1. INTRODUCTION
The assessment of lipid level is a key tool in the determination of hyperlipidemic status of individual in disease conditions. Elevated levels of serum lipids are established risk factors for assessing cardiovascular diseases [1]. Dyslipidemia in diabetes mellitus is characterized by increase in plasma lipids; triacylglycerols (TRIG) and cholesterol, including very-low-density lipoprotein cholesterol (VLDLc) and LDLc, and reduced high-density lipoprotein cholesterol (HDLc) levels [2,3]. High levels of cholesterols and TRIG in the blood, which are known as hypercholesterolemia and hypertriacylglycerolemia, respectively, are the major causes of atherosclerosis, which is strongly associated with ischemic heart disease [4]. Furthermore, high levels of plasma cholesterols are reported to cause over four million deaths in a single year [5]. Hyperlipidemia relates to increased oxidative stress in biological system causing significant increases in oxygen-free radicals that may lead to modifications (oxidative) in important molecules such as LDLc [6]. These modifications play significant roles in the initiation and progress of atherosclerosis with other associated cardiovascular diseases [6].
Oftentimes, statins are the preferred group of drugs prescribed for the treatment of hyperlipidemia [1]. Their mechanism of action involves the reduction in total circulating lipids cholesterol in the system by acting as inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase enzyme, the rate-limiting enzymes in the biosynthetic pathway for cholesterol. Statins are important in treatment of hyperlipidemia and thus reduce the rate of reported cardiovascular diseases cases [7]. The undesirable side effects associated with statins including muscle damage and skeletal muscle disorder [7,8] as well as abnormalities in some liver enzymes and increased risk of diabetes mellitus [9] are causes of concern. This has necessitated the search for alternative treatments that are cheap, potent with minimum side effects. Several indigenous plants have been used for their beneficial effects in the treatment of diabetes mellitus and hyperlipidemia [10].
Daucus carota L. (Carrot) is a popular vegetable, which is grown throughout the world. D. carota seed has been used medicinally for the treatment of a spectrum of diseases, including diabetes mellitus [11], which is known to be associated with oxidative stress and hyperlipidemia. Carrot seed has cardioprotection and muscle contraction regulation effects [12]. They also have anti-inflammatory, analgesic [13], hepatoprotective activities [14], and can improve antioxidant status by inhibiting peroxidation activity in liver tissue [15]. The present study evaluates the antioxidant and antihyperlipidemic activities of D. carota seed in triton ×100-induced hyperlipidemic condition in mice.
2. MATERIALS AND METHODS
2.1. Plant Material
Rohini carrot (D. carota L.) seed was purchased from Hooghly, West Bengal, India and identified by Mr. Azila Joseph, a curator with the Federal College of Forestry, Jos, Plateau State, Nigeria.
2.2. Chemicals and Reagents
Triton ×100 used in the study was purchased from Technico Laboratory Chemicals, Coimbatore. Simvastatin (SIMCARD) was a product of Swiss Pharma, Gujarat, India. Ammonium molybdate, ascorbic acid, methanol, naphthylethylenediamine, potassium ferricyanide, sodium azide, sodium hydroxide, sodium nitroprusside, sucrose, sulphanilamide, sulphosalicylic acid, tetraoxosulphate (VI) acid, trichloroacetic acid, KH2PO4, KOH, MgCl2, and ZnSO4 were purchased from Merck Pharmaceutical Company, Darmstadt Germany. Triacylglycerol, total cholesterol, and HDLc kits were products of Randox Laboratories, Ardmore, Co. Antrim, UK, while 2,2-diphenyl-1-picrylhydrazyl (DPPH), reduced glutathione (GSH), ethylenediaminetetraacetic acid (EDTA), 1-Chloro-2,4-dinitrobenzene, GSH, acetic acid, epinephrine, sodium nitrite, thiobarbituric acid, and Tris buffer used in the study were purchased from Sigma Chemical Company, St. Louis, Mo, USA. All other used chemicals were of analytical grades.
2.3. Extract Preparation
The dry D. carota seed was pulverized, and 167 g dissolved in 250 ml distilled water for 24 h with intermittent shaking. The sample was filtered and then concentrated at 40°C using a rotary evaporator to obtain the aqueous seed extract (AQEDCS, 29.42 g, 17.62% yield). The AQEDCS was then stored in an airtight container, after drying in a desiccator until required for use.
