1. INTRODUCTION
Chilli peppers (Capsicum spp.) are known to be the most important spice crop all across the world and India is one of the richest centers of pepper diversity. The genus Capsicum of the Solanaceae family consists of approximately 25 wild and 5 domesticated species [1]. Among the 5 species namely C. annuum L., C. chinense Jacq., C. frutescens L., C. baccatum L., and C. pubescens, the first three are cultivated extensively in India. The crop although is self-pollinating, the rates of out-crossing (7% to 91%) recorded by several investigators argue that Capsicum should be considered to be facultative cross-pollinating species in field research [2,3,4,5].
The pungency of flavor is one typical attribute for which all pepper plants are valued commercially. It is produced by capsaicinoids, the alkaloid compounds that are found only in the genus Capsicum [6]. Capsaicin is the principle capsaicinoid. Pepper sprays containing capsicum oleoresin provide ingredients for a non-lethal deterrent to some human and animal behavior and are useful riot control agents and self-defense tools [7]. Capsaicin is being used to alleviate pain. Its mode of action is thought to be from nerve endings releasing a neurotransmitter called substance P [2]. Lota Bhot, a C. chinense variety indigenous to Assam, is known for its extreme pungent nature. Despite having tremendous commercial value, very little scientific progress has been made on it. In addition to that, capsaicin content has been deteriorating day by day due to cross-pollination and genetic mutation. In this context, it is considered as important as to conserve the original germplasm and maintain a constant level of capsaicin through biotechnological intervention, more precisely, micropropagation technique.
A number of literature is available on in vitro propagation of various Capsicum species. Several of these reports suggest a strong influence of genotype and media on the regeneration process [8,9,1,10]. The function of silver nitrate (AgNO3) in the regeneration of micropropagated plantlets has been thoroughly analyzed in several crops including Capsicum. Addition of AgNO3 to the culture medium induced shoot regeneration in distal cotyledons of cucumber [11], enhanced callus development in Brassica [12] and improved embryogenic callus frequency in immature wheat embryos [13], symptoms like hyperhydricity and leaf epinasty caused by ethylene disappeared when potato cultivars were cultured in medium supplemented with AgNO3 [14]. It is by far known that AgNO3 is a potent ethylene inhibitor. In C.frutescens, the combined effect of AgNO3 and CoCl2 caused extensive flower induction and shoot proliferation in in vitro raised plantlets [15]. Another vital nutrient source which is widely used in plant tissue culture is glutamine. It is supplemented as an organic nitrogen source [16]. The inclusion of glutamine in the callus induction medium has been shown to promote callusing in rice [16,17,18,19]. However, there is no single report till date in Lota Bhot, of a standard in vitro regeneration protocol elucidating the role of glutamine.
Therefore keeping the above points in mind, an experiment was designed to optimize a regeneration protocol in the said variety and analyze the functions of AgNO3 and glutamine throughout the process of in vitro organogenesis. Much emphasis was given on the reproductive phase i.e. in vitro flower induction and fruit development. Direct regeneration via seeds and shoot tips was followed considering the facts that seeds show a high percentage of germination and meristems are actively dividing cells with less pathogenic susceptibility.
2. MATERIALS AND METHODS
2.1. Explant Collection and Disinfection
2.1.1. Seed explants
Healthy seeds of C. chinense variety ‘Lota Bhot’ cultivated in the experimental area under the Division of Biotechnology at Defence Research Laboratory (DRL), Tezpur, were collected and chosen for the said investigation. Seeds were placed and thoroughly washed under running tap water for about an hour followed by fungicidal treatment with 0.1% Bavistin (w/v) (Biostadt India Limited) for another 30 minutes. Thereafter seeds were washed properly with distilled water 6-7 times and brought inside the laminar airflow cabinet for decontamination by rinsing with 70% ethyl alcohol (3-5 seconds), followed by surface sterilization with an aqueous solution of 0.1% HgCl2 (w/v) (Himedia, India) for 3 minutes. Surface sterilized seeds were rinsed 8-10 times with double distilled water to remove any traces of HgCl2.
