The present study focused on the variations of morphological and cerebellar histological structures of five strains of adult male Columba livia domestica (n = 5) and their genetic polymorphism using RAPD-PCR technique to differentiate between them and find the best strains that can adapt to different circumstances. In each strain, the beak length and the eye diameter were measured in relation to the head length. Specimens of the cerebellum were fixed and processed for histological investigations. The thickness of the cerebellar cortex layers and the numbers of Purkinje cells was determined. For genetic polymorphism, 20 RAPD primers were used to determine the genetic diversity between the studied strains. Balady strain could express the best adaptation if subjected to feed restrictions; however, its low Purkinje cells number may reveal its low cognitive ability compared to the other studied strains. Primers OPA- 1, OPA-3, and OPA-6 are the best used primers to differentiate between the studied strains. The highest similarity coefficient was detected between Halaby and Gamey strains, while, Balady strain was completely separated and independent. In conclusion, in addition to the morphological and histological variations between studied strains, the RAPD markers are recommended as a valuable tool to verify the genetic polymorphism of Columba livia domestica strains.
Sabry DA, Fouda YA. Comparative morphological, histological, and RAPD analysis of Columba livia domestica strains. J App Biol Biotech. 2020;8(6):13-20. https://dx.doi.org/10.7324/JABB.2020.80603
Domestic pigeons are widely abundant old domesticated bird. There are more than 350 different pigeon strains worldwide which are bred as a hobby and for other purposes [1]. Furthermore, the domestic pigeons characterized by having a short cycle for reproduction and an economic importance [2]. Shephard et al. [3] mentioned that morphometric analysis is not enough to differentiate between strains due to alterations in the color of feathers and body size in different geographical regions. Martinez-Abrain et al. [4] and Liordos and Goutner [5] reported that both morphometric analysis and DNA fingerprinting should be taken into consideration to differentiate between pigeon populations. These could be useful for zoos, museum collections, or even at the level of enthusiasts [6]. Cerebellum is an important part of the brain. It acts as a coordinator of impulses comes to and from the cerebral cortex [7]. Hence, cerebellar abnormalities may lead to impairment of cognitive functions [8] motor coordination, smooth movement [9], and vision ability [10]. Purkinje cells are the most important cells of the cerebellum because of their functional capability. Also, they are the most emphasized cells in cognitive and behavioral studies [11,12]. Assessments of genetic diversity of farm animals allow breeders to identify, choose, and develop new breeds to face the changing conditions of climate, human needs of nutrition, and disease threats [13].
There is no distinct clarification between the cultured pigeons. Although the presence of some morphological variations between the pigeons’ strains and all of them is under the family Columbidae and genus Columba, RAPD markers are settled for characterization of different strains for breeding programs [14]. Hence, studying phenotypic and genetic characterization helps for the production of more efficient breeding programs and improves the production systems [15] by mating of different populations [16].
Random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) has been evidenced to be valuable in assessment the inter-population variations and phylogenetic relationships [17-22]. It is the most widely used molecular technique which produce a PCR products fingerprint to distinguish between species and characterized by its simple, rapid, and inexpensive processing. Furthermore, it does not require previous knowledge and the sequences of the target DNA [19,23-25].
There is a deficiency in information about the genetic diversity of the Columba livia domestica strains. It was proved that RAPD technique is very valuable in the genetic studies of other avian population [26-30]. Hence, the present study is carried out to outline the genetic diversity of different Columba livia domestica strains collected from some bird breeders in Egypt.
In the present study, five strains of adult male Columba livia domestica (each strain consists of five individuals) (family Columbidae, genus Columba) (AL-Louse, 1960 and Chiasson, 1984) were collected from a known Farm of Dakahlea Governorate. The collected strains were about 1 year old, they were transferred in cages to the lab and they were free from any pathological lesions or abnormal morphological appearance. These strains are Halaby (1), Gamey (2), Australy (3), White Tumbler (Shaklabaz) (4), and Baladi (5). They were euthanized by overdose ether (according to the local experimental animal ethics committee, code number RZ 19003) and sacrificed by decapitation. The heads were dissected and the brains were removed and exposed to the following investigations.
The head and the brain of each strain were examined and photographed [Figure 1]. In each strain, the percentages of both the eye diameter and the beak length (from the beak tip until its base) to the head length (from the beak base until the neck corner) were determined. Furthermore, the beak overhang was measured. The dissected whole brains were photographed and differences between strains were recorded. Specimens of the cerebellum of the selected strains were fixed in 10% phosphate-buffered formalin (pH 7.4), dehydrated in ascending concentrations of ethyl alcohol, cleared in xylene, and mounted in molten paraffin wax (58–62°C). Five mm histological sections were cut, stained with hematoxylin and eosin stain and examined under bright field light microscope. The thickness of the cerebellar cortex layers and the numbers of Purkinje cells were measured.
 | Figure 1: Photomicrograph of lateral view of the head region and dorsal view of the brain of the different Columba livia strains; Halaby (A&A1), Gamey (B&B1), Australy (C&C1), Shaklabaz (D&D1), and Balady (E&E1). Curved beak in Australy (C) and straight in the others. Narrowest and shortest beak in Gamey (B) compared to the other strains, the longest beak in Balady strain and the most prominence cerebellar folds in Shaklabaz. [Click here to view] |
Specimens of the brains of the studied strains were kept in the refrigerator at −20°C. The frozen brains were grinded and DNA extraction was carried out using QIAamp DNA Mini Kit (Cat. No. 51304, Qiagen, Germany). These were done by grinding about 25 mg of frozen tissue with liquid nitrogen in a mortar and pestle and transfer in micro-centrifuge tube containing 180 mL buffer ATL, 20mL proteinase K (600 mAU/ml solution), and incubate for 2 h at 56° C till complete lysis. After centrifugation, 200 mL ATL was added and incubated at 70°C for 10 min, followed by adding of 200 mL ethanol (96–100%). The mixture was applied to QIAamp mini spin, centrifuged at 6000 ×g (8000 rpm) for 2 min then the filtrate was discarded. 500 mL buffer AW2 was added to the column then centrifuged at 20000 ×g (14000 rpm) followed by adding 200 mL buffer AE and incubate at room temperature and centrifuge at 6000 ×g (8000 rpm) for 3 min.
