1. INTRODUCTION
The traditional Indian system of medicine, known as Ayurveda, has been used for generations in treatment of diseases associated with the immune system. Unlike the modern system of medicine, Ayurveda is more preventive in nature and is centered on maintaining a balance in the body. Medicinal plants prescribed by Ayurveda are known for enhancing the body’s defense mechanism through their rejuvenating properties and immunomodulatory effect on the immune system [1]. Immunomodulation is the regulation of host responses by stimulation or suppression according to different pathological or biological changes. The proper functioning of the immune system requires a balance between these two [2].
Immunostimulation is activation of an immune response or the function of the immune system either specific or nonspecific manner, such as the antigen dependent activation of function and efficiency of colony stimulating factor 2, natural killer cells, etc., whereas immunosuppression is the process of deactivating an immune response in hyperactive or autoimmune disorders [3]. On immunostimulation pro-inflammatory cytokines such as IL-6, IL-1, IL-8, and TNFα recognized as integral mediators for the development of suitable host defense mechanism for infections [4,5]. The above cytokines activates macrophages, polymorphonuclear neutrophils, and monocytes which involves in destruction of pathogens and phagocytosis. Previous studies have reported that few antibiotics along with antimicrobial activity can affect the production of cytokines in immune responses [6,7].
Zebrafish model was selected for the evaluation because it has small size, fast to develop, economical to maintain and has high fecundity. Transparent body of zebrafish embryos in their early stages helps to collect various data through high quality images. The annual zebrafish maintenance cost is less when compared to rodents [8]. The cost advantage is highly increased when we use zebrafish embryo as test animal, due to their ability to lay up to ten thousand eggs annually. Embryo of zebrafish can fulfill the needs of biomedical research with low cost and high throughput screening. Administration of drugs directly in the swimming water is easier and quicker compared to rodents [9].
At present, immunomodulators obtained from different sources are used to treat pathological conditions with altered immune response such as disorders of autoimmune, asthma, and carcinoma [10]. Although a number of synthetic drugs are being used in immunotherapeutics, the adverse side effects associated with their usage such as nephrotoxicity, anemia, thrombocytopenia, and bone marrow suppression have produced an awareness to limit their usage and to search for safe alternatives [11,12]. Use of immunomodulators from plant-based medicines has enhanced due to broad safety margin compared to synthetic drugs.
Immusante® also known as IM-133N is a proprietary formulation of The Himalaya Drug Company, Bengaluru, India, which is a mixture of aqueous extracts of Symplocos racemosa and Prosopis glandulosa in a ratio of 2:3, respectively. The finger printing and identification of various phytochemical components of Immusante® were reported and identified by LC-MS/MS method [13]. Briefly, it was found to have Symploveroside, Apigenin, Mesquitol, Chaulmoogric acid, Quercetin, Locoracemoside B, Symphoxanthone, Symconoside A, Salireposide, Symplocomoside, β-Sitosterol, Symponoside, Symplososide, Nonaeicosanol, oleanolic acid, betulinic acid, and β-Amyrin.
In this context, the present study was carried to evaluate the immunomodulatory activity of Immusante® against A. hydrophila (Ah) induced bacterial infection in zebrafish.
2. MATERIALS AND METHODS
2.1. Materials
Immusante® was obtained from The Himalaya Drug Company, Makali, Bengaluru. Zebrafish primers were procured from Sigma-Aldrich and cDNA synthesis kit from Invitrogen Bengaluru.
2.2. Zebra Fish
Adult zebrafish (Danio rerio) of both sex around 3-4 months old weighing about 400–500 mg were procured from aquarium pet store (Varalikas Aqua and Pets, Hyderabad, India) and kept in acrylic housing tanks of 30 cm × 15 cm × 15 cm dimensions filled with RO water at a density of 5 fish/L. 14:10 h light and dark cycle was maintained with continuous aeration. Fishes were fed two times daily with Guppy pro. Fishes were kept for 15 days acclimatization period to the experimental conditions.
2.3. Bacterium
A. hydrophila MCC 2052 was obtained from Microbial Culture Collection, Pune. A. hydrophila was cultured at 28°C in Tryptone Soya Broth medium for 16 h followed by harvesting by centrifuging at 3000 rpm for 15 min at 4°C. The obtained bacterial pellet was suspended in 20 mM sterilized PBS to get a concentration of 6 × 107 cells/ml which was then stored at 4°C until further use [14].