2.1.2. Shoot tip explants
The young healthy shoot tips (1.5-2 cm) trimmed from 2-year-old mother plant (maintained in the experimental area at DRL, Tezpur) were collected and washed thoroughly with tap water for an hour. Leaves were treated with fungicide 0.1% Bavistin (w/v) for around 15 minutes and rinsed (6-7 times) with distilled water before they were taken to the laminar air flow cabinet. Decontamination was carried out in 70% ethanol for 3 seconds which was followed by surface sterilization with an aqueous solution of 0.1% HgCl2 (w/v) (Himedia, India) for another 2-3 minutes. Subsequently, leaves were rinsed thoroughly with double distilled water to remove any traces of HgCl2.
2.2. Explant Preparation, Inoculation and Culture Conditions
Surface sterilized seeds and shoot tips were aseptically inoculated onto Murashige and Skoog’s (MS) medium [20] [Plate 1(a), (b)]. The medium was supplemented with no plant growth hormones. The basal MS medium was augmented with 3% (m/v) sucrose (Himedia, Mumbai, India) and solidified by 0.8% (m/v) agar. The pH of all the media was maintained at 5.8 and autoclaved at 121°C and 15 pounds per square inch pressure for 15 minutes prior to inoculation. The cultures were incubated at a temperature of 25 ± 2°C and 16-hour photoperiod using cool white fluorescent tubes.
2.3. Multiple Shoot Proliferation
To encourage multiple inductions, the in vitro regenerated primary shoots from both the explants (seed and meristems) were isolated, rinsed with double distilled water and excised into 1-1.5 cm long shoots. The shoots were transferred onto fresh MS medium supplemented with plant hormones: N6-benzylaminopurine (BAP) (2-4 mg/L), 1-naphthaleneacetic acid (NAA) (1.5 mg/L) with/without AgNO3 (2.5-3.5 mg/L) and glutamine (1 mg/L). Healthy shoots were sub-cultured thrice onto the medium with the same hormonal composition at an interval of 4 weeks. The cultures were kept at a temperature of 25 ± 2°C and 16-hour photoperiod.
2.4. In Vitro Flower Induction and Fruit Development
For induction of flower under in vitro condition, healthy shoot buds were selected from six-week-old cultures and shifted to flower induction medium containing MS supplemented with BAP (3.5 mg/L), GA3 (2-6 mg/L), glutamine (0.5 mg/L) and enriched with/without AgNO3 (3.5-4.5 mg/L). In vitro flowering was observed after 35 days of inoculation. Terminal shoots with in vitro flowers were carefully transferred to fruit induction medium which consists of MS fortified with AgNO3 (3.5-4.5 mg/L) and with/without glutamine. All cultures were subjected to a 16/8 h light/dark cycle for fruit development.
2.5. In Vitro Root Induction and Acclimatization
In vitro raised shoots were successfully transferred to rooting medium containing indole-3-butyric acid (IBA) (0.5-2 mg/L) along with BAP (2.5 mg/L), NAA (0.5-2 mg/L), glutamine (1 mg/L) and with/without AgNO3 (1.5-3 mg/L). Culture conditions were kept same as described in previous section (section 2.4). After successful root initiation, plantlets were carefully taken out of the medium and washed gently under tap water to remove any traces of hormones, agar, and medium. For acclimatization of in vitro raised plantlets, plastic cups containing sterile soil and vermicompost (1:1) were used and plantlets were carefully transferred to plastic cups thereafter taken to polyhouse. The cups were covered with polythene bags initially for 10-15 days. To maintain high relative humidity, plantlets were watered once in three days with sterile distilled water, after which humidity was reduced by making small perforations in the polythene bags. Twelve-week-old hardened plants were finally transferred to the open field. The percentage of plant survival was recorded.
2.6. Statistical Analysis
All the experiments were conducted with Complete Randomized Block Design (CRD) and repeated thrice with each treatment having 10 replicates. Significance between treatments was calculated using One-Way ANOVA and differences among different treatment means were based on Turkey’s Honesty Significant difference (HSD at 0.05).