To determine the genetic diversity between the different studied strains of Columba livia domestica, 20 RAPD primers were carried out for all the individuals (OPA1to OPA10 and OPB1 to OPB10). All primers were purchased from Metabion International AG, Germany. The primers list with their sequence is shown in Table 3. The isolated DNA from the studied strains is considered as a template of DNA. The PCR mixture consists of Emerald Amp GT PCR master mix (2X Premix) 25 ml, template DNA (<500 ng), forward primer (0.2 mM), reverse primer (0.2 mM), and 50 mL distilled water. The PCR amplification conditions were 94°C for 5 min (initial denaturation), then 40 cycles of denaturation at 94°C for 30 s, followed by 30 s annealing at 35°C (for OPA3-OPA5,OPA7,OPA8,OPA10,OPB1,OPB2,OPB4) or at 37°C (for OPA1,OPA2,OPA6,OPA9,OPB3,OPB5-OPB10) after that extension was made at 72°C for 30 s. Finally, final extension at 72°C for 7 min.
Table 1: Morphometric analysis of the beak and eye region of the studied strains.
| Strains | The percentage of eye diameter to head length (%) | The percentage of beak length to head length (%) | Overhang length (mm) |
|---|---|---|---|
| (1) Halaby | 21.44±2.5a | 32.37±0.83a | 2±0.2a |
| (2) Gamey | 15±0.83ab | 22.92±0.84ab | 2±0.2b |
| (3) Australy | 20.98±1.49bc | 27.27±1.14ac | 4±0.3abc |
| (4) White Tumbler (Shaklabaz) | 17.36±1.13ac | 27.17±1.13ad | 3±0.5abc |
| (5) Balady | 17.39±0.87ac | 33.48±1.31bcd | 2±0.2c |
Data are presented as means ±SD. The mean difference between strains is significant at p<0.05. The same superscript letters mean significant difference
Table 2: Morphometric analysis of the cerebellar cortex of the pigeon strains.
| Strains | PC Number | Thickness of ML (µm) | Thickness of IGL (µm) | Thickness of cerebellar cortex (µm) |
|---|---|---|---|---|
| (1) Halaby | 8.75±0.5a | 147.50±20.62 | 140.00±67.3 | 296.25±52.1 |
| (2) Gamey | 10.00±1.4b | 135.00± 19.15 | 105.00±46.6 | 250±45.8a |
| (3) Australy | 8.75±1c | 135.00± 36.97 | 107.50±59.1 | 251.25±57.7b |
| (4) White Tumbler (Shaklabaz) | 9.50±1d | 167.50±20.6 | 145.00±71.4 | 322±65.2 |
| (5) Balady | 6.75±1abcd | 162.50±37.7 | 167.00±2 | 334.25±45.3ab |
The mean difference is significant between strains at P <0.05. The same superscript letters mean significant. PC: Purkinje cell, ML: Molecular layer, IGL: Internal granular layer. Data are presented as means ±SD
Table 3: Numerical data based on the RAPD-PCR technique among the studies strains.
| primer | Sequence | TB | MB | PB | UB | %PB |
|---|---|---|---|---|---|---|
| OPA-1 | CAGGCCCTTC | 6 | 0 | 6 | 2 | 100 |
| OPA-2 | TGCCGAGCTG | 5 | 3 | 2 | 2 | 40 |
| OPA-3 | AGTCAGCCAC | 6 | 0 | 6 | 2 | 100 |
| OPA-4 | AATCGGGCTG | 7 | 4 | 3 | 0 | 43 |
| OPA-5 | AGGGGTCTTG | 6 | 0 | 6 | 0 | 100 |
| OPA-6 | GGTCCCTGAC | 5 | 0 | 5 | 3 | 100 |
| OPA-7 | GAAACGGGTG | 3 | 0 | 3 | 0 | 100 |
| OPA-8 | GTGACGTAGG | 3 | 1 | 2 | 1 | 67 |
| OPA-9 | GGGTAACGCC | 4 | 0 | 4 | 0 | 100 |
| OPA-10 | GTGATCGCAG | 6 | 2 | 4 | 0 | 67 |
| OPB-1 | GTTTCGCTCC | 7 | 1 | 6 | 2 | 86 |
| OPB-2 | TGATCCCTGG | 2 | 0 | 2 | 2 | 100 |
| OPB-3 | CATCCCCCTG | 6 | 1 | 5 | 0 | 83 |
| OPB-4 | GGACTGGAGT | 3 | 3 | 0 | 0 | 0 |
| OPB-5 | TGCGCCCTTC | 5 | 3 | 2 | 0 | 40 |
| OPB-6 | TGCTCTGCCC | 8 | 2 | 6 | 0 | 75 |
| OPB-7 | GGTGACGCAG | 5 | 4 | 1 | 0 | 20 |
| OPB-8 | GTCCACACGG | 3 | 1 | 2 | 0 | 67 |
| OPB-9 | TGGGGGACTC | 1 | 0 | 1 | 1 | 100 |
| OPB-10 | CTGCTGGGAC | 4 | 4 | 0 | 0 | 0 |
| Total | 95 | 30 | 65 | 15 | 69 |
TB: Total bands number, MB: Monomorphic bands, PB: Polymorphic bands, UB: Unique bands, %PB: Percent of polymorphic bands
The data were presented as means ± standard deviation using ANOVA test and post hoc analysis using SPSS and expressed in Tables 1 and 2. The base pair values were used to perform agglomerative hierarchical clustering (ACH) of the different studied strains using similarity Pearson correlation coefficient using XLSTAT software (2015). Bands are considered as “polymorphic bands” if they are present in some strains and absent in another. If the polymorphic band is present in only one strain and absent in the others, it will be called a unique band. If the band is present in all strains, it will be considered as “monomorphic” band.
From Table 1, the studied strains showed significant differences in both eye diameter and beak lengths in-relation to head length. Halaby exhibited a significantly (P < 0.05) widest relative eye diameter compared to the other strains while Gamey showed the narrowest one [Figure 1]. Concerning the relative beak length, Gamey strain had the shortest beak while Balady had a significantly (P < 0.05) longest one compared to the other strains. The studied strains showed a similar horny operculum covering the base of the beak. However, both Australy and Shaklabaz showed significantly (P < 0.05) the longest maxillary overhangs compared to the other studied strains. Feathers like bristles were detected at the base of the beak of Shaklabaz strain [Figure 1].