2.4. Methods
2.4.1. Optimization of A. hydrophila dose
To determine the A. hydrophila dose for the study, 30 zebrafish of either sex were divided into 5 groups of 6 each and then intra-peritoneal injected with 10 μl of 6.0 × 106, 6.0 × 205, 6.0 × 105, 6.0 × 104, and 6.0 × 103 cells/ml of live A. hydrophila suspension to get the concentration of 6.0 × 104, 6.0 × 203, 6.0 × 103, 6.0 × 102, and 6.0 × 101 cells/fish followed by continuous culture. For control, zebrafish were injected 10 μl of 20 mM sterilized PBS alone. The zebrafish mortality was recorded at every 6 h up to 48 h. The concentration of bacteria causing at least 80% mortality was determined and that was used for the further study. The LD50 was calculated by the method of bliss [15].
2.4.2. Acute toxicity study of Immusante®
Acute toxicity study was done as per the procedures described in OECD 203 guideline [16], a limit test was performed for the polyherbal formulation-Immusante® to select the test drug concentration for the study. Immusante® was tested at 1000 mg/L and tested concentration was prepared by homogenizing the required amount with 100 ml RO water followed by its dilution in the required volume with RO water.
The fishes were separated into 2 groups of 7 each. Group 1 was control and Group 2 was treated with Immusante®. The fish/water ratio was 1 g/1.8–2 L. The fishes were kept in 25 ± 1°C water with pH 7.3, dgH = 15 N° was the total hardness of water, 14:10 h light and dark regimen was maintained, dissolved concentration of oxygen was > 60%. All toxicity tests were semi-static. The exposure of sexually mature zebrafish individuals to the water with or without the Immusante® was performed in covered 7 L acrylic tanks for 4 days. Any signs of mortality and sublethal effects were observed during the entire study period. The experiment was repeated 3 times.
2.4.3. Zebrafish survival assay
2.4.3.1. Method 1
To examine the protective role of Immusante® and to check the survival rate, 36 zebrafish of either sex were taken and divided into 4 equal groups of 9 each. Group 1 was control, group 2-4 were administered with A. hydrophila; Group 3 and 4 were treated with Immusante® at a dose of 200 and 400 mg/L, respectively.
Before experimentation groups of 9 zebrafish each were fasted for 12 h, and exposed to Immusante® at different concentrations 200 mg/L and 400 mg/L for 4 days, and then intraperitoneal injected 10 μl of 6.0 × 203 cells/ml (causing 80% mortality) of live A. hydrophila suspension. To path control, zebrafish were similarly injected with 10 μl of sterilized 20 mM PBS alone. Mortality was recorded at 6 h, 12 h, 24 h, and 48 h after bacterial injection, the calculation of total mortality was done. All the experiments were repeated three times. The relative percent survival (RPS) or relative level of protection was calculated using the following formula.
RPS = [1 - (mortality of experimental group/mortality of control group)] × 100
2.4.3.2. Method 2
To test the longevity in vivo, 4 groups of 9 zebrafish were fasted for 12 h, anesthetized by Tricaine. Group 1 was control, Group 2-4 were administered with A. hydrophila. Fifteen microliters per oral of Immusante® with different concentrations of 6.6 and 13.2 mg/ml were given to Groups 3 and 4 for 4 days (to give a final amount of 1.3 g and 2.6 g/kg fish respectively) followed by intraperitoneal injection of 10 μl of 6.0 × 205 cells/ml of live A. hydrophila suspension. To the path control (group 2), zebrafishes were injected with 10 μl of sterilized 20 mM PBS then injected same amount of live A. hydrophila as in treated groups. Mortality was observed up to 72 h at the intervals of every 6 h after the bacterial challenge and longevity graph was plotted. All the experiments were carried out for 3 times.
2.4.4. Effect of Immusante® on A. hydrophila infection in zebrafish
The zebrafish survival assay revealed that 400 mg/L concentration is the minimum dose of Immusante® to have maximum RPS, thus to test the effect of Immusante® on A. hydrophila infection, 16 zebrafish of either sex were taken, divided into 2 groups of 8 each and proceeded for the following treatment exposures accordingly.