3. RESULTS AND DISCUSSION
3.1. In Vitro Proliferation and Induction of Multiple Shoots
Our investigation revealed a linear relationship between multiple shoots proliferation from seed explants of C. chinense variety Lota Bhot and concentrations of different plant growth promoting substances [Table 1]. The highest number of multiple shots (7.5 ± 0.52) (mean value) was recorded in the treatment where BAP (3 mg/L) and NAA (1.5 mg/L) were combined with AgNO3 and glutamine at concentrations 3 mg/L and 1 mg/L respectively [Fig. 1(c)] The same treatment also generated the longest shoot of about 7.2 cm length. In the whole experiment, the concentration of NAA was kept fixed at 1.5 mg/L. A slight alteration in its concentration drastically affected multiple shoot induction. The shoots observed were unhealthy and short when cultured on medium containing NAA at a concentration higher than 1.5 mg/L. This result contradicts the findings of song et al.[21]. According to the authors, NAA at higher concentration exerts a positive impact not only on shoot proliferation but also the percentage of shoot regeneration. This discrepancy might have occurred owing to differences in responsiveness of pepper genotypes. Moreover, excessive auxin concentration might have caused an endogenous hormonal imbalance which in turn had drastically reduced shoot length and proliferation. The treatment comprising of BAP (2 mg/L) and NAA (1.5 mg/L) without any incorporation of AgNO3 and glutamine generated the lowest number of multiple shoot (2.3 ± 0.21). Data presented in Table 1 indicate that the highest regeneration percentage (96.8%) from shoot tip explants was recorded in treatment where BAP and AgNO3 concentrations were slightly increased to 4 mg/L and 3.5 mg/L respectively, keeping the other values same as the previous treatment. Withdrawal of AgNO3 and glutamine from the medium reduced the percentage to 58.9%.
 | Table 1: Effects of various media composition on in vitro regeneration, multiple shoot induction and shoot length on seed explants of Lota Bhot. [Click here to view] |
 | Table 2: Effects of various media composition on in vitro regeneration, multiple shoot induction and shoot length on shoot tip explants of Lota Bhot. [Click here to view] |
Data obtained from shoot tip explants slightly differed from the one obtained from seed explants [Table 2]. According to our experiment MS medium supplemented with BAP (4 mg/L), NAA (1.5 mg/L), AgNO3 (3.5 mg/L) and glutamine (1 mg/L) generated a maximum of 8.3 shots (mean value) per shoot tip and highest percentage of shoot regeneration (98.3%). The same treatment also generated the longest shoot of 7.6 cm. On the other hand, the number of multiple shoots got reduced to a mean of 3.2 ± 0.52 in the absence of AgNO3. Similarly, the regeneration percentage dropped down to 50% when both AgNO3 and glutamine were taken out of the medium.
 | Figure 1: In vitro propagation of C. chinense variety Lota Bhot (a) Seed explant (b) Shoot tip explant (c) Multiple shoot induction (d) Flower Induction (e) Fruit Formation (f) Root induction (e) Ex vitro acclimatization (Soil + vermicompost in 1:1 ratio). [Click here to view] |
The only preferred cytokinin used in the entire investigation was BAP. However, initially, the experiment was conducted with Thidiazuron (TDZ) and the combination of BAP and TDZ. But the results obtained were not very convincing. Plantlets cultured on a medium supplemented with TDZ (with/without auxin) were produced short with thin shoots which would easily fall off. Our observation complies with the result obtained by Ostroshy et al. [22]. In their report, authors claimed BAP to have the best cytokinin activity at a concentration around 2 mg/L when applied without auxin. In the said investigation, BAP at a slightly higher dose (3-4 mg/L) generated highest multiple shoot induction in plantlets derived from seed and shoot tip explants. This marginal difference in BAP concentrations might have occurred due to recalcitrant nature of pepper genotypes. On the other hand, the positive response of TDZ towards shoot induction and proliferation of different Capsicum species has been cited in the experiments conducted by Song et al. [21], Santana-Buzzy et al. [23], Kehia et al. [24] and Bhutia et al. [25].