The cerebellar cortex of the examined Columba livia strains composed of a molecular layer situated externally followed by internal granular layer and between the two layers there is a Purkinje cell layer of one cell thick [Figure 2]. The cerebellar cortex of Balady strain appeared significantly (P < 0.05) thicker than the cortex in Gamey and Australy, while, Purkinje cells in Balady strain had significantly (P < 0.05) the fewest number compared to the other four strains [Table 2 and Figure 2].
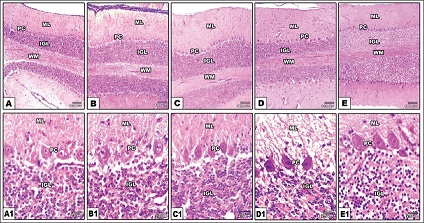 | Figure 2: Photomicrograph of sagittal histological sections of cerebellar cortex of different Columba livia strains; Halaby (A&A1), Gamey (B&B1), Australy (C&C1), Shaklabaz (D&D1), and Balady (E&E1). Note: Comparative differences in the thickness of the molecular and internal granular layers between the studied strains. The least Purkinje cell number in Balady strain. Abbreviations: PC, Purkinje cell; ML, Molecular layer; IGL, Internal granular layer; WM, White matter. [Click here to view] |
The used 20 primers produced 95 RAPD bands (The total number of produced bands) with an average 4.75 per primer. The produced bands ranged from 235 and 1732 bp (base pair). Primer OPB-6 produced the highest number of bands (8). The prepared specimen of Halaby produces the highest total bands number with the used primers (75 DNA bands), followed by Shaklabaz (73 DNA bands), Gamey (71 DNA bands), and then Balady (67), while Australy had the least number (47 DNA bands) [Figures 3-6]. Table 3 shows that primers altogether produced 30 monomorphic bands for the studied five strains, while, the number of polymorphic bands was 65. The polymorphic bands included (15) polymorphic unique bands and (50) polymorphic without unique bands.
.png) | Figure 3: (a and b) Agarose gel electrophoresis of RAPD banding of Columba Livia domestica strains using primers OPA-1 to OPA-6. Lanes from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). L represent ladder at100–3000 bp. OPA-1 produces a specific unique marker for both Halaby and Balady. Primers OPA-2 produce two specific DNA bands for Balady. Balady strain exhibited no DNA bands with primer OPA-5. Primer OPA-6 produces three unique bands; one for Shaklabaz and two for Balady. [Click here to view] |
.png) | Figure 4: (a and b) Agarose gel electrophoresis of RAPD banding of Columba Livia domestica strains using primers OPA-7 to OPA-10. Lanes from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). L represents ladder at100–3000 bp. Primer OPA-7 expressed no DNA bands with Australy and Shaklabaz while the other strains exhibited three bands at the same base pair. Primer OPA-8 exhibited a unique band only with Balady strain. Primer OPA-10 expressed three polymorphic bands and three monomorphic bands. [Click here to view] |
.png) | Figure 5: (a and b) Agarose gel electrophoresis of RAPD banding of Columba Livia domestica strains using primers OPB-1to OPB-6. Lanes from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). L represents ladder at100–3000 bp. Primer OPB-1 produces 2 unique DNA bands for Halaby. Also, primer OPB-2 produces two unique DNA bands for Shaklabaz. [Click here to view] |
.png) | Figure 6: (a and b) Agarose gel electrophoresis of RAPD banding of Columba Livia domestica strains using primers OPB-7to OPB-10. Lanes from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). L represents ladder at100–3000 bp. Primer OPB-9 expressed one polymorphic band at 500 bp and a unique band for Shaklabaz strain. Primer OPB-10 exhibited four monomorphic bands ranged between 400 bp and 1200 bp. [Click here to view] |
Concerning primer OPA-1, the studied strains exhibited wide expression of DNA bands of varying densities ranged from 260 bp to 96o bp (Halaby - 5 bands, Gamey - 3 bands, Australy - 0 bands, Shaklabaz - 2 bands, and Balady - 3 bands). The studied strains exhibited three monomorphic DNA bands with primer OPA-2 while Balady strain exhibited additional two unique bands. With regard to OPA-3, Balady, Gamey, and Shaklabaz strains expressed four bands at similar base pair, while, Australy expressed only one band and Balady exhibited five bands [Figure 3a]. Primer OPA-4 showed four monomorphic bands and three polymorphic bands with the studied strains. Balady strain exhibited no DNA bands with primer OPA-5 while, there was a varying in the expression of DNA bands density and number between the studied strains (Halaby - 4 bands, Gamey - 6 band, and both Australy and Shaklabaz - 5 bands). In primer OPA-6, all of Halaby, Gamey, and Australy expressed no DNA bands [Figure 3b]. Primer OPA-7 showed no DNA bands with Australy and Shaklabaz while the other strains exhibited three bands at the same base pair.
Primer OPA-8 expressed one monomorphic band at 1000 bp, one polymorphic band and a single unique band for Balady strain [Figure 4a]. Primer OPA-9 expressed 4 DNA bands for Gamey, three bands for both Halaby and Balady and only one band for both Australy and Shaklabaz. The studied strains exhibited varying densities and band numbers with primer OPA-10, which expressed six bands three polymorphic bands and three monomorphic bands [Figure 4b].
Concerning primer OPB-1, Halaby strain exhibited a considerable number of DNA bands (7 bands), Gamey expressed three bands, both Australy and Shaklabaz expressed four bands, and the least number of bands expressed by Balady strain (two bands). All studied strains exhibited no DNA bands with primer OPB-2 except Shaklabaz which showed two specific bands. Australy produced only one DNA band using primer OPB-3 while both Halaby and Balady produced five bands and both Gamey and Shaklabaz produced six DNA bands [Figure 5a]. Primer OPB-4 exhibited three monomorphic bands with all studied strains. Primer OPB-5 expressed 5 DNA bands classified into three monomorphic bands and two polymorphic bands. Primer OPB-6 expressed a varying number of DNA bands among the studied strains. Halaby exhibited the highest number of bands (Halaby - eight bands, Gamey and Shaklabaz - seven bands, Balady - five bands, and finally Australy- three bands) [Figure 5b]. Primer OPB-7 exhibited four monomorphic bands and one polymorphic band.