Group 1: PBS and A. hydrophila
Group 2: Immusante® (400 mg/L) and A. hydrophila
In Group 1, zebrafish were injected with PBS followed by immediately injecting 10 μl of 6.0 × 205 cells/ml (the dose that causes 80% mortality) of live A. hydrophila intraperitoneally. While in Group 2, zebrafish were exposed to 400 mg/L of Immusante® for 4 days, and then challenged i.p. with the same amount of live A. hydrophila suspension. The fish culture was done at 27 ± 1°C. At 6 h and 12 h, 4 fishes were sacrificed after the bacterial challenge then blood was withdrawn by cardiac puncture into K2-EDTA-coated Eppendorf tubes and serial dilution was done 5 times with PBS. Subsequently, kidney was dissected out, washed with sterile PBS with pH 7.4, and weighed. Each tissue was homogenized in 10 volumes (volume/weight) of 20 mM PBS and serially diluted to 10 times with PBS. One hundred microliters aliquots of diluted blood and 1000 μl of diluted tissue homogenate had taken and spread onto TSA medium and allowed to culture at 28°C for 48 h. All the experiments were carried out for three times. The resulting CFUs were counted and calculation of CFUs/ml was done using following formula.
CFU/ml= No. of colonies X Dilution factor/ Volume of culture plated
2.4.5. Differential WBC count in the zebrafish infected with A. hydrophila
To see the effect of Immusante® on differential WBC count in zebrafish infected with A. hydrophila, 15 zebrafish of either sex were taken, divided equally into three groups of 5 each as follows and treated respectively
- Group 1: (Control): PBS
- Group 2: (Path Control): PBS and A. hydrophila
- Group 3: Immusante® (2.6 g/kg) and A. hydrophila.
In control group, the zebrafishes were injected with 20 μl PBS alone and in path control group, the zebrafishes were injected with 10 μl of sterilized 20 mM PBS followed by 10 μl injection of 6.0 × 205 cells/ml of live A. hydrophila. Whereas, Group 3 received 15 μl of the Immusante® with the same concentration of 13.2 mg/ml (2.6 g/kg) orally for 4 days, and then injected intraperitoneally same amount of live A. hydrophila suspension. Six hours after the bacterial challenge fish were bleed through cardiac puncture using 1 ml tuberculin syringe fitted with 24G needle and around 10 μl blood was collected into glass slide using micropipette with tip rinsed previously with K2-EDTA solution and then stained with Leishman’s stain. Differential WBC count was done after selecting about 100 leukocytes from each smear under oil immersion objective. Percentage of lymphocytes, monocytes, and neutrophils were calculated. All the experiments were carried out three times.
2.4.6. Gene expression analysis
To test Immusante® act as an immunomodulator, 15 zebrafish of either sex were taken and divided into 3 equal groups of 5 each as follows:
- Group 1: (Control): PBS
- Group 2: (Path Control): PBS & A. hydrophila
- Group 3: Immusante® (2.6 g/kg) and A. hydrophila.
In the control group, zebrafishes were injected 10 μl of PBS alone. In the path control group, zebrafishes were injected 10 μl of sterilized 20 mM PBS followed by 10 μl injection of 6.0 × 205 cells/ml of live A. hydrophila suspension and Group 3 were treated orally with 15 μl of Immusante® of concentration 13.2 mg/ml (2.6 g/kg) for 4 days, and then challenged i.p. with the same amount of live A. hydrophila suspension. Zebrafishes were sacrificed at 12 h after the bacterial challenge and kidney was dissected out from all the fishes in the different groups, weighed individually, and stored immediately at −80°C followed by RNA isolation.
2.4.6.1. Step 1: Total RNA isolation from kidney tissues
The pooled frozen kidney samples each weighing around 50 mg were homogenized individually with limited speed (to avoid RNA degradation) in 500 μl of Ribox, a monophasic solution contains guanidine salt and phenol that quickly lyses the cells and inactivates nucleases. The homogenized samples were incubated for 5 min at room temperature and centrifuged at 12,000 rpm, 4°C for 10 min. To the supernatant almost 400 μl collected, 100 μl of chloroform was added and shaken for 15 s. Separation of the homogenate into aqueous and organic phases by done was by addition of chloroform. As RNA gets separated in the aqueous phase; therefore, aqueous phase (200 μl) was collected and mixed with equal amount of RB1 buffer. Four hundred microliters of the mixture were transformed to the mini spin column and centrifuged at 10,000 rpm for 1 min at RT. Filtrate was discarded completely and the mini spin column was reinserted and added with 500 μl of SW1 buffer, then centrifuged, filtrate was discarded and 500 μl of RNW buffer was added to mini spin column and the above steps were repeated again. The upper tube of mini spin column was transferred to RNase free vials and 80 μl of nuclease free water was mixed (to prevent genomic contamination) and centrifuged at 10000 rpm for 1min at RT. The filtrate containing only RNA was collected into separate vials with labeling the required details and immediately stored in −20°C until used [17].