2.4. Phytochemical Studies
Phytochemical analysis was done using the methods of Odebiyi and Sofowora [16].
2.5. Experimental Animals
Twenty-five Wister albino mice of both sexes with average weight of 20 ± 3 g were purchased from the animal holding unit, University of Jos, Nigeria. The mice were acclimatized for 14 days to the laboratory conditions of 12 h light-dark cycles, at 25 ± 2°C in standard plastic cages. They were also allowed free access to standard mice chow and water ad libitum. Research ethical approval was obtained from the University of Jos Ethical Review Committee (UJ/FPS/F17-00379) which is in accordance with the ethical standards as reviewed in the Principles of Laboratory Animal Care (NIH publication no. 85 23, revised 1985).
2.5.1. Triton ×100 treatment
Triton ×100 was used to induce hyperlipidemia in twenty experimental mice by a single injection (intraperitoneal) of 0.2 ml freshly prepared solution of triton ×100 (100 mg/kg body weight) in normal saline (0.9% NaCl solution) after an 18 h overnight fasting [17]. The induction of hyperlipidemia was done 30 min after mice received respective dosages of treatments (p.o).
2.5.2. Animal grouping
The control group received 0.2 ml distilled water (p.o), 30 minutes before intraperitoneal injection of saline (2.5 ml/kg body weight). The other groups (2–5) received 0.2 ml oral dosage of distilled water, simvastatin (10 mg/kg body weight), AQEDCS extract (50 mg/kg body weight), and AQEDCS extract (100 mg/kg body weight) respectively, 30 minutes before induction of hyperlipidemia. All animals remain in the fasting state for the period of this experiment [18].
2.5.3. Preparation of blood samples and liver homogenates
Twenty-four hours after triton ×100 treatment, the mice were sacrificed under diethyl ether anesthesia and the jugular veins were incised to collect blood samples into EDTA bottles. The plasma was separated by centrifugation at 2400 ×g for 15 min. The liver was excised, cleaned of blood stains, weighed, and thereafter homogenized in ice cold 0.25 M sucrose solution (1:5 w/v). The homogenates obtained were centrifuged at 3000 ×g for 15 min to get clear supernatants, which were kept in plain specimen bottles and maintained frozen until required for analysis.
2.6. In Vitro Antioxidant Studies
The in vitro scavenging activity against DPPH radical was determined according to the method described by McCune and Johns [19], while the total antioxidant capacity (TAC) was evaluated using the phosphomolybdenum assay as described by Prieto et al. [20]. The ferric reducing antioxidant property was determined according to the method reported by Girgih et al. [21] and nitric oxide (NO) scavenging capacity was measured according to the method of Fiorentino et al. [22].
2.7. Lipid Profile Status
Plasma total cholesterol, TRIG, and HDLc were estimated using the procedure described in Randox kits (Randox Laboratories Ltd., Antrim, UK). The fecal matters collected within the past 24 h were dried at 40°C until a constant weight was achieved and they were ground to fine powder using pestle and mortar. They were then diluted using phosphate buffer saline (pH 7.1) solution (1:5 w/v). The solutions were centrifuged at 1000 ×g for 5 min to obtain the supernatants, which were kept in plain specimen bottles for analysis. The fecal cholesterol and TRIG content were measured using the procedure described in Randox kits (Randox Laboratories Ltd., Antrim, UK). The following formula was used to calculate LDLc = (TC/2.2) − HDLc (mmol/L) [23], Coronary artery index = LDLc/HDLc [24], Cardiac index = total cholesterol/HDLc [25], and Atherogenic index = (total cholesterol - HDLc)/HDLc [26].
2.8. In Vivo Antioxidant Studies
The superoxide dismutase (SOD) activity was determined by the method described by Misra and Fridovich [27], while catalase (CAT) activity was determined as described by Sinha [28]. Glutathione peroxidase (GPx) activity was determined by the method described by Rotruck et al. [29] and glutathione-S-transferase (GST) activity was determined using the method described by Habig et al. [30]. The levels of lipid peroxidation in plasma and liver homogenates were estimated by determining the concentration of malondialdehyde (MDA) as described by Varshney and Kale [31]. Nitrite concentrations (NO) and reduced GSH concentrations in plasma and liver homogenates were, respectively, determined as described by Green et al. [32] and Beutler et al. [33].
