1. INTRODUCTION
The genus Launaea (tribe Lactuceae, family Asteraceae) contains about 40 species in the Algerian flora, where nine species of them grow mostly within the Sahara [1,2]. They are the following species: L. acanthoclada, L. angustifolia, L. anomala, L. arborescens, L. cassiniana, L. glomerata, L. nudicaulis, L. quercifolia, and L. resedifolia [3,4]. The regional name of Launaea resedifolia is “laadid, Azim” [4], synonym Scorzonera resedifolia is a perennial herb, it has length up to 40 cm, exceedingly distributed in the wetland of the arid zones of Mediterranean area, and as well existing in many several countries, e.g., Algeria, Libya, Tunisia, Pakistan, and India [5].
Many species of this genus are utilized in alternative medicine in bitter stomach, skin diseases, and reported to have antitumor, insecticide, and cytotoxic activities [6]. The antimicrobial activities of coumarin constituents [7] and the neuropharmacological properties [5] have been investigated as well.
Previous works on parts of this species revealed the presence of compounds from the following families: flavonoids [8], phenolics [9], coumarins [10,11], and terpenoids [12,13]. Therefore, we investigated the antioxidant capacity of L. resedifolia from Algeria-Sahara.
2. MATERIALS AND METHODS
2.1. Plant Material
The L. resedifolia was collected in the flowering duration (March 2013) from the region of Bashar, which is located in the south-west of Algeria. The aerial part of L. resedifolia was identified by Doctor Halis Youcef researcher in Scientific and Technical Research Centre for Arid Areas-Touggourt.
2.2. Chemicals and Reagents
Folin-Ciocalteu’s reagent was purchased from Sigma-Aldrich, and sodium carbonate (Na2CO3) (99.8%), aluminum chloride (97%), 1,1-diphenyl-2-picrylhydrazyl (DPPH) (99%), potassium ferricyanide (99%), ferric chloride (99%), trichloroacetic acid (99%), disodium phosphate (99%), ammonium molybdate (99%), gallic acid (99%), quercetin (99%), catechin (99%), ascorbic acid (99.7%), BHA (98%), and BHT (98%) were obtained from Sigma-Aldrich and Biochem. All chemicals and reagents used are of analytical grades.
2.3. Preparation of Extracts
A 100 g of L. resedifolia powder was macerated in MeOH-H2O (7:3, v/v) and left at room temperature and then was filtered after 48 hours (repeated three times). The filtrates are collected and evaporated in the rotary evaporator under 40°C then recovered with warm distilled water (40–60 ml distilled water per 100 g of plant powder) and kept in the dark for a full night and then filtered. The filtrate was partitioned successively using chloroform, ethyl acetate, and n-butanol. The extracts and also the remaining water fraction are concentrated under reduced pressure and then re-dissolved with a minimum of ethanol or water and kept at 4°C.
2.4. Yield of Extracts
Extraction yield was calculated following the equivalent of %yield = (mextract/mdried sample) × 100, where mextract, mdried sample are the weight of extract and weight of the dried sample, respectively
2.5. Phytochemical Investigation
2.5.1. Total phenolic content (TPC)
TPC of L. resedifolia’s extracts was determined with the Folin-Ciocalteu reagent [14,15]. Briefly; 0.1 ml of extract sample was jumbled with 0.5 ml of 10% Folin-Ciocalteu reagent. Then 2.0 ml of 20% aqueous Na2CO3 solution was added after 5 minutes, the mixture was kept for 30 minutes in the dark at room temperature, and then the absorbance was read at 760 nm versus prepared blank solution. Results were expressed as mg gallic acid equivalents/g of the plant (mg GAE/g P).
2.5.2. Total flavonoid content
Total flavonoid content (TFC) of L. resedifolia’s extracts was determined following the method described by Kumazawa et al. [14]. Briefly, a mixture of 0.5 ml of extract and 0.5 ml of 2% AlCl3 ethanol solution was prepared and allowed to stand for 30 minutes at room temperature. The absorbance was recorded at 430 nm against a blank solution. Results were presented as mg quercetin equivalent/g of the plant (mg QE/g P).