Primer OPB-8 exhibited three bands; one monomorphic band and two polymorphic bands at 470 and 1040 bp [Figure 6a]. Primer OPB-9 showed one polymorphic band at 500 bp and one band specific for Shaklabaz at 1730 bp. Primer OPB-10 exhibited four monomorphic bands ranged between 400 bp and 1200 bp [Figure 6b].
Focusing on the unique bands; Primer OPA-1 produces a specific unique band for both Halaby and Balady at base pair 650 and 960, respectively. Each of primers OPA-2 and OPA-3 showed two unique bands for Balady strain. These bands produced at base pair 710 and 1130 for the first primer and at base pair 470 and 763 for the second one [Figure 3a]. OPA-6 produced three unique bands; one band for Shaklabaz at 1370 bp and two bands for Balady at 750, 800 bp [Figure 3b]. Primer OPA-8 produced only a unique band for Balady at 470 bp [Figure 4a]. Primer OPB-1 produced two specific DNA bands for Halaby at 260, 370 bp. Furthermore, primer OPB-2 produced two specific DNA bands for Shaklabaz at 560,730 bp [Figure 5a]. Primer OPB-9 produced a unique band for only Shaklabaz strain at 1730 bp [Figure 6b]. The other 12 used primers generated no specific band markers.
Figure 7 and Table 4 illustrated the genetic relationship between the strains. The similarity matrix between the studied samples ranged from 0.22 to 0.67. The highest similarity coefficient was detected between Halaby and Gamey strains and reached (0.67) while the lowest one was between Australy and Balady (0.22). Balady strain was completely separated and independent from the other strains.
.png) | Figure 7: Dendrogram of five strains of Columba livia domestica depending on their RAPD-PCR data generated by Pearson correlation method showing the genetic Proximity. Bar at the right side refers to similarity coefficient. Numbers from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). Balady was independently separated from the other strains. The highest similarity coefficient was detected between Halaby and Gamey strains. [Click here to view] |
Table 4: The genetic relationship between the studied strains.
| Samples | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.00 | ||||
| 2 | 0.67 | 1.00 | |||
| 3 | 0.49 | 0.41 | 1.00 | ||
| 4 | 0.33 | 0.54 | 0.36 | 1.00 | |
| 5 | 0.38 | 0.40 | 0.22 | 0.23 | 1.00 |
Numbers from 1 to 5 refer to the different strains; Halaby (1), Gamey (2), Australy (3), Shaklabaz (4), and Balady (5). The highest similarity coefficient was detected between Halaby and Gamey strains and reached (0.67) while the lowest one was between Australy and Balady (0.22)
The present work shows that the studied Columba livia domestica strains are significantly varied from each other depending on morphological criteria such as eye diameter and beak lengths in-relation to head length. The variations of beak morphology may be related to genetic history, phylogeny development on skull components [31], feeding ecology, and adaptive capability [32]. Savas et al. [33] reported that pigeons of short beaks spent longer time during food consumption and preening behavior (as the releasing of parasites and dirt) if compared to birds of long beaks. They added that, during feeding restrictions, pigeons of short beaks show aggressive pecking and loss of weight. The studied Balady strain expressed a significant increase in the relative beak length compared to the other studied strains while Gamey showed the shortest one. Hence, Gamey might face feed intake problems if subjected to feed restrictions more than the other studied strains, while Balady strain could express the best adaptation.
The Australian pigeons exhibited the longest overhang which seemed to be helping it for controlling against their body lice, as ectoparasites, more than the other studied strains with shorter overhang, this explanation agrees with Clayton et al. [34] who illustrated that, birds trimmed by removing their overhangs had significant loss of their feather mass.
Brooke et al. [35] reported that speed is not the only significant factor of eye size. Behavior and ecology are also determinant. He added that owl has a large eye to provide wide pupil that enhance vision at night, while, raptors have big eyes that provide an acute vision to detect preys. Thomas et al. [6] explained that birds adapt their eyes depending on their habitat, size of preferred food, and the cycle of diurnal activity. In the present work, Halapy strain showed a significantly widest relative eye diameter if compared to the other studied strains which could refer to its higher speed or acute vision.
The cerebellum manages the function of locomotor system as well as control the relation between the motor and sensory pathways [11,36]. The thickness of the molecular and internal granular layers in the cerebellar folia of the studied strains ranged from 135 µ–167.5 µ to 105 µ–167 µ, respectively. Pal et al. [37] recorded that the thickness of the molecular and granular layers in the cerebellum of human was 227 m–343.5 m and 389.5 m–215.2 m, respectively, while hen values were 196 m–294 m and 330 m–187 m for molecular and granular layer thickness, respectively. Sur et al. [38] reported that pigeon had the highest thickness of folium summit molecular and granular layers if compared with turkey, duck, and starling. In the present study, the highest thickness of the cerebellar cortex was for Balady strain. Iwaniuk et al. [12] recorded that altricial strains would have higher degrees of foliation and thus cortex width than precocial strains, and those longer periods of embryonic and postembryonic development will be positively correlated with the cortex width. The indicated higher thickness of cerebellar cortex of Balady strain compared to Gamey and Australy strains could refer to the longer time required by hatched Balady for parent’s care.
The present study showed that Balady strain exhibited the least number of Purkinje cells in comparison with the other strains. Iwaniuk et al. [12] explained that cognitive ability, managing functional activity, and behavioral complexity increase as a result of increasing number of Purkinje cell. This may reveal that Balady strain had the least cognitive ability if compared to the other studied strains. Sur et al. [38] attributed the variations of cerebellar structure of bird strains to ecological, behavioral, and flying skills variations. Hence, the present data could be useful to comparative and behavioral biologists.
RAPD-PCR analysis revealed that primers OPA-1, OPA-3 (expressed six polymorphic bands including two unique bands with the studied strains), and OPA-6 (expressed five polymorphic bands including three unique bands with the studied strains) are the best used primers to differentiate between the studied strains. Patel et al. [39] explained that the appeared polymorphism is predominating due to the genetic variations which represent a number of alleles at a specific locus in a population. The studied Halaby and Gamey strains were clustered together with the highest similarity coefficient, which may be attributed to their close genetic relationship, while the most far genetic relationship was for Australy and Balady. The genetic diversity may be due to the climatic and geographical distribution [40] or even due to mutations, migration, and strains adaptation [41]. El-Mergawy et al. [42], Hameed et al. [43], Singha et al. [44], Chowdhury et al. [14], and El-Bakatoshi and Ahmed [45] recommended RAPD-PCR for the assessment of genetic diversity in genus Rhynchophorus ferrugineus, Bemisia tabaci, Fusarium sp., and Ocimum and Peganum harmala L., respectively.