2.9. Statistical Analysis
Data were subjected to one-way analysis of variance followed by Duncan multiple range test (SPSS version 20, SPSS Inc., Chicago. IL, USA). Data are also presented as means ± SEM. Significant levels were considered at P < 0.05. Graphs were, respectively, generated using GraphPad Prism 6 software (GraphPad Software, California, USA).
.png) | Table 1: Phytochemical status of aqueous seed extract of Daucus carota (AQEDCS). [Click here to view] |
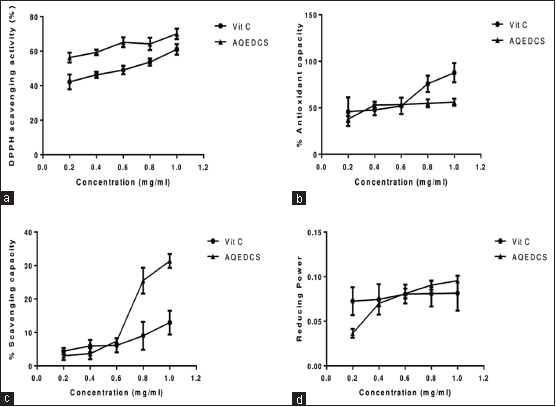 | Figure 1: In vitro antioxidant effects of aqueous seed extract of Daucus carota (AQEDCS) (a) 2,2-diphenyl-1-picrylhydrazyl scavenging activities (b) Total antioxidant capacity (c) Nitric oxide scavenging capacity (d) Ferric ion reducing properties. Values are means ± SEM of triplicate determinations. Vit C = Vitamin C = Ascorbic acid, AQEDCS=Aqueous extract [Click here to view] |
3. RESULTS
3.1. Phytochemical Status of Aqueous Seed Extract of D. carota
Phytochemical analysis indicated the presence of alkaloids, tannins, and phenols [Table 1], while glycosides, saponins, flavonoids, steroids, and phlobatannins were not detected.
3.2. In Vitro Antioxidant Effects of Aqueous Seed Extract of D. carota
The aqueous seed extract of D. carota expressed significant DPPH scavenging activities for the various concentrations with IC50 of 0.29 mg/ml compared with Vitamin C with IC50 of 0.49 mg/ml [Figure 1]. The aqueous seed extract and Vitamin C at concentrations of 0.2–0.6 mg/ml expressed similar total TAC and NO scavenging capacity [Figure 1]. However, at concentration of 0.6 mg/ml and above, Vitamin C had higher TAC while AQEDCS had higher NO scavenging capacity. The ferric ion reducing properties of AQEDCS and Vitamin C at the concentration range of 0.4–1.0 mg/ml were similar [Figure 1].
3.3. Effects of Aqueous Seed Extract of D. carota on Oxidative Stress Biomarkers
Administration of AQEDCS expressed a significant decrease (P < 0.05) in plasma SOD, CAT, and GPx activity at 50 and 100 mg/ kg body weight when compared with untreated hyperlipidemic mice [Figures 2-4]. Likewise, significant decrease (P < 0.05) was observed in liver SOD at 100 mg/kg body weight. However, no significant alteration (P > 0.05) was observed in liver CAT and GST activities compared with untreated hyperlipidemic mice [Figures 3 and 5].
The increase in MDA observed in the plasma of untreated hyperlipidemic mice was significantly reduced (P < 0.05) by treatment with AQEDCS and simvastatin. However, no significant difference (P > 0.05) was observed in the liver when compared with the untreated hyperlipidemic mice [Figure 6].