2.5.3. Total tannin content
Total tannin content (TTC) of L. resedifolia’s extracts was determined by a colorimetric method [15,17]. Briefly; the 0.3 ml of extract sample was jumbled with 1.8 ml of 4% ethanol vanillin solution and 0.9 ml of concentrated hydrochloric acid (HCl). The mixture was allowed to stand for 15 minutes in the dark at room temperature. The absorbance was recorded at 500 nm versus prepared blank solution. Results were expressed as mg catechin equivalent/g of the plant (mg CE/g P).
2.6. Antioxidant Capacities
2.6.1. DPPH radical scavenging capacity
The DPPHâ— scavenging capacity of extracts of L. resedifolia was assayed by Thaipong et al. [18] with some modifications. A 0.14 ml of diluted plant extract was mixed with 2.66 ml of 0.1 mM DPPHâ— ethanol solution. The mixture was saved for 30 minutes in the darkish chamber then the absorbance was recorded at 517 nm and ascorbic acid was used as a positive control. The DPPH radical inhibition was calculated as
Where A0 and As are the absorbance of the control and the sample extracts, respectively.
2.6.2. Ferric reducing power (FRP)
The FRP of the different extracts of L. resedifolia was examined following the method of Kumaran et al. [19] with a small modification. Two hundred microliters of the extracts, 0.5 ml of phosphate buffer (0.2 M, pH 6.6), and 0.5 ml of potassium ferricyanide [K3Fe(CN)6] (1%) were prepared and saved at 50°C for 20 minutes. Then 0.5 ml of trichloroacetic acid solution (10%) was added. The resulting mixture (1.7 ml) was blended with 1.7 ml of distilled water and 0.34 ml of ferric chloride (1%). The absorbance was read at 700 nm, using ascorbic acid as a positive control, and the results were expressed as mM equivalents to ascorbic acid.
2.6.3. Total antioxidant capacity
The total antioxidant capacities of the different extracts of L. resedifolia were assayed by the method of Prieto et al. S. 0.2 ml of sample extracts was jumbled with 2 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The resulting solutions were saved in a water bath (95°C) for 90 minutes. Then the mixture was cooled to room temperature and the absorbance of the mixture was read at 695 nm, using ascorbic acid as a positive control, and the results were expressed as mM equivalent to ascorbic acid.
2.7. Statistical Analysis
The results were expressed as mean f three replicates together with standard deviations. Statistical calculations were done by Microsoft Excel 2010. IC50 was calculated from linear regression.
3. RESULTS AND DISCUSSION
3.1. Yield of Extracts
The yield of the crude extract (CE) of L. resedifolia was 11.93%. As for the fractions, the elevated yield was in water fraction (WF) (8.17%), followed by butanol fraction (BF) (2.49%) then by ethyl acetate fraction (AF) (0.27%) and the lowest yield of extraction was in chloroform fraction (CF) (0.16%) (Table 1).
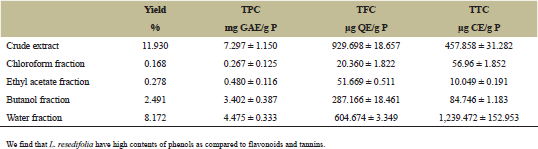 | Table 1. Yield of extracts and phytochemical contents. [Click here to view] |
 | Figure 1: Phytochemical contents of L. resedifolia’s extracts. [Click here to view] |
3.2. Phytochemical Contents
The amount of TPC from L. resedifolia’s extracts was ranging from 7.297 ± 1.150 to 0.267 ± 0.125 mg GAE/g of the plant. Where the extracts gave the following order: CF < AF < BF < WF < CE with values 0.267 ± 0.125 mg GAE/g < 0.480 ± 0.116 mg GAE/g < 3.402 ± 0.387 mg GAE/g < 4.475 ± 0.333 mg GAE/g < 7.297 ± 1.150 mg GAE/g of the plant (Table 1).