2.4.6.2. Step 2: cDNA synthesis by reverse transcription (RT) reaction
Twenty-four-microliter RNA was incubated with 2.25 μl of Hexamer Primer and 7.5 μl RNase free water at 70°C for a minute to denature RNA 2° structure and then immediately chilled on ice for 10 min to let the primer anneal to the RNA. Other RT components were added to the reaction including 2.5 μl dNTPs, 3 μl RTase, and 9 μl of 5x RT buffer. Then, RT reaction was extended to 1 h at 42°C and was heated for 10 min at 70°C to inactivate the enzyme [18].
2.4.6.3. Step 3: Real-time polymerase chain reaction (RT-PCR)
Specific forward and reverse primers for pro-inflammatory cytokine genes such as IFN-γ, TNF-α, IL-1β, and anti-inflammatory cytokine genes such as IL-4 were designed using primer 5 program based on the D. rerio gene sequences deposited in the GenBank (Table 1). Internal standardization was done using β-actin gene. Efficiency of primer was determined by performing serial dilutions of reference cDNA. Semi-quantitative RT-PCR was performed using CFX Connect RT-PCR Detection System after the quantification of cDNA templates and primers. Reaction (22.5 μl total volumes) contained 4.5 μl of water, 10 μl of 2 x SYBR green mixes, 2 μl of 10 μM forward and reverse primer of a gene, and 4 μl cDNA. The following 3 step qRT-PCR reaction was performed: Pre-denaturation at 95°C for 30 s, followed by 35 cycles of denaturation at 95°C for 5 min, annealing at 60°C for 30 s, and elongation at 72°C for 20 s. The expression levels of the cytokine genes relative to β - actin were calculated by the comparative Ct method (2-ΔΔCt) [19,20].
2.4.7.Statistical analysis
Data of half lethal dose in optimization of A. hydrophila dose were analyzed by the method of logit-probit analysis and all the other data were determined by One-way ANOVA followed by Dunnett’s test using GraphPad Prism 3.0. Difference at P < 0.05 was considered statistical significant. All the data were expressed as mean ± SD.
3. RESULTS
3.1. Optimization of A. hydrophila Dose
To determine the dose of live A. hydrophila in zebrafish, different doses of live A. hydrophila (6.0 × 101, 6.0 × 102, 6.0 × 103, 6.0 × 203, 6.0 × 104 cells/fish) were injected in zebrafish and generated 48 h cumulative mortalities of 0%, 16.6%, 50%, 83.3%, and 100%. As shown in Table 2, LD50 dose of live A. hydrophila calculated was 7.4 × 103 cells/fish and the concentration of bacteria causing at least 80% mortality was 6.0 × 203 cells/fish. Death of zebrafish by the injection of higher concentrations of A. hydrophila was faster when compared to lower concentrations of A. hydrophila. Zebrafishes injected with A. hydrophila showed symptoms like weak swimming, swelling of coelomic cavity with red or dark brown lesions near the injection site. There was no disease symptoms and mortality found in zebrafishes injected with PBS.
3.2. Acute Toxicity Study of Immusantereg
Immusante® (1000 mg/L) showed no mortality, no signs of abnormal behavior and sublethal effects. This indicates that the LC50 of Immusante® would be greater than this concentration used in the limit test and these observations seem to justify that Immusante® up to 1000 mg/L is safe and nontoxic to zebrafish according to OECD 203. The concentrations of Immusante® selected for the study was 200 mg/L and 400 mg/L.