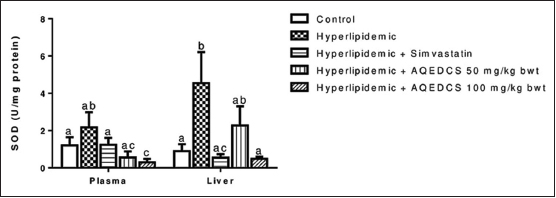 | Figure 2: Superoxide dismutase activity in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (p<0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
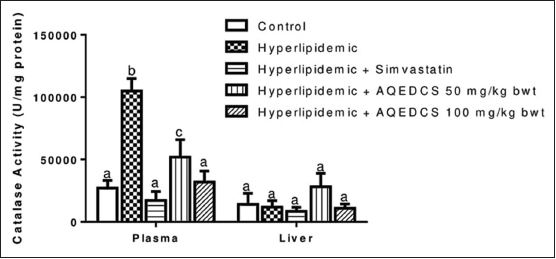 | Figure 3: Catalase activity in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
.png) | Figure 4: Glutathione peroxidase activity in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
The extract produced no significant change (P > 0.05) of nitrite levels in plasma and liver of treated hyperlipidemic mice when compared with untreated hyperlipidemic mice except at the dosage of 50 mg/kg body weight that resulted in a significant increase [Figure 7]. Treatment with 100 mg/kg body weight AQEDCS and simvastatin caused no significant alterations (P > 0.05) in plasma GSH when compared with control mice. Furthermore, no significant change (P > 0.05) was observed in GSH levels of the liver for all treatments when compared with untreated hyperlipidemic mice [Figure 8].
3.4. Effects of Aqueous Seed Extract of D. carota on Dyslipidemia
Treatment with AQEDCS significantly decreased (P < 0.05) total cholesterol, TRIG, and LDL when compared with untreated hyperlipidemic mice. Furthermore, treatment with AQEDCS improved the concentration of circulating HDL when compared with untreated hyperlipidemic mice [Table 2]. Furthermore, treatment with AQEDCS significantly (P < 0.05) decreased hepatic cholesterol but caused no significant alteration in hepatic TRIG when compared with untreated hyperlipidemic mice. However, treatment with 50 mg/kg body weight AQEDCS increased the concentration of hepatic proteins when compared with normal control mice [Table 3]. The coronary artery, atherogenic, and cardiac indices were significantly decreased (P < 0.05) for hyperlipidemic mice treated with AQEDCS when compared with untreated hyperlipidemic mice [Table 4]. Analysis of the fecal matter revealed increase fecal cholesterol and protein at 50 mg/kg body weight dose, while treatment with 100 mg/kg body weight AQEDCS resulted in decreased level of fecal TRIG when compared with untreated hyperlipidemic mice [Table 5].
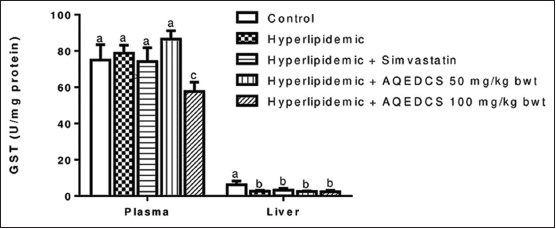 | Figure 5: Glutathione-S-transferase activity in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
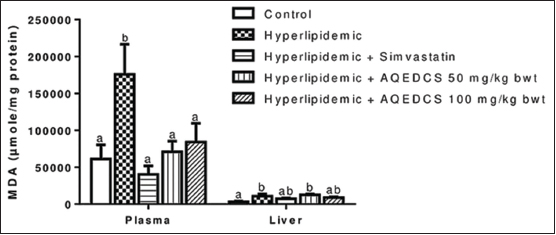 | Figure 6: Malondialdehyde concentration in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
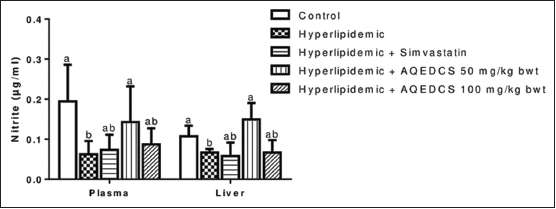 | Figure 7: Nitrite concentration in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
4. DISCUSSION
The aqueous seed extract of D. carota (AQEDCS) expressed in vitro antioxidant activities and these could be due to the phytochemicals such as phenols, tannins, and alkaloids detected in the extract. Factors modulating antioxidant activities of phytochemicals could include the number of hydroxyl groups, methoxy esters, and/or phenolic units [34,35]. The antioxidant ability of the identified phenolic compound could be majorly due to the ability of the hydrogen atom attached to their aromatic hydroxyl (OH) group to release as free radical [36] and the ease of their aromatic rings to support unpaired electron state due to the delocalize micro-electron system [36].