The amount of TFC from L. resedifolia’s extracts was ranging from 929.698 ± 18.657 μg QE/g to 20.360 ± 1.822 μg QE/g of the plant. Where the extracts gave the following order: CF < AF < BF < WF < CE with values 20.360 ± 1.822 μg QE/g < 51.669 ± 0.511 μg QE/g < 287.166 ± 18.461 μg QE/g < 604.674 ± 3.349 μg QE/g < 929.698 ± 18.657 μg QE/g of the plant (Table 1).
The amount of TFC from L. resedifolia’s extracts varied between 1,239.472 ± 152.953 μg CE/g and 10.049 ± 0.191 μg CE/g of the plant. Where the extracts gave the following order: AF < CF < BF < CE < WF with values 10.049 ± 0.191 μg CE/g < 56.96 ± 1.852 μg CE/g < 84.746 ± 1.183 μg CE/g < 457.858 ± 31.282 μg CE/g < 1,239.472 ± 152.953 μg C/g of plant (Table 1)
 | Figure 2: Percentage scavenging of DPPH in L. resedifolia’s extracts [Click here to view] |
 | Table 2. DPPH scavenging, reducing power, and total antioxidant capacity. [Click here to view] |
We have determined the amount of phenolic and flavonoids contents present in different extracts of L. resedifolia. However, we have observed that TPC and TFC are greater in the polar fractions (WF and BF), this suggests that these polyphenol compounds are more hydroxylated and/or glycosylated. The phenolic or flavonoid compounds contained in extracts were influenced by their solubility in the solvent used for extraction. Polyphenols are present in polar fractions more than non-polar fractions, Results similar to those found in Nagalapur and Paramjyothi [21] and Belboukhari et al. [22].
3.3. Antioxidant Activities
3.3.1. DPPH radical scavenging capacity
The antiradical capacity of the extracts of L. resedifolia was assayed by using the DPPH. This method is based on the granting of hydrogen from phenolic hydroxyl groups to reduction DPPHâ—. This reduction is accompanied by a color change of DPPHâ— (violet) to DPPH-H (yellow), where reading was done at 517 nm. Figure 2 shows the percentage of scavenging DPPH in extracts of L. resedifolia. The values of IC50 ranged from 437.999 ± 26.097 μg/ml to 4,226.41332 ± 81.5639287 μg/ml (Table 2). The highest capacity recorded at AF and BF with an IC50 value of 437.999299 ± 26.0969056 μg/ml and 976.193157 ± 36.1905122 μg/ml, respectively, followed by WF and CE with an IC50 value of 1,528.45228 ± 46.9357862 μg/ml and 2,673.95055 ± 24.9000979 μg/ml, respectively, and the lowest capacity recorded at a CF with an IC50 value of 4,226.41332 ± 81.5639287 μg/ml.
The fractions showed better scavenging capacity than the CE, except for the CF. IC50 values of all these compounds were higher than that of BHA and ascorbic acid where IC50 was achieved at 83.34988 ± 4.1495129 μg/ml and 83.1162377 ± 3.74982633 μg/ml, respectively..
3.3.2. Ferric reducing power (FRP)
The antioxidant capacity of this method depends on the mechanisms which measure the transformation of a ferricyanide complex (Fe3+) to the ferrous (Fe2+) form by granting an electron according to the chemical reaction (Eq. 1) [23]:
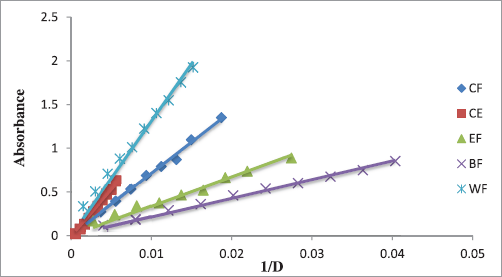
Figure 3 shows the reducing power capacities of extracts of L. resedifolia expressed as absorbance in terms of the inverse of the dilution factor.
 | Figure 3: Reducing power capacities of L. resedifolia’s extracts. [Click here to view] |
 | Figure 4: Total antioxidant capacity of L. resedifolia’s extracts. [Click here to view] |
Ferric reducing capacity of the various extracts of L. resedifolia varied between 17.772 ± 0.722 mM and 156.799 ± 22.795 mM. EF had the best-reducing capacity with a value of 156.799 ± 22.795 mM, followed by WF and CE with values of 116.701 ± 2.414 Mm and 112.126 ± 0.483 mM, respectively. The lowest reducing capacity was registered in BF and CF (45.922 ± 2.158 and 17.772 ± 0.722 mM, respectively). All extracts showed a ferric reducing capacity bested than BHT and BHA (1.960 ± 0.031 and 1.126 ± 0.041 mM, respectively).