3.3. Zebrafish Survival Assay
3.3.1. Method 1
There was no mortality observed among all the groups in first 6 h, as shown in Figure 1. Maximum mortality was found in zebrafishes injected with PBS and A. hydrophila within 12-48 h, while gradual mortality found in zebrafishes exposed to different concentrations of Immusante®. The Immusante® action was found in a concentration dependent manner. Cumulative mortality of zebrafishes exposed with Immusante® at the dose of 200 mg/L and 400 mg/L were 55.55 ± 9.61% and 22.21 ± 9.62%, respectively, at 48 h, whereas the cumulative mortality of zebrafishes injected with PBS was found 88.88 ± 9.62%. The RPS of Immusante® at concentrations of 200 mg/L and 400 mg/L were 36.66% and 74.44% individually (Table 3). These data indicate that Immusante® can protect zebrafish from the pathogenic attack of A. hydrophila and significantly enhanced the survival rate. 400 mg/L was the effective concentration of Immusante® to get maximum RPS.
3.3.2. Method 2
No mortality was observed among all the groups of zebrafishes in first 12 h. High mortality was found in zebrafishes injected with PBS and A. hydrophila within 8–24 h, while gradual mortality found in zebrafishes exposed to different concentrations of Immusante®. The percentage survival of zebrafishes at different time points in groups treated with Immusante® at dose of 1.3 g/kg and 2.6 g/kg was shown in Figure 2 and the percentage survival of zebrafishes was more in Immusante® (2.6 g/kg), then Immusante® (1.3 g/kg) when compared to path control group. This longevity in zebrafish treated with Immusante® followed by bacterial challenge indicates Immusante® can protect zebrafish from pathogenic attack of A. hydrophila.
3.4. Effect of Immusante® on A. hydrophila Infection in Zebrafish
To verify the effect of Immusante® on A. hydrophila infection, zebrafish were given oral dose of Immusante® then injected live A. hydrophila followed by measuring bacterial numbers in kidney and blood of the zebrafishes at 6 and 12 h as shown in Figure 3A and B. A. hydrophila was found in blood and kidney tissues from zebrafishes treated with Immusante® (400 mg/L) at 6 h and 12 h. The CFUs in blood and kidney tissues at 12 h was found higher compared to those at 6 h, showing that until 12 h A. hydrophila did not effectively traffic to tested tissues. The CFUs in Immusante® treated zebrafish tissues were significantly reduced at 12 h compared to control group (Figure 3A and B). This denotes that Immusante® was able to stop the multiplication/dissemination of A. hydrophila in zebrafish.
3.5. Effect of Immusante® on Differential WBC Count in the Zebrafish Infected with A. hydrophila
Lymphopenia and neutrophilia were observed in the path control group as compared to control group. Immusante® treated groups showed significantly increase in the lymphocytic count and decrease in neutrophil count when compared to path control group, but there was no significant difference in monocytes count among different groups (Figure 4).
 | Table 1: The specific primers sequence of zebrafish used for RT-PCR. [Click here to view] |