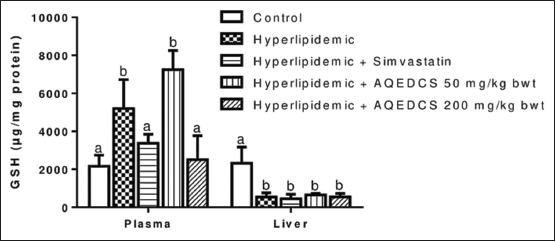 | Figure 8: Reduced glutathione concentration in plasma and liver of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). Values are mean ± SEM of five replicates. Bars with different alphabets are significantly different (P < 0.05). AQEDCS=Aqueous extract, mg/kg bwt=mg/kg body weight [Click here to view] |
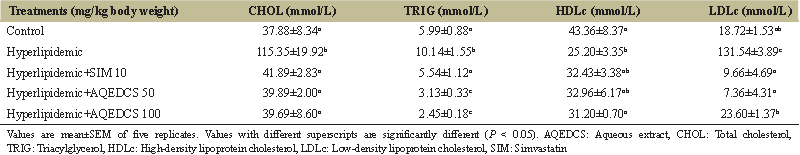 | Table 2: Lipid profile of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). [Click here to view] |
 | Table 3: Hepatic cholesterol, triacylglycerols, and protein concentrations of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). [Click here to view] |
 | Table 4: Coronary artery, atherogenic, and cardiac indices of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). [Click here to view] |
 | Table 5: Fecal cholesterol, triacylglycerols, and protein of hyperlipidemic mice treated with aqueous seed extract of Daucus carota (AQEDCS). [Click here to view] |
The antioxidant enzymes system is important in the prevention or worsening of several disease conditions such as atherosclerosis [37], heart disease [38], cancer [39], stroke [40], and diabetes [41]. Antioxidants are helpful in neutralizing the possible negative effects of excess reactive species. Progression of some disease conditions such as diabetics and malaria is associated with increase in generation of reactive oxygen species. These reactive species target lipids in the body, causing their peroxidation, thereby leading to the formation of MDA (an aldehyde) which is a secondary product of polyunsaturated fatty acid peroxidation. The interaction of this aldehyde with DNA and protein is potentially mutagenic and atherogenic [42]. The ability of AQEDCS to significantly reduce the concentration of MDA in hyperlipidemic mice suggest the extract was able to mop up radicals that can cause peroxidation, or prevented the accumulation of the peroxidation product, which could cause several harmful effects.
Nitrite is an oxidative maker which forms peroxynitrite (ONOO-) in a reaction between NO and superoxide anion. This product is lipid-soluble and similar in their reactivity to hypochlorous acid. Peroxynitrous acid (ONOOH) can be formed from it through protonation and can undergo hemolytic cleavage to form nitrogen dioxide and hydroxyl radical [43]. The non-significant change observed in the concentration of nitrite suggest AQEDCS was able to prevent adverse effects that could result from excessive production of peroxynitrite or peroxynitrous acid.
The antioxidant molecules and enzymes serve to regulate the level of oxidants in biological tissue. However, when the antioxidants networks are could not produce the require protection against free radicals, the tissue is damaged through oxidative stress by the free radicals. The moderation of these antioxidant enzymes activities in AQEDCS treated hyperlipidemic mice compared to untreated hyperlipidemic mice suggest that AQEDCS could protect tissues from oxidative stress. This is in agreement with Singh et al. [14] who reported in vivo antioxidant effect of methanolic extract of D. carota seed in thioacetamide-induced oxidative stress. The effects on the enzymes are more likely related to the antioxidant properties of AQEDCS.
