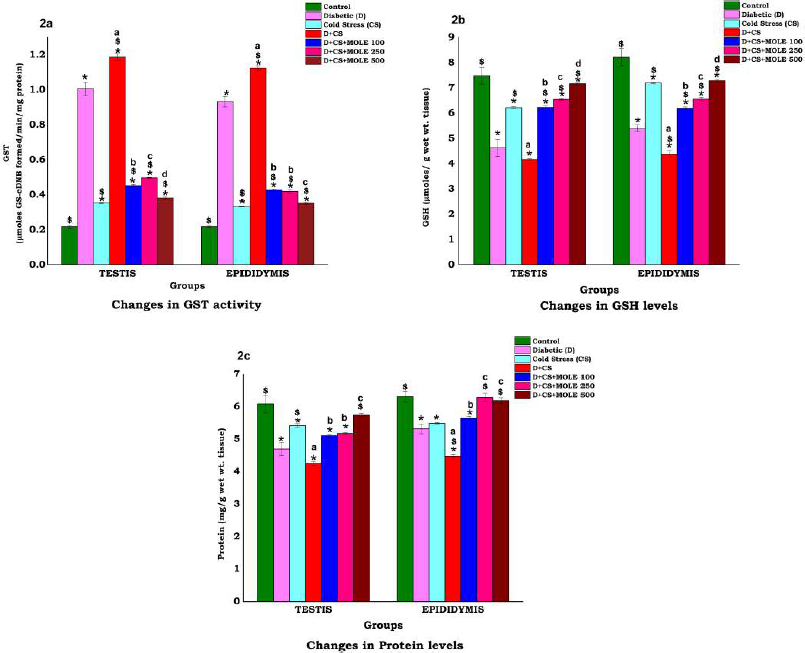 | Figure 2: Dose-dependent effect of MOLE on oxidative stress indices in testis and epididymis of cold-exposed diabetic rats. (a) Changes in GST activity; (b) changes in GSH levels; (c) changes in protein levels. *p < 0.05 significantly different from diabetic control; $p < 0.05 significantly different front diabetic control (D) by using Bonferroni post-hoc. Different superscripts (a, b, c, and d) indicate significant (p < 0.01) differences among antioxidant treatments compared to positive control (D + CS) using DMRT post-hoc. [Click here to view] |
The GSH levels (Fig. 2b) were found to be decreased (p < 0.05) upon exposure to cold stress. In the testis, the co-exposure caused a significant (p < 0.05) exacerbation by suppressing the GSH content. Among MOLE-supplemented groups, 500 mg/kg bw/day dose helped to augment GSH levels.
4. DISCUSSION
Reactive oxygen species (ROS) are byproducts of the mitochondrial respiratory chain that are physiologically neutralized by intracellular antioxidants. The abundance of lipids and proteins makes cellular membranes more vulnerable to the attack of ROS leading to oxidative injury by lipid peroxidation. The presence of ROS such as superoxide (O2 −) and hydrogen peroxide (H2O2) elicits the antioxidant system involving superoxide dismutase, catalase, and glutathione-dependent enzymes to scavenge the ROS and reduce the oxidative damage. The tissue damage due to ROS and lipid peroxidation has been observed in DM. Hyperglycemia often leads to the formation of H2O2 and ketoaldehydes which enhances the production of advanced glycation end products, which in turn results in cellular damage due to the ROS. When the rate of ROS generation surpasses the protective capacity of antioxidants, it ensures the development of insulin resistance and oxidative stress [2,21]. Moreover, the findings of this study revealed that diabetes induction in rats caused the critical oxidative injury in the male testicular milieu which is evident from increased MDA levels in testis and epididymis. Furthermore, the altered antioxidant enzyme activity, such as CAT and SOD, can be attributed to hyperglycemia-induced production of O2 − and H2O2, respectively. Excessive ROS production might have enhanced glutathione-dependent enzymes such as peroxidase and transferase that may lead to the depletion of a non-enzymatic antioxidant, glutathione.
The global prevalence of diabetes has raised the concern of diabetics in temperate and continental zones too. People living in those zones, where the annual average temperature is 10°C or lesser, are more susceptible to health complications. Cold temperature is associated with raised metabolic rate and metabolic heat production to prevent the decline in core temperature [5]. The elevated metabolic rate could generate ROS [22]. Studies of Basha and Poojary [23] have revealed that subjecting CPF-intoxicated rats to CS (15°C and 20°C) could trigger unbalanced oxidative status in discrete brain regions. Similarly, in the present investigation, enhanced LPO, SOD, CAT, GPx, and GST levels reveal oxidative alterations in testis and epididymis of cold-exposed rats which indicate a possible testicular dysfunction. Earlier reports revealed that the diabetic population exposed to cold climate may have poor glycemic control and raised glycosylated hemoglobin levels [5], but the underlying mechanism is still unclear. According to our study, co-exposure to cold stress and diabetes led to exacerbated oxidative stress indices and lipid peroxidation in testis and epididymis. Therefore, corroborating the studies of Saumya and Basha [2], alterations in ROS and antioxidant defenses could influence the spermatogenetic process, leading to testicular dysfunction in cold-stressed diabetics.