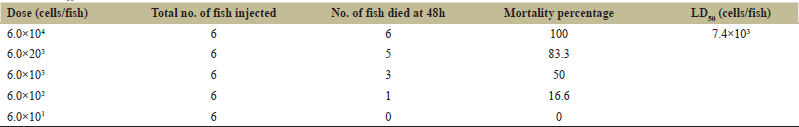 | Table 2: LD50 of A. hydrophila in zebrafish by i.p. injection. [Click here to view] |
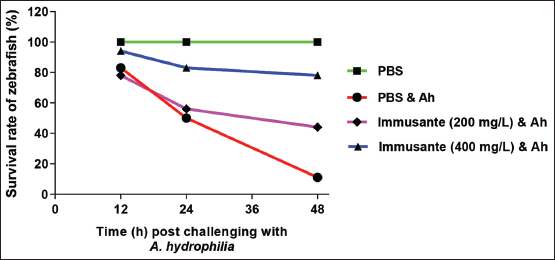 | Figure 1: Effect of Immusante® on survival rate of zebrafish infected with A. hydrophila. [Click here to view] |
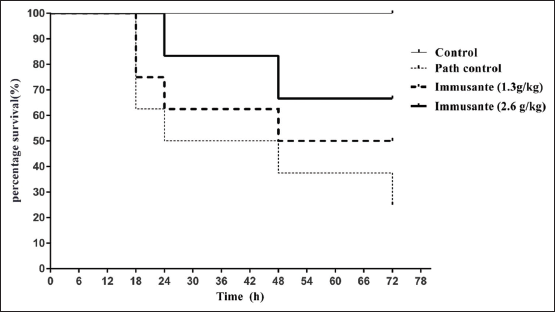 | Figure 2: Effect of Immusante® on longevity of infected zebrafish. [Click here to view] |
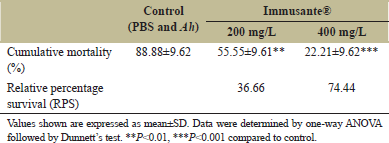 | Table 3: Effect of Immusante® on cumulative mortality and RPS of zebrafish infected with A. hydrophila. [Click here to view] |
3.6. Effect of Immusante® on Gene Expression
To evaluate the immunomodulatory activity of Immusante®, we have checked the expression of pro-inflammatory cytokine genes such as IFN-γ, TNF-α, IL-1β, and anti-inflammatory cytokine gene like IL-4. Immusante® was found to significantly decrease the expression of TNF-α, IL-1β, and IFN-γ in kidney at 12 h after injecting A. hydrophila when compared to path control group (Figure 5a-c). By contrast, there was no significant difference in IL-4 expression among different groups (Figure 5d). These data revealed that Immusante® could suppress the expression of pro-inflammatory cytokine genes such as TNF-α, IFN-γ, and IL-1β indicating that Immusante® can be an immunomodulator.
4. DISCUSSION
In present study, an experimental model of A. hydrophila-induced infection in zebrafish is used to evaluate Immusante® for immunomodulatory activity. A. hydrophila and several other motile Aeromonas are infectious bacteria which can cause fatal septicemia in fishes as well as in human which is more harmful than previously believed [21], because of it can produce a number of virulence factors such as lipases, proteases, enterotoxins, and its resistance to many antibiotics such as ampicillin and penicillin [22]. This bacterium is capable of causing cytotoxicity and massive inflammation. The fish infected with A. hydrophila shows clinical symptoms such as swelling of abdomen and hemorrhages [23].
The experimental results demonstrated the beneficial effect of Immusante® against the A. hydrophila infection and also significantly enhanced the infected zebrafish survival rate. Increased survival was also confirmed by the fact that Immusante® able to decrease markedly A. hydrophila multiplication in zebrafish.
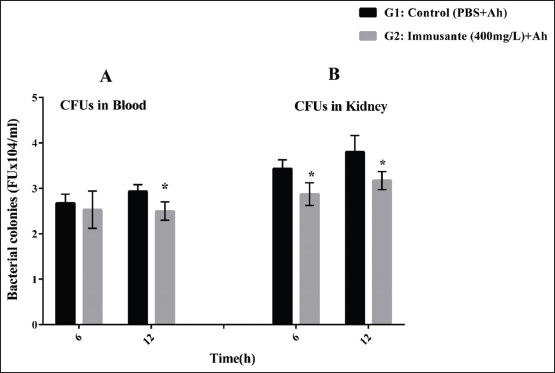 | Figure 3: (A and B) Bactericidal activity of Immusante® on A. hydrophila. The results shown are in mean ± SD. Data were determined by one-way ANOVA followed by Dunnett’s test.*P < 0.05 compared to control. [Click here to view] |
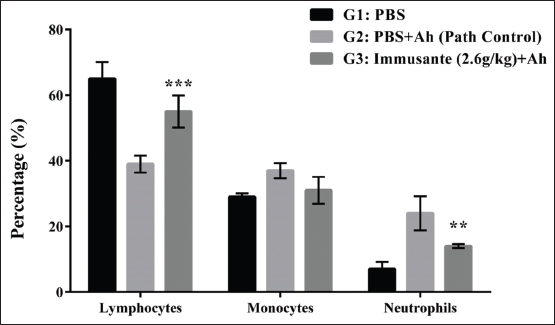 | Figure 4: Effect of Immusante® on lymphocytes, monocytes, and neutrophil count of zebrafish infected with A. hydrophila. Values are expressed as mean ± SD. Data were determined by one-way ANOVA followed by Dunnett’s test. *P < 0.05 compared to path control group. [Click here to view] |
Moreover, a significant decrease of lymphocytes and increase in neutrophil count is clearly associated with the decreased survival rate in pathological control group. The treatment groups produced an opposite effect, which may be considered as a sign of improvement in non-specific immune response.