3.3.3. Total antioxidant capacity
The total antioxidant capacity is based on the reduction of molybdenum hexavalent oxidation state Mo (VI) to molybdenum pentavalent Mo (V) by the effect of the electron donor by the antioxidant and formation of molybdenum complex colored green to acid pH, according to the chemical reaction (Eq. 2) [23]:
Figure 4 shows the total antioxidant capacity of L. resedifolia’s extracts expressed as absorbance in terms of the inverse of the dilution factor.
Total antioxidant capacity of the different extracts of L. resedifolia varied between 25.778 ± 0.721 mM and 157.928 ± 1.270 mM. WF and CE had the best-reducing capacity (157.928 ± 1.270 mM and 125.763 ± 8.119 mM, respectively), followed by CF with a value of 86.758 ± 7.748 mM. The lowest reducing capacity was registered in EF and BF (40.807 ± 0.367 mM and 25.778 ± 0.721 mM, respectively). All extracts showed a ferric reducing capacity better than BHT and BHA (1.211 ± 0.171 mM and 0.535 ± 0.034 mM, respectively).
The antioxidant capacity demonstrated by these methods used in this study possibly mainly due to the existence of phenolic compounds in these polar extracts. Thus, in a study conducted earlier, the phytochemical studies of this genus showed that this genus was rich in phenolics, flavonoids, and tannins [24–27]. In addition, BF of the aerial parts of L. resedifolia gave four flavonoids [8]. Finally, it is well known that the presence of polyphenolic compounds increases antioxidant activities [22].
4. CONCLUSION
The various extracts of L. Resedifolia were examined for their phytochemical contents and antioxidant capacities. The results obtained from this present work showed that the extracts of L. resedifolia have high antioxidant capacity. The strong antioxidant capacity of L. resedifolia extracts’ shown in this study encourages further studies such as anti-corrosion [28] and anti-bacterial activity; isolation and identification of active compounds present in these extracts.
REFERENCES
1. Quezel P, Santa S. Nouvelle flore de l’Algérie et des régions désertiques méridionale. Tome 2. Ed CNRS, Paris, France, 1963.
2. Gherraf N, El-Bassuony AA, Zellagui A, Rhouati S, Ahmed AA, Ouahrani MR. Isolation of coumarins and coumarin glucoside from Launaea resedifolia. Asian J Chem 2006;18(3):2348.
3. Ozenda P. Flore du Sahara septentrional. Ed CNRS, Paris, France, 1983.
4. Zellagui A, Gherraf N, Ladjel S, Hameurlaine S. Chemical composition and antibacterial activity of the essential oils from Launaea resedifolia L. Organ Med Chem Lett 2012;2(1):2. CrossRef
5. Auzi ARA, Hawisa NT, Sherif FM, Sarker SD. Neuropharmacological properties of Launaea resedifolia. Rev Brasil Farmacogn 2007;17(2):160–5. CrossRef
6. Rashid S, Ashraf M, Bibi S, Anjum R. Insecticidal and cytotoxic activities of Launaea nudicaulis (Roxb.) and Launaea resedifolia (Linn.). Pak J Biol Sci 2000;3(5):808–9. CrossRef
7. El-Bassuony AA, Abdel-Hamid N. Antibacterial coumarins isolated from Launaea resedifolia. Ð¥Ð¸Ð¼Ð¸Ñ Ñ€Ð°Ñтительного ÑÑ‹Ñ€ÑŒÑ 2006;1: 65–8.