The present study revealed that although Balady strain could express the best adaptation if subjected to feed restrictions; however, its low number of Purkinje cells may reveal that it had the least cognitive ability if compared to the other studied strains. Australian pigeon characterized by having the highest control against their ectoparasites compared to the other studied strains. This was assessed by its long overhang. Hatched Balady strain needs longer time for parent’s care compared to Gamey and Australy as a result of its higher cerebellar cortex thickness. Furthermore, primers OPA-1, OPA-3, and OPA-6 are the best used primers to differentiate between the studied strains. Also, the highest similarity coefficient was detected between Halaby and Gamey strains, while, Balady strain was independent. Hence, the findings of the present study indicate that, in addition to the variations of morphological and histological structures, the RAPD markers are recommended as a valuable tool to verify the genetic polymorphism of Columba livia domestica strains.
The authors would like to thank molecular biology lab and electron microscope unit in Mansoura University for the cooperation to support research.
Authors declared that they do not have any conflicts of interest.
1. Shapiro MD, Kronenberg Z, Li C, Domyan ET, Pan H, Campbell M, et al. Genomic diversity and evolution of the head crest in the rock-pigeon. Science 2013;339:1063-7. [CrossRef]
2. Omar AS, Abd El-Rahim SA, Abdel-Aziz YA, Sammour HB, Aggour MG. A field study on pigeon production systems in the rural sector of El-Sharkia governorate. Egypt Poult Sci J 2014;34: 1030-53.
3. Shephard JM, Catterall CP, Hughes JM. Discrimination of sex in the white bellied sea-eagle, Haliaeetus leucogaster, using genetic and morphometric techniques. Emu 2004;104:83-7. [CrossRef]
4. Martinez-Abrain A, Oro D, Velando A, Genovart M, Gerique C, Bartolome MA, et al. Morphometric similarities between central and peripheral populations of the European shag Phalacrocorax aristotelis. Mar Ornithol 2006;34:21-4.
5. Liordos V, Goutner V. Sex determination of great cormorants (Phalacrocorax carbosinensis) using morphometric measurements. Water Birds 2008;31:203-10. [CrossRef]
6. Thomas RJ, Széskely T, Cuthill IC, Harper DG, Newson SE, Frayling TD, et al. Eye size in birds, and the timing of song at dawn and dusk. Proc R Soc 2002;269:831-7. [CrossRef]
7. Kalanjati VP, Dewi AK, Santoso MW. Quantitative study on human cerebellar cortex from anatomy cadaver preparations. Int J Morphol 2017;35:167-71. [CrossRef]
8. Glickstein M, Strata P, Voogd J. Cerebellum: History. Neuroscience 2009;162:549-59. [CrossRef]
9. Bastian AJ. Learning to predict the future: The cerebellum adapts Feed forward movement control. Curr Opin Neurobiol 2006;16:645-9. [CrossRef]
10. Crossman AR, Neary D. Neuroanatomy. An Illustrated Colour Text. 5th ed. Edinburgh: Churchill Livingstone/Elsevier; 2015. p. 111-8.
11. Mondal RK. Comparative Gross Anatomical and Histomorphological Studies on Cerebellum of Fish, Amphibia, Reptilia and Mammalian. Koklata, West Bengal, India: PhD Thesis West Bengal, University of Animal and Fisheries Science; 1997.
12. Iwaniuk AN, Hard PL, Wylie DR. The comparative morphology of the cerebellum in caprimulgiform birds: Evolutionary and functional implications. Brain Behav Evol 2006;67:53-68. [CrossRef]
13. Boettcher PJ, Hoffmann I, Baumung R, Drucker AG, McManus C, Berg P, et al. Genetic resources and genomics for adaptation of livestock to climate change. Front Genet 2014;5:461. [CrossRef]
14. Chowdhury T, Mandal A, Roy S, Sarker DD. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC-MS) analysis. J Genet Eng Biotech 2017;15:275-86. [CrossRef]
15. Kristensen TN, Hoffmann AA, Pertoldi C, Stronen AV. What can livestock breeders learn from conservation genetics and vice versa? Front Genet 2015;6:38. [CrossRef]
16. Laxuman L, Patil S, Salimath P, Dharmatti P. Study on genetic diversity and its relation to heterosis in bitter gourd (Momordica charantia L.). Karnataka J Agric Sci 2012;25:14-7.
17. Gasser RB. Molecular tools-advances, opportunities and prospects. Vet Parasitol 2005;136:69-89. [CrossRef]
18. Mas-Coma S, Bargues MD, Valero MA. Fasciolosis and other plant borne trematode zoonoses. Int J Parasitol 2005;35:1255-78. [CrossRef]
19. Nuchprayoon S, Jupnee A, Poovorawan Y. Random amplified polymorphic DNA (RAPD) for differentiation between Thai and Myanmar strains of Wuchereria bancrafti. Filaria J 2007;6:4-8. [CrossRef]
20. Rokni MB, Mirhendi H, Behnia M, Haranol MF, Jalalizand N. Molecular characterization of Fasciola hepatica isolates by RAPD PCR and ribosomal ITS1 sequencing. Iran Red Crescent Med J 2010;12:27-32.
21. Khoshbakht R, Seifi S, Tabatabaei M, Aski HS, Ranjbar V, Abdi Hacheso B. Mycoplasma gallisepticum strains with identical random amplified polymorphic DNA (RAPD) patterns in chukar partridges (Alectoris chukar) and broilers: A case report. Vet Med 2013;58: [CrossRef]
22. Dilipan E, Papenbrock J, Thangaradjou T. Random amplified polymorphic DNA (RAPD) finger prints evidencing high genetic variability among marine angiosperms of India. J Mar Biol Assoc UK 2017;97:1307-15. [CrossRef]
23. Mohammadzadeh T, Sadjjadi SM, Motazedian MH, Mowdavi GR. Study on the genomic diversity of Hymenolepis nana between rat and mouse isolates by RAPD PCR. Iran J Vet Res 2007;8:16-22.