As a form of traditional approach to drug discovery, several plant-based extracts have been studied and some have been widely accepted and used in the treatment of diseases [44,45]. Endowment of plants with varieties of bioactive compounds that can act and protect against different disease [46] with various modes and mechanisms make them a valuable source of antihyperlipidemic agent. Hyperlipidemia is characterized by an increase in total cholesterol, TRIG, VLDLc, and decreases HDL cholesterol [47]. Treatment of hyperlipidemic mice with AQEDCS improved the lipid profile. It reversed the accumulation of LDLc and improved the concentration of circulating HDLc, in a manner similar to the reference drug simvastatin. The cholesterol-lowering effect of AQEDCS could be due to catabolism of LDLcholesterol through hepatic receptors for final elimination from bile acid. HDLc facilitates the translocation of lipids from peripheral tissue, such as arterial walls to the liver for their catabolism, and reduction in plasma HDLc is a risk factor for developing atherosclerosis while increase in HDLc has been reported to slow down atherosclerotic process [48].
Dietary sources are the major sources of lipids in the body aside from their biosynthesis by the liver. Treatment of hyperlipidemic mice with 50 mg/kg body weight AQEDCS improved fecal cholesterol, fecal TRIG excretion, and protein biosynthesis. The increased level of HDLc and decreased total cholesterol level along with their LDLc fraction may also be due to decreased in cholesterol absorption through gastrointestinal tract and increased in cholesterol excretion. This suggests that treatment with the extract at that dosage may not enhance the accumulation of lipids from dietary source.
5. CONCLUSION
The results of the study indicated that aqueous seed extract of D. carota (AQEDCS) possesses in vitro antioxidant activities, which is comparable to Vitamin C, and with ameliorative effects on deleterious free radicals in vivo. The antihyperlipidemic potential of the extract could also be beneficial during its traditional usage in treatment of diabetes mellitus. The medicinal values of the aqueous seed extract may be related to the phytochemical constituents that were detected.
6. AUTHORS’ CONTRIBUTION
HT, AM, CDL, APA, and AJA conceived and designed the experiment; SM1, HT, and AM performed the phytochemical studies; SM2, HT, and EBJ performed the antioxidant studies; YA, HT, and AAI performed the antihyperlipidemic studies; and HT and APA analyzed the data, prepared the manuscript. All authors read and approved this final manuscript.
7. REFERENCES
1. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013;40:195-211. CrossRef
2. Gadi R, Samaha FF. Dyslipidemia in Type 2 diabetes mellitus. Curr Diab Rep 2007;7:228-34. CrossRef
3. Mooradian AD. Dyslipidemia in Type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009;5:150-9.
4. Brouwers MC, van Greevenbroek MM, Stehouwer CD, de Graaf J, Stalenhoef AF. The genetics of familial combined hyperlipidaemia. Nat Rev Endocrinol 2012;8:352-62. CrossRef
5. Kumar D, Parcha V, Maithani A, Dhulia I. Effect and evaluation of antihyperlipidemic activity guided isolated fraction from total methanol extract of Bauhinia variegata (Linn) in Triton WR–1339 induced hyperlipidemic rats. Asian Pac J Trop Dis 2012;2:909-13. CrossRef
6. Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, et al. Oxidative stress-mediated atherosclerosis: Mechanisms and therapies. Front Physiol 2017;8:600. CrossRef
7. Ito MK. Dyslipidemia: Management using optimal lipid-lowering therapy. Ann Pharmacother 2012;46:1368-81. CrossRef
8. Abd TT, Jacobson TA. Statin-induced myopathy: A review and update. Expert Opin Drug Saf 2011;10:373-87. CrossRef
9. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 2013;6:390-9. CrossRef
10. Parikh NH, Parikh PK, Kothari C. Indigenous plant medicines for health care: Treatment of diabetes mellitus and hyperlipidemia. Chin J Nat Med 2014;12:335-44. CrossRef
11. Pouraboli I, Ranjbar B. The effect of Daucus carota seeds extract on lipid profile, LFT and kidney function indicators in streptozocin-induced diabetic rats. Int J Plant Sci Ecol 2015;3:84-7.
12. Muralidharan P, Balamurugan G, Kumar P. Inotropic and cardioprotective effects of Daucus carota Linn on isoproterenol-induced myocardial infarction. Bangladesh J Pharmacol 2008;3:74-9. CrossRef
13. Vasudevan M, Parle M, Ramasamy K, Majeed AB. Anti-dementia potential of Daucus carota seed extracts in rats. Pharmacologyonline 2010;1:552-65.