Previously, a study reported beneficial effect of IM-133N (Immusante®) in various experimental rodent models for antisepsis and phagocytic, immunoglobulin enhancing potential in mice and rats, respectively, through immunotherapeutic mechanisms [13].
In this present study, Immusante® has shown to regulate immunity by altering the host cytokine gene expression. It was able to significantly decrease the elevated expression of pro-inflammatory cytokines genes such as IL-1β, IFN-γ, and TNF-α (a macrophage activator and the key cytokine of Th1 cell immune responses in infections) in the kidney of A. hydrophila infected zebrafish and there was no significant difference in IL-4 expression among different groups as it is an anti-inflammatory cytokine.
The significant suppression of pro-inflammatory cytokines such as TNF-α and IFN-γ by the treatment with IM-133N in RAW264.7 cell line were also reported [24].
The down regulation of expression of IFN-γ, IL-1β, and TNF-α with the treatment of Immusante® could prevent autoimmune disorder from uncontrolled induction of these cytokines and by activating phagocytosis. Unlike pro-inflammatory cytokines, the expression of anti-inflammatory cytokine IL-4 (may contribute to reduce inflammation) was not affected.
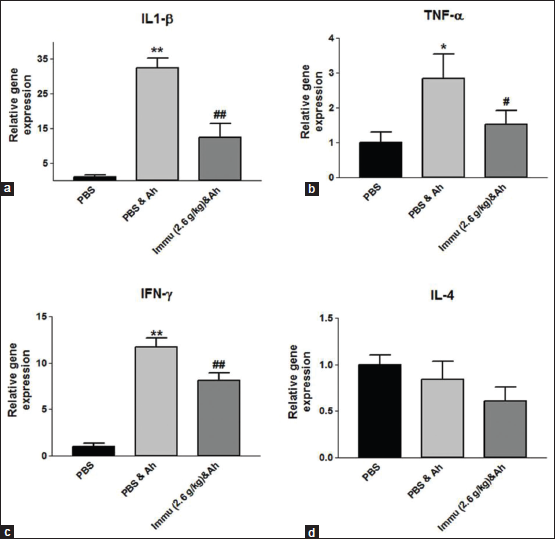 | Figure 5: (a-d) Gene expression profile of the cytokines. Values are expressed as mean ± SD. Data were determined by one-way ANOVA followed by Dunnett’s test. *P < 0.05, **P < 0.01 compared to control (PBS). #P < 0.05, ##P < 0.01 compared to path control (PBS and Ah). [Click here to view] |
5. CONCLUSION
Immusante® regulates the host immune response through ameliorating pro-inflammatory cytokine gene expression, thereby providing the protection to host from harmful effects of an excessive inflammatory response. From the above results obtained from zebrafish studies, it is proved that Immusante® is an effective immunomodulator and it validates our previous findings both in clinical and preclinical studies involving rodents.
6. ACKNOWLEDGMENT
Authors are thankful to Himalaya Drug Company, Makali, Bengaluru and JSS College of Pharmacy, JSS Academy of Higher Education and Research, Mysuru, Karnataka, India, for supporting and carrying out this research work.