24. Sripalwit P, Wongsawad C, Wongsawad P, Anuntalabhochai S. High annealing temperature random amplified polymorphic DNA (HAT-RAPD) analysis of three paromphistome flukes from Thailand. Exp Parasitol 2007;115:98-102. [CrossRef]
25. Ganie SH, Upadhyay P, Das S, Sharma MP. Authentication of medicinal plants by DNA markers. Plant Gene 2015;4:83-99. [CrossRef]
26. Khaliq I, Babar M, Riaz M, Khan AA. Genetic diversity in see-see partridge (Ammoperdix griseogularis, Galliformes) populations from sub-Himalayan Mountain ranges of Pakistan. Belg J Zool 2010;140:227-32.
27. Muhammad S, Khan AA, Babar M, Riaz M, Akhtar N, Khaliq I. Population genetic structure of Rufous-Vented Prinia (Prinia burnesii) in Pakistan. Afr J Biotechnol 2010;9:9077-81.
28. Akhter N, Khan AA, Babar, M, Riaz M, Imtiaz A, Khaliq I. Evaluation of the genetic structure of the urban dwelling species of Bank Myna (Acridotheres ginginisnus) using random amplified polymorphic DNA (RAPD) analysis. Afr J Biotechnol 2011;10:6342-7.
29. Imtiaz A, Khan AA, Babar M, Riaz M, Akhtar N, Arshad M, et al. Genetic diversity of Pakistani common myna (Acridotheres tristis) revealed by RAPD-PCR. Afr J Biotechnol 2011;10:7751-5. [CrossRef]
30. Riaz M, Khan AA, Babar M, Akhtar N, Muhammad S, Khaliq I. High genetic diversity revealed by RAPD markers in the black francolin (Francolinus francolinus, Galliformes) of Pakistan. Pak J Zool 2011;43:889-96.
31. Bright JA, Marugán-Lobón J, Cobb S, Rayfield EJ. The shapes of bird beaks are highly controlled by nondietary factors. Proc Natl Acad Sci USA 2016;113:5352-7. [CrossRef]
32. Olsen AM. Feeding ecology is the primary driver of beak shape diversiï¬cation in waterfowl. Funct Ecol 2017;3:1985-95. [CrossRef]
33. Savas T, Konyali C, Das G, Yurtman IY. Effect of beak length on feed intake in pigeons (Columba livia f. domestica). Anim Welfare 2007;16:79-86.
34. Clayton D, Moyer BR, Bush SE, Jones TG, Gardiner DW, Rhodes BB, Goller F. Adaptive significance of avian beak morphology for ectoparasite control. Proc R Soc Lond B 2005;272:811-7. [CrossRef]
35. Brooke ML, Hanley S, Laughlin SB. The scaling of eye size with body mass in birds. Proc R Soc Lond B 1999;266:405-12. [CrossRef]
36. Masabanda JS, Burt DW, O'brien PC, Vignal A, Filon V, Walsh PS. Molecular cytogenetic definition of the chicken genome, the first complete avain karyotype. Genetics 2004;166:1367-73. [CrossRef]
37. Pal B, Chowdhury S, Ghosh RK. Comparative anatomical study of the cerebellum of man and fowl. J Anat Soc Ind 2003;52:32-7.
38. Sur E, Öznurlu Y, Özaydin T, Çolakoglu F, Ünsal S, Yener Y. Comparative histological study of the cerebellum and the determination of some Agnor parameters in different avian species. Bull Vet Inst Pulawy 2011;55:261-5.
39. Patel P, Rajkumar BK, Parmar P, Shah R, Krishnamurthy R. Assessment of genetic diversity in Colletotrichum falcatum Went accessions based on RAPD and ISSR markers. J Genet Eng Biotechnol 2017;16:153-9. [CrossRef]
40. Mei Z, Zhang X, Khan MA, Imani S, Liu X, Zou H, et al. Genetic analysis of Penthorum Chinense Pursh by improved RAPD and ISSR in China. J Genet Eng Biotechnol 2017;30:6-11. [CrossRef]
41. Zarringhabai GE, Javanmard A, Pirahary O. Random amplified polymorphic markers as indicator for genetic conservation program in Iranian pheasant (Phasianus colchicus). Sci World J 2012;2012:640381. [CrossRef]
42. El-Mergawy RA, Al-Ajlan AM, Abdallah NA, Nasr MI, Silvain JF. Determination of different geographical populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using RAPD-PCR. Int J Agric Biol 2011;13:227-32.
43. Hameed S, Hameed S, Sadia M, Malik SA. Genetic diversity analysis of Bemisia tabaci populations in Pakistan using RAPD markers. Electron J Biotechnol 2012;15:1-9. [CrossRef]
44. Singha IM, Kakoty Y, Unni BG, Das J, Kalita MC. Identification and characterization of Fusarium sp. using ITS and RAPD causing Fusarium wilt of tomato isolated from Assam, North East India. J Genet Eng Biotechnol 2016;14:99-105. [CrossRef]
45. El-Bakatoushi R, Ahmed DG. Evaluation of genetic diversity in wild populations of Peganum harmala L., a medicinal plant. J Genet Eng Biotechnol 2017;16:143-51. [CrossRef]
1. Shapiro MD, Kronenberg Z, Li C, Domyan ET, Pan H, Campbell M, et al. Genomic diversity and evolution of the head crest in the rock-pigeon. Science 2013;339:1063-7. https://doi.org/10.1126/science.1230422 | |
2. Omar AS, Abd El-Rahim SA, Abdel-Aziz YA, Sammour HB, Aggour MG. A field study on pigeon production systems in the rural sector of El-Sharkia governorate. Egypt Poult Sci J 2014;34: 1030-53. | |
3. Shephard JM, Catterall CP, Hughes JM. Discrimination of sex in the white bellied sea-eagle, Haliaeetus leucogaster, using genetic and morphometric techniques. Emu 2004;104:83-7. https://doi.org/10.1071/MU03043 | |
4. Martinez-Abrain A, Oro D, Velando A, Genovart M, Gerique C, Bartolome MA, et al. Morphometric similarities between central and peripheral populations of the European shag Phalacrocorax aristotelis. Mar Ornithol 2006;34:21-4. | |
5. Liordos V, Goutner V. Sex determination of great cormorants (Phalacrocorax carbosinensis) using morphometric measurements. Water Birds 2008;31:203-10. https://doi.org/10.1675/1524-4695(2008)31[203:SDOGCP]2.0.CO;2 | |