8. Moussaoui F, Zellagui A, Segueni N, Touil A, Rhouati S. Flavonoid constituents from Algerian Launaea resedifolia (OK) and their antimicrobial activity. Records Nat Prod, 2010;4(1):91–5.
9. El-Bassuony A, Kabbash A. Biological activity of Coumarins from Launaea resedifolia. Pharmacogn Mag 2008;4(16):249.
10. Saleh M, Habib A, Al-Ghazooly M, Ghabar D, Al-Fiky F. Chemical constituents from Launaea resedifolia. Egypt J Pharm Sci 1988;29:507–13.
11. Giner RM, Díaz J, Máñez S, Recio MC, Soriano C, Ríos J. Phenolics of Spanish Launaea species. Biochem Systemat Ecol 1992;20(2):187–8. CrossRef
12. Abd-el-Fattah H, Zaghloul A, Halim A, Waight E. Steroid and triterpenoid constituents of Launaea resedifolia (L.) Kuntze. Egypt J Pharm Sci 1990;31(1–4):81–91.
13. Bitam F, Ciavatta ML, Manzo E, Dibi A, Gavagnin M. Chemical characterisation of the terpenoid constituents of the Algerian plant Launaea arborescens. Phytochemistry 2008;69(17):2984–92. CrossRef
14. Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem 2004;84(3):329–39. CrossRef
15. Belguidoum M, Dendougui H, Kendour Z. In vitro antioxidant properties and phenolic contents of Zygophyllum album L. from Algeria. J Chem Pharm Res 2015;7(1):510–4.
16. Wang H, Gao XD, Zhou GC, Cai L, Yao WB. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem 2008;106(3):888–95. CrossRef
17. Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 1998;46(10):4267–74. CrossRef
18. Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Composit Anal 2006;19(6–7):669–75. CrossRef
19. Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol 2007;40(2):344–52. CrossRef
20. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 1999;269(2):337–41. CrossRef
21. Nagalapur SK, Paramjyothi S. In vitro antioxidant activity of ctivity of ctivity of launaea pinna unaea pinna unaea pinnatifida cass leaves. In Vitro 2010;5(1):105–8.
22. Belboukhari M, Cheriti A, Belboukhari N. In vitro antioxidant activity of Launaea nudicaulis (Asteraceae) growing in Southwest of Algeria. Ann Sci Technol 2014;6(1):52–5. CrossRef
23. Keffous F, Belboukhari N, Sekkoum K, Djeradi H, Cheriti A, Aboul-Enein HY. Determination of the antioxidant activity of Limoniastrum feei aqueous extract by chemical and electrochemical methods. Cogent Chem 2016;2(1):1186141. CrossRef
24. Misonge JO, Kinyanjui JG, Kingori WM, Mwalukumbi JM. Phytochemical screening and cytotoxicity evaluation of Launaea cornuta H. (Asteraceae) using brine shrimp. Merit Res J Med Med Sci 2015;3(4):116–20.
25. Koukoui O, Agbangnan P, Boucherie S, Yovo M, Nusse O, Combettes L, et al. Phytochemical study and evaluation of cytotoxicity, antioxidant and hypolipidemic properties of Launaea taraxacifolia leaves extracts on cell lines HepG2 and PLB985. Amer J Plant Sci 2015;6(11):1768–79. CrossRef
26. Arawande JO, Amoo IA, Lajide L. Chemical and phytochemical composition of wild lettuce (launaeataraxacifolia). J Appl Phytotechnol Environ Sanitat 2013;2(1):25–30.
27. Adinortey MB, Sarfo JK, Quayson ET, Weremfo A, Adinortey CA, Ekloh W, et al. Phytochemical screening, proximate and mineral composition of Launaea taraxacifolia leaves. Res J Med Plants 2012;6(2):171–9. CrossRef
28. Dekmouche M, Saidi M, Hadjadj M, Ghiaba Z, Yousfi M. Green approach to corrosion inhibition by ethyl acetate extract from Pistacia atlantica gals in hydrochloric acid solution. Int J Elect Sci 2014;9(7):3969–78.