The preponderance of studies indicated that phytochemicals present in M. oleifera offered protection from oxidative stress [24,25]. Liu et al. [26] ascertained that the Moringa leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxideinduced oxidative stress. Likewise, the findings of Mohamed et al. [27] advocated the efficacy of M. oleifera extract in reversing high fructose diet-induced insulin resistance and improvement in testicular function in hyper-insulinemic male rats. Relevant studies also demonstrated that M. oleifera leaves extract, rich in polyphenols, delays the onset of diabetes [8,28–30]. Although beneficial effects of M. oleifera are confirmed by various studies [28,31–32], there is a need to understand the modulatory actions of phytoextract on diverse pathways and specific enzymes. The MOLE extract used in the present study was previously assessed and it exhibited potential in vitro free radical scavenging ability, and phenolic and flavonoid contents (results not shown). After 60 days of in vivo studies, the supplementation of MOLE extracts at 250 and 500 mg/kg bw/day was found to exhibit a glucose-lowering effect in CS-exposed diabetic rats (Table 1) and the hypoglycemic ability of MOLE extract can be ascribed to the actions of quercetin and kaempferol-O-glycosides [8–12]. The preponderance of studies supports the connection between kaempferol-O-glycoside molecules and glucose uptake in rat muscle via the PI3K and PKC pathways as they improve insulin resistance [8–12]. Quercetin was shown to lower blood glucose uptake due to the downregulation of muscle and hepatic phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (G6Pase) which explains the hypoglycemic ability [33]. The findings of this research are consistent with the results of previous studies [8,28–30] on the anti-oxidative abilities of phytoextracts. Our results confer the antioxidant ability of M. oleifera and its exposure helps to decrease oxidative stress. Moreover, polyphenols in MOLE might be responsible for quenching free radical malactions in CS diabetic rats. In short, the pharmacological actions of M. oleifera extract are ascribed to its active polyphenolic derivatives such as quercetin-3-glycoside, rutin, and glycosides, whose actions proved to quench malactions of free radicals by donating hydrogen and forming stable radical intermediates. Furthermore, these phenols besides the neutralization of free radicals also helped to regulate dismutase enzymes, as well as peroxidases [34]. The flavonoids and triterpenoids of M. oleifera seem to be helpful in regulating the ROS production to minimize the oxidative damage in reproductive organs of cold-stressed diabetic rats [35]. These findings in brief corroborate with the inferences drawn in the studies of Nizio?-?ukaszewska et al. [36].
5. CONCLUSION
Repeated exposure to cold temperatures produces stress in animals/humans and stressed subjects display various abnormalities including hyperglycemia. In a given situation, if the subject is already suffering from diabetes and exposure to cold stress further aggravates the complication in causing an elevation of pro-oxidants with consistent depletion in antioxidant defense leading to oxidative stress and related changes. The findings advocate exacerbated oxidative stress in male reproductive organs (testis and epididymis), which could pay a way to testicular dysfunction. The phenolic and flavonoids contents present in MOLE helped to regulate the enzymatic antioxidants and consumption of MOLE extracts at 500 mg/kg bw/day for 60 days offered protection to cold-stressed diabetic rats. However, further studies are warranted to formulate the dose regimen required for the human population.
6. ACKNOWLEDGMENTS
The help rendered in the screening of biochemical compounds of phytoextract by Rizwan Sharief is acknowledged.
7. CONFLICT OF INTEREST
Declared no conflicts.
8. FUNDING
Monetary support in the form of Rajiv Gandhi National Fellowship (RGNF) funded by the Ministry of Tribal Affairs.
9. AUTHORS’ CONTRIBUTIONS
Basha PM designed the protocol and drafted the manuscript. Rakesh H carried out the experiments and assimilated data. Saumya SM analyzed data and edited the manuscript.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Feng SL, Li SH, Wang L, Chen CC, Gao B. Effect of ligustrum fruit extract on reproduction in experimental diabetic rats. Asian J Androl 2001;3(1):71–3.
2. Saumya SM, Basha PM. Fluoride exposure aggravates the testicular damage and sperm quality in diabetic mice: protective role of ginseng and banaba. Biol Trace Elem Res 2017;177(2):331–44. CrossRef
3. Liu IM, Niu CS, Chi TC, Kuo DH, Cheng JT. Investigations of the mechanism of the reduction of plasma glucose by cold-stress in streptozotocin-induced diabetic rats. Neurosci 1999;92(3):1137–42. CrossRef
4. Nishanta, Gora P. Assessment of impact of cold stress on heart rate and blood pressure in healthy offspring with and without parental history of Type 2 diabetes mellitus: a comparative study. Int J Med Res Prof 2020;6(1):64–7.