6. Thomas RJ, Széskely T, Cuthill IC, Harper DG, Newson SE, Frayling TD, et al. Eye size in birds, and the timing of song at dawn and dusk. Proc R Soc 2002;269:831-7. https://doi.org/10.1098/rspb.2001.1941 | |
7. Kalanjati VP, Dewi AK, Santoso MW. Quantitative study on human cerebellar cortex from anatomy cadaver preparations. Int J Morphol 2017;35:167-71. https://doi.org/10.4067/S0717-95022017000100027 | |
8. Glickstein M, Strata P, Voogd J. Cerebellum: History. Neuroscience 2009;162:549-59. https://doi.org/10.1016/j.neuroscience.2009.02.054 | |
9. Bastian AJ. Learning to predict the future: The cerebellum adapts Feed forward movement control. Curr Opin Neurobiol 2006;16:645-9. https://doi.org/10.1016/j.conb.2006.08.016 | |
10. Crossman AR, Neary D. Neuroanatomy. An Illustrated Colour Text. 5th ed. Edinburgh: Churchill Livingstone/Elsevier; 2015. p. 111-8. | |
11. Mondal RK. Comparative Gross Anatomical and Histomorphological Studies on Cerebellum of Fish, Amphibia, Reptilia and Mammalian. Koklata, West Bengal, India: PhD Thesis West Bengal, University of Animal and Fisheries Science; 1997. | |
12. Iwaniuk AN, Hard PL, Wylie DR. The comparative morphology of the cerebellum in caprimulgiform birds: Evolutionary and functional implications. Brain Behav Evol 2006;67:53-68. https://doi.org/10.1159/000089120 | |
13. Boettcher PJ, Hoffmann I, Baumung R, Drucker AG, McManus C, Berg P, et al. Genetic resources and genomics for adaptation of livestock to climate change. Front Genet 2014;5:461. https://doi.org/10.3389/fgene.2014.00461 | |
14. Chowdhury T, Mandal A, Roy S, Sarker DD. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC-MS) analysis. J Genet Eng Biotech 2017;15:275-86. https://doi.org/10.1016/j.jgeb.2016.12.004 | |
15. Kristensen TN, Hoffmann AA, Pertoldi C, Stronen AV. What can livestock breeders learn from conservation genetics and vice versa? Front Genet 2015;6:38. https://doi.org/10.3389/fgene.2015.00038 | |
16. Laxuman L, Patil S, Salimath P, Dharmatti P. Study on genetic diversity and its relation to heterosis in bitter gourd (Momordica charantia L.). Karnataka J Agric Sci 2012;25:14-7. | |
17. Gasser RB. Molecular tools-advances, opportunities and prospects. Vet Parasitol 2005;136:69-89. https://doi.org/10.1016/j.vetpar.2005.12.002 | |
18. Mas-Coma S, Bargues MD, Valero MA. Fasciolosis and other plant borne trematode zoonoses. Int J Parasitol 2005;35:1255-78. https://doi.org/10.1016/j.ijpara.2005.07.010 | |
19. Nuchprayoon S, Jupnee A, Poovorawan Y. Random amplified polymorphic DNA (RAPD) for differentiation between Thai and Myanmar strains of Wuchereria bancrafti. Filaria J 2007;6:4-8. https://doi.org/10.1186/1475-2883-6-6 | |
20. Rokni MB, Mirhendi H, Behnia M, Haranol MF, Jalalizand N. Molecular characterization of Fasciola hepatica isolates by RAPD PCR and ribosomal ITS1 sequencing. Iran Red Crescent Med J 2010;12:27-32. | |
21. Khoshbakht R, Seifi S, Tabatabaei M, Aski HS, Ranjbar V, Abdi Hacheso B. Mycoplasma gallisepticum strains with identical random amplified polymorphic DNA (RAPD) patterns in chukar partridges (Alectoris chukar) and broilers: A case report. Vet Med 2013;58: 284-8. https://doi.org/10.17221/6811-VETMED | |
22. Dilipan E, Papenbrock J, Thangaradjou T. Random amplified polymorphic DNA (RAPD) finger prints evidencing high genetic variability among marine angiosperms of India. J Mar Biol Assoc UK 2017;97:1307-15. https://doi.org/10.1017/S0025315416000631 | |
23. Mohammadzadeh T, Sadjjadi SM, Motazedian MH, Mowdavi GR. Study on the genomic diversity of Hymenolepis nana between rat and mouse isolates by RAPD PCR. Iran J Vet Res 2007;8:16-22. | |
24. Sripalwit P, Wongsawad C, Wongsawad P, Anuntalabhochai S. High annealing temperature random amplified polymorphic DNA (HAT-RAPD) analysis of three paromphistome flukes from Thailand. Exp Parasitol 2007;115:98-102. https://doi.org/10.1016/j.exppara.2006.05.005 | |
25. Ganie SH, Upadhyay P, Das S, Sharma MP. Authentication of medicinal plants by DNA markers. Plant Gene 2015;4:83-99. https://doi.org/10.1016/j.plgene.2015.10.002 | |
26. Khaliq I, Babar M, Riaz M, Khan AA. Genetic diversity in see-see partridge (Ammoperdix griseogularis, Galliformes) populations from sub-Himalayan Mountain ranges of Pakistan. Belg J Zool 2010;140:227-32. | |
27. Muhammad S, Khan AA, Babar M, Riaz M, Akhtar N, Khaliq I. Population genetic structure of Rufous-Vented Prinia (Prinia burnesii) in Pakistan. Afr J Biotechnol 2010;9:9077-81. | |
28. Akhter N, Khan AA, Babar, M, Riaz M, Imtiaz A, Khaliq I. Evaluation of the genetic structure of the urban dwelling species of Bank Myna (Acridotheres ginginisnus) using random amplified polymorphic DNA (RAPD) analysis. Afr J Biotechnol 2011;10:6342-7. | |
29. Imtiaz A, Khan AA, Babar M, Riaz M, Akhtar N, Arshad M, et al. Genetic diversity of Pakistani common myna (Acridotheres tristis) revealed by RAPD-PCR. Afr J Biotechnol 2011;10:7751-5. https://doi.org/10.5897/AJB11.151 | |
30. Riaz M, Khan AA, Babar M, Akhtar N, Muhammad S, Khaliq I. High genetic diversity revealed by RAPD markers in the black francolin (Francolinus francolinus, Galliformes) of Pakistan. Pak J Zool 2011;43:889-96. | |