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Mitra MP, Pattnayak S, Parvani H, Sasmal D, Rathinavelusamy P. Evaluation of immunomodulatory activity of Glycyrhiza glabra L roots in combination with zing. Asian Pac J Trop Biomed 2012;2:15-20. CrossRef
2. Mukherjee PK, Nema NK, Bhadra S, Mukherjee D, Braga FC, Matsabisa MG. Immunomodulatory leads from medicinal plants. Indian J Tradit Knowl 2014;13:235-56. CrossRef
3. Aranha I, Clement F, Venkatesh YP. Immunostimulatory properties of the major protein from the stem of the Ayurvedic medicinal herb, guduchi (Tinospora cordifolia). J Ethnopharmacol 2012;139:366-72. CrossRef
4. Zhang S, Li Z, Wang X, An L, Bao J, Zhang J, et al. Isolation, structural elucidation, and immunoregulation properties of an arabinofuranan from the rinds of Garcinia mangostana. Carbohydr Polym 2020;246:116567. CrossRef
5. Liu WS, Kuan YD, Chiu KH, Wang WK, Chang FH, Liu CH, et al. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar Drugs 2013;11:1899-908. CrossRef
6. Praveen K, Evans DL, Jaso-Friedmann L. Constitutive expression of tumor necrosis factor-alpha in cytotoxic cells of teleosts and its role in regulation of cell-mediated cytotoxicity. Mol Immunol 2006;43:279-91. CrossRef
7. Abe T, Mikekado T, Haga S, Kisara Y, Watanabe K, Kurokawa T, et al. Identification, cDNA cloning, and mRNA localization of a zebrafish ortholog of leukemia inhibitory factor. Comp Biochem Physiol B Biochem Mol Biol 2007;147:38-44. CrossRef
8. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001;25:402-8. CrossRef
9. Lessman CA. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res Part C Embryo Today Rev 2011;93:268-80. CrossRef
10. Wang J, Xiang M. Targeting potassium channels Kv1.3 and KCa3.1: Routes to selective immunomodulators in autoimmune disorder treatment? Pharmacotherapy 2013;33:515-28. CrossRef
11. Zhang S, An L, Li Z, Wang H, Shi L, Zhang J, et al. An active heteropolysaccharide from the rinds of Garcinia mangostana Linn.: Structural characterization and immunomodulation activity evaluation. Carbohydr Polym 2020;235:115929. CrossRef
12. Li Z, Shi Y, Zhang X, Xu J, Wang H, Zhao L, et al. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo zebrafish assays. Front Pharmacol 2020;11:1-19. CrossRef
13. Firashathulla S, Inamdar MN, Rafiq M, Viswanatha GL, Kumar LM, Babu UV, et al. IM-133N a useful herbal combination for eradicating disease-triggering pathogens in mice via immunotherapeutic mechanisms. J Pharmacopuncture 2016;19:21-7. CrossRef
14. Ding Y, Liu X, Bu L, Li H, Zhang S. Antimicrobial-immunomodulatory activities of zebrafish phosvitin-derived peptide Pt5. Peptides 2012;37:309-13. CrossRef
15. BLISS CI. The calculation of the dosage mortality curve. Ann Appl Biol 1935;22:134-67. CrossRef
16. OECD. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals. Vol. 203. Section 2. OECD Guidel Test Chem; 2019.
17. Rougeot J, Torraca V, Zakrzewska A, Kanwal Z, Jansen HJ, Sommer F, et al. RNAseq profiling of leukocyte populations in zebrafish larvae reveals a cxcl11 chemokine gene as a marker of macrophage polarization during mycobacterial infection. Front Immunol 2019;10:1-17. CrossRef
18. Kirsten K, Pompermaier A, Koakoski G, Mendonça-Soares S, da Costa RA, Maffi VC, et al. Acute and chronic stress differently alter the expression of cytokine and neuronal markers genes in zebrafish brain. Stress 2020;2020:1-6. CrossRef
19. Yang LE, Wang J, Wang D, Hu G, Liu ZY, Yan D, et al. Delayed behavioral and genomic responses to acute combined stress in zebrafish, potentially relevant to PTSD and other stress-related disorders: Focus on neuroglia, neuroinflammation, apoptosis and epigenetic modulation. Behav Brain Res 2020;389:112644. CrossRef
20. Terriente J, Pujades C. Use of zebrafish embryos for small molecule screening related to cancer. Dev Dyn 2013;242:97-107. CrossRef
21. Cao Y, He S, Zhou Z, Zhang M, Mao W, Zhang H, et al. Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp. strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol 2012;78:1899-908. CrossRef
22. Rodríguez I, Novoa B, Figueras A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol 2008;25:239-49. CrossRef
23. da Silva BC, Mouriño JL, Vieira FN, Jatobá A, Seiffert WQ, Martins ML. Haemorrhagic septicaemia in the hybrid surubim (Pseudoplatystoma corruscans×Pseudoplatystoma fasciatum) caused by Aeromonas hydrophila. Aquac Res 2012;43:908-16. CrossRef
24. Varma RS, Guruprasad KP, Satyamoorthy K, Kumar LM, Babu UV, Patki SP. IM-133N modulates cytokine secretion by RAW264.7 and THP-1 cells. J Immunotoxicol 2016;13:217-25. CrossRef