5. Kenny GP, Sigal RJ, Ryan R. Body temperature regulation in diabetes. Temperature 2016;3(1):119–45. CrossRef
6. Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes: the global Burden. Evid Based Complement Alternat Med 2014;2014:596071. CrossRef
7. Singh RK, Sharma B. Certain traditional Indian plants and their therapeutic applications: a review. Vri Phytomedicine 2013;1(1):1–11. CrossRef
8. Misrha G, Singh P, Verma R, Kumar S, Srivastav S, Jha KK, et al. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. Der Pharmacia Lett 2011;3(2):141–64.
9. Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Antifungal activity of crude extracts and essential oils of Moringa oleifera Lam. Bioresour Technol 2007;98(1):232–6. CrossRef
10. Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-ngam L, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol 2008;116(3):439–46. CrossRef
11. Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycaemic rats. J Ethnopharmacol 2009;123(3):392–6. CrossRef
12. Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed Res Int 2015;2015:381040. CrossRef
13. Ma S, Morilak DA. Chronic intermittent cold-stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol 2005;17(11):761–9. CrossRef
14. Niehaus WG, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6(1):126–30. CrossRef
15. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170–5. CrossRef
16. Aebi H. Catalase In vitro. In: Packer L (ed.). Methods in enzymology, Academic Press, San Diego, CA, pp 121–6, 1984. CrossRef
17. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976;71(4):952–8. CrossRef
18. Habig WH, Pabst MJ, Jakoby WB, Glutathione S-transferases, The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249(22):7130–9. CrossRef
19. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–7. CrossRef
20. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265–75. CrossRef
21. Ramalho-Santos J, Amaral S, Oliveira PJ. Diabetes and impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 2008;4(1):46–54. CrossRef
22. Selman C, McLaren JS, Himanka MJ, Speakman JR. Effect of long-term cold exposure on antioxidant enzyme activities in a small mammal. Free Radic Biol Med 2000;28(8):1279–85. CrossRef
23. Basha PM, Poojary A. Oxidative macromolecular alterations in the rat central nervous system in response to experimentally co-induced chlorpyrifos and cold stress: a comparative assessment in aging rats. Neurochem Res 2012;37(2):335–48. CrossRef
24. Sánchez-Muñoz MA, Valdez-Solana MA, Campos-Almazán MI, Flores-Herrera O, Esparza-Perusquía M, Olvera-Sanchez S, et al. Streptozotocin-induced adaptive modification of mitochondrial super-complexes in liver of Wistar rats and the protective effect of Moringa oleifera Lam. Biochem Res Int 2018;2018:5681081. CrossRef
25. Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med 2013;6(6):426–32. CrossRef
26. Liu J, Ma G, Wang Y, Zhang Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod Domest Anim 2020;55(6):711–9. CrossRef
27. Mohamed MA, Ahmed MA, El Sayed RA. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J Diabetes Metab Disord 2019;18(2):487–94. CrossRef
28. Ahmad J, Khan I, Blundell R. Moringa oleifera and glycemic control: a review of current evidence and possible mechanisms. Phytother Res 2019;33(11):2841–8. CrossRef
29. Ma ZF, Ahmad J, Zhang H, Khan I, Muhammad S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. S Afr J Bot 2020;129:40–6. CrossRef
30. Sierra-Campos E, Valdez-Solana M, Avitia-Domínguez C, Campos-Almazán M, Flores-Molina I, García-Arenas G, Téllez-Valencia A. Effects of Moringa oleifera leaf extract on diabetes-induced alterations in paraoxonase 1 and catalase in rats analyzed through progress kinetic and blind docking. Antioxidants (Basel) 2020;9(9):840. CrossRef
31. Vongsak B, Mangmool S, Gritsanapan W. Antioxidant activity and induction of mRNA expressions of antioxidant enzymes in HEK293 cells of Moringa oleifera leaf extract. Planta Med 2015;81(12– 13):1084–9. CrossRef
32. Abdelazem, H. Effect of Moringa oleifera on antioxidant enzymes and oxidative stress induced by aluminium exposure in male albino rat testes. Int J Cancer Biomed Res 2019;3(3):34–41.
33. Eid HM, Nachar A, Thong F, Sweeney G, Haddad PS. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag 2015;11(41):74–81. CrossRef
34. Fukumoto LR, Mazza G. Assessing antioxidant and pro-oxidant activities of phenolic compounds. J Agric Food Chem 2000;48(8):3597–604. CrossRef
35. Nunthanawanich P, Sompong W, Sirikwanpong S, Mäkynen K, Adisakwattana S, Dahlan W, et al. Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springerplus 2016;5(1):1098. CrossRef
36. Nizio?-?ukaszewska Z, Furman-Toczek D, Bujak T, Wasilewski T, Hordyjewicz-Baran Z. Moringa oleifera L. extracts as bioactive ingredients that increase safety of body wash cosmetics. Dermatol Res Pract 2020;1:8197902. CrossRef