31. Bright JA, Marugán-Lobón J, Cobb S, Rayfield EJ. The shapes of bird beaks are highly controlled by nondietary factors. Proc Natl Acad Sci USA 2016;113:5352-7. https://doi.org/10.1073/pnas.1602683113 | |
32. Olsen AM. Feeding ecology is the primary driver of beak shape diversification in waterfowl. Funct Ecol 2017;3:1985-95. https://doi.org/10.1111/1365-2435.12890 | |
33. Savas T, Konyali C, Das G, Yurtman IY. Effect of beak length on feed intake in pigeons (Columba livia f. domestica). Anim Welfare 2007;16:79-86. | |
34. Clayton D, Moyer BR, Bush SE, Jones TG, Gardiner DW, Rhodes BB, Goller F. Adaptive significance of avian beak morphology for ectoparasite control. Proc R Soc Lond B 2005;272:811-7. https://doi.org/10.1098/rspb.2004.3036 | |
35. Brooke ML, Hanley S, Laughlin SB. The scaling of eye size with body mass in birds. Proc R Soc Lond B 1999;266:405-12. https://doi.org/10.1098/rspb.1999.0652 | |
36. Masabanda JS, Burt DW, O'brien PC, Vignal A, Filon V, Walsh PS. Molecular cytogenetic definition of the chicken genome, the first complete avain karyotype. Genetics 2004;166:1367-73. https://doi.org/10.1534/genetics.166.3.1367 | |
37. Pal B, Chowdhury S, Ghosh RK. Comparative anatomical study of the cerebellum of man and fowl. J Anat Soc Ind 2003;52:32-7. | |
38. Sur E, Öznurlu Y, Özaydin T, Çolakoglu F, Ünsal S, Yener Y. Comparative histological study of the cerebellum and the determination of some Agnor parameters in different avian species. Bull Vet Inst Pulawy 2011;55:261-5. | |
39. Patel P, Rajkumar BK, Parmar P, Shah R, Krishnamurthy R. Assessment of genetic diversity in Colletotrichum falcatum Went accessions based on RAPD and ISSR markers. J Genet Eng Biotechnol 2017;16:153-9. https://doi.org/10.1016/j.jgeb.2017.11.006 | |
40. Mei Z, Zhang X, Khan MA, Imani S, Liu X, Zou H, et al. Genetic analysis of Penthorum Chinense Pursh by improved RAPD and ISSR in China. J Genet Eng Biotechnol 2017;30:6-11. https://doi.org/10.1016/j.ejbt.2017.08.008 | |
41. Zarringhabai GE, Javanmard A, Pirahary O. Random amplified polymorphic markers as indicator for genetic conservation program in Iranian pheasant (Phasianus colchicus). Sci World J 2012;2012:640381. https://doi.org/10.1100/2012/640381 | |
42. El-Mergawy RA, Al-Ajlan AM, Abdallah NA, Nasr MI, Silvain JF. Determination of different geographical populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using RAPD-PCR. Int J Agric Biol 2011;13:227-32. | |
43. Hameed S, Hameed S, Sadia M, Malik SA. Genetic diversity analysis of Bemisia tabaci populations in Pakistan using RAPD markers. Electron J Biotechnol 2012;15:1-9. https://doi.org/10.2225/vol15-issue6-fulltext-8 | |
44. Singha IM, Kakoty Y, Unni BG, Das J, Kalita MC. Identification and characterization of Fusarium sp. using ITS and RAPD causing Fusarium wilt of tomato isolated from Assam, North East India. J Genet Eng Biotechnol 2016;14:99-105. https://doi.org/10.1016/j.jgeb.2016.07.001 | |
45. El-Bakatoushi R, Ahmed DG. Evaluation of genetic diversity in wild populations of Peganum harmala L., a medicinal plant. J Genet Eng Biotechnol 2017;16:143-51. https://doi.org/10.1016/j.jgeb.2017.11.007 | |
Year
Month
Identification, evaluation and optimization of a minimum simple sequence repeat marker set for triticale breeding
W.C. Botes and D. BitaloRepetitive PCR based detection of Genetic Diversity in Xanthomonas axonopodis pv citri Strains
Minhaj Arshiya, Alka Suryawanshi, Digamber More, Mirza Mushtaq Vaseem BaigGenetic diversity and phylogenetic analyses of culturable extremely haloarchaea isolated from marine solar saltern pond in Mumbai, India
Dipak T. Nagrale , Renu, Priyanka DasPhenotypic and genotypic diversity of Xanthomonas axonopodis pv. manihotis causing bacterial blight disease of cassava in Kenya
Mary N. Chege , Fred Wamunyokoli, Joseph Kamau, Evans N. NyabogaAssessment of genetic diversity in Shorea robusta: an economically important tropical tree species
Giridara-Kumar Surabhi, Subhankar Mohanty, Rajesh Kumar Meher, Arup Kumar Mukherjee, Lakshmi Narayana R.VemireddyHeritability, genetic advance, and correlation studies of morpho-agronomic traits and brix in Burkina Faso sweet stalk sorghum genotypes
Nerbéwendé Sawadogo, Inoussa Drabo, Nofou Ouédraogo, Wendmanegda Hermann Tondé, Tewendé Lionel Kevin Béré, Josiane Tiendrébéogo, Gilbert Compaoré, Mahamadi Hamed Ouédraogo, Kiswendsida Romaric Nanema, Pauline Bationo-KandoPhenotypic characterization and genetic diversity of the Khiew-Phalee chicken (Gallus gallus): A fighting cock originating from Uttaradit, Thailand
Siriwadee Phromnoi, Preeda Lertwatcharasarakul, Wallaya Phongphaew, Pisit PoolprasertPhylogenetic analysis of Omicron subvariants in Vietnam
Phuoc Huynh, Huyen Thi Thuong Nguyen, Quan Ke ThaiPhylogenetic study of some major Dendrobium species of Eastern Himalaya using internal transcribed spacer marker
Animesh Mondal, Kalyan Kumar DeUsing RAPD and ISSR markers to assess genetic diversity and conservation of Bruguiera gymnorrhiza (L.) Lam. for sustainable management on Kerala's West Coast
Sreeram Sudhir, Sankara Velmani Vel, Hariharan Selvam, Arumugam ArunprasathGenetic diversity investigation of some Darjeeling Himalayan Rhododendron species based on RAPD method
Animesh Mondal, Kalyan Kumar De