14. Singh K, Singh N, Chandy A, Manigauha A. In vivo antioxidant and hepatoprotective activity of methanolic extracts of Daucus carota seeds in experimental animals. Asian Pac J Trop Biomed 2012;2:385-8. CrossRef
15. Rezaei-Moghadam A, Mohajeri D, Rafiei B, Dizaji R, Azhdari A, Yeganehzad M, et al. Effect of turmeric and carrot seed extracts on serum liver biomarkers and hepatic lipid peroxidation, antioxidant enzymes and total antioxidant status in rats. Bioimpacts 2012;2:151-7.
16. Odebiyi OO, Sofowora EA. Phytochemical screening of Nigerian medicinal plants II. Lloydia 1978;41:234-46.
17. Gundamaraju R, Hwi KK, Singla RK, Vemuri RC, Mulapalli SB. Antihyperlipidemic potential of Albizia amara (Roxb) boiv. Bark against triton X-100 induced hyperlipidemic condition in rats. Pharmacognosy Res 2014;6:267-73. CrossRef
18. da Rocha JT, Sperança A, Nogueira CW, Zeni G. Hypolipidaemic activity of orally administered diphenyl diselenide in triton WR-1339-induced hyperlipidaemia in mice. J Pharm Pharmacol 2009;61:1673-9. CrossRef
19. McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol 2002;82:197-205. CrossRef
20. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal Biochem 1999;269:337-41. CrossRef
21. Girgih AT, Udenigwe CC, Aluko RE. Reverse-phase HPLC separation of hemp seed (Cannabis sativa L.) protein hydrolysate produced peptide fractions with enhanced antioxidant capacity. Plant Foods Hum Nutr 2013;68:39-46. CrossRef
22. Fiorentino A, Ricci A, D’Abrosca B, Pacifico S, Golino A, Letizia M, et al. Potential food additives from Carex distachya roots: Identification and in vitro antioxidant properties. J Agric Food Chem 2008;56:8218-25. CrossRef
23. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
24. Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757-65. CrossRef
25. Kang MJ, Lee EK, Lee SS. Effects of two P/S ratios with same peroxidizability index value and antioxidants supplementation on serum lipid concentration and hepatic enzyme activities of rats. Clin Chim Acta 2004;350:79-87. CrossRef
26. Kayamori F, Igarashi K. Effects of dietary nasunin on the serum cholesterol level in rats. Biosci Biotechnol Biochem 1994;58:570-1. CrossRef
27. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170-5.
28. Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389-94. CrossRef
29. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG, et al. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973;179:588-90. CrossRef
30. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130-9.
31. Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 1990;58:733-43. CrossRef
32. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR, et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982;126:131-8. CrossRef
33. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-8.
34. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002;13:572-84. CrossRef
35. Kumar S, Mishra A, Pandey AK. Anti-oxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Alternat Med 2013;13:120. CrossRef
36. Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Lipidol 2000;11:43-7. CrossRef
37. Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 2006;185:219-26. CrossRef
38. Elahi MM, Matata BM. Free radicals in blood: Evolving concepts in the mechanism of ischemic heart disease. Arch Biochem Biophys 2006;450:78-88. CrossRef
39. Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 2004;44:239-67. CrossRef
40. Alexandrova M, Bochev P, Markova V, Bechev B, Popova M, Danovska M, et al. Dynamics of free radical processes in acute ischemic stroke: Influence on neurological status and outcome. J Clin Neurosci 2004;11:501-6. CrossRef
41. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84. CrossRef
42. Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005;15:316-28. CrossRef
43. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315-424. CrossRef
44. Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 2013;10:210-29. CrossRef
45. Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK, et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med 2013;2013:627375. CrossRef
46. Willett WC. Diet and health: What should we eat? Science 1994;264:532-7. CrossRef
47. Ajiboye TO, Akinpelu SA, Muritala HF, Ogunbode SM, Adeleye AO, Oladiji AT, et al. Trichosanthes cucumerina fruit extenuates dyslipidemia, protein oxidation, lipid peroxidation and DNA fragmentation in the liver of high-fat diet-fed rats. J Food Biochem 2014;38:480-90. CrossRef
48. Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur Heart J Suppl 2005;7:4-8. CrossRef