The principal components for the said investigation that regulated the in vitro regeneration processes were AgNO3 and glutamine. Several sets of experiments were set up with/without AgNO3. Data presented in Table 1 and Table 2 clearly showed the positive response of AgNO3 towards in vitro shoot proliferation and percentage of shoot induction. Considering multiple shoot induction from seed explants [Table 1], the highest number of shoots per explant (7.5 ± 0.52) was obtained when AgNO3 at 3.0 mg/L was combined with glutamine (1 mg/L) along with BAP and NAA at 3 mg/L and 1.5 mg/L respectively. The same set of treatment also generated the longest shoot of approximately 7.2 cm. However, the percentage of shoot induction was found the maximum (96.8%) at a slightly higher concentration (3.5 mg/L) of AgNO3. Results obtained from shoot tip explants [Table 2] also followed a similar pattern. Shoot tips responded best in terms of multiple shoot induction, shoot length and shoot induction percentage in the medium supplemented with BAP (4 mg/L), NAA (1.5 mg/L) AgNO3 (3.5 mg/L) and glutamine (1 mg/L). In the said treatment approximately a mean of 8.3 numbers of shoots were produced from a single explant, which is quite a satisfactory figure. The shoots attained a length of 7.6 cm with a very high percentage (98.3%) of shoot induction. In both the cases (seed and shoot tips), withdrawal of AgNO3 from shoot proliferation medium, caused a drastic reduction in the number of multiple shoot induction and shoot regeneration percentage [Table 1 and Table 2]. AgNO3, an ethylene inhibitor in the plant tissue culture system, is found to be an essential component in the induction and elongation of shoots in pepper [26,27]. In the absence of AgNO3, the number of multiple shoots from seed and shoot tips was reduced to (mean value) 2.6 ± 0.61 and 3.2 ± 0.52 respectively. Silver ions are known to protect the plants from senescence caused by ethylene thus preventing the shoots from falling off. A similar trend was observed in the work of Sheeja and Mandal [28] in tomato, Anantasaran and Kanchanapoom [29] in Zinnia, Sandra and Maira [14] in potato and Ashrafuzzaman atal. in Capsicum species[27].
Our investigation also examined and focused on the role of glutamine in the process of shoot regeneration and multiple shooting. Glutamine has proved to be a good source of nitrogen supply in various in vitro processes. Glutamine supports cell growth which has high energy demands and synthesizes large amounts of nucleic acids, proteins etc. Exogenous supply of glutamine is found beneficial in tissue culture as it increases biomass of explants and rate of regeneration of in vitro plantlets. Pawar et al. [16] observed very constructive response of glutamine towards callus formation and shoot proliferation in rice. However, in our study we were not able to see any strong correlation between glutamine and shoot proliferation. Exclusion of glutamine did affect the regeneration process in seed explants to certain extent but failed to generate positive impact on shoot multiplication from shoot tips. Therefore, it could be suggested that not all explants show similar response but sensitivity towards glutamine varies from explants to explant within the same genotype. Another important observation was reported by Shahsavari [30] in rice. In the report the author claimed that glutamine did support callus induction but could not enhance shoot regeneration suggesting that juvenile stage requires more nitrogen supply than does the mature stage. Conversely, such observation was not recorded in our investigation.
3.2. In Vitro Flower and Fruit Induction
The most crucial period of any in vitro regeneration process is the reproductive phase i.e. flower formation and fruit development stage. Data shown in Table 3 and Table 4 represented direct correlation between growth hormones and induction of flower buds and formation of fruits. We replaced NAA with GA3. Concentration of BAP was kept constant at 3.5 mg/L throughout the experiment. From the data shown in Table 3 highest production of flower per seed explant (8.4 ± 0.53) was recorded in the treatment where GA3 at 2 mg/L was supplemented along with AgNO3 and glutamine at 3.5 and 0.5 mg/L respectively [Fig. 1(d)]. However, maximum fruit formation per explant (8.1 ± 0.21) was seen in the treatment when doses of AgNO3 and GA3 were slightly raised to 4 mg/L each [Fig. 1(e)]. All flowers were off-white which remained open for almost two weeks. Poorest results in terms of flower induction (2.0 ± 0.72) and fruit formation (2.0 ± 0.94) were obtained when AgNO3 was withdrawn from the medium. Exactly similar trend was noticed in case of shoot tip explants. Figures shown in Table 4 represent the status of reproductive stages of C. chinense variety Lota Bhot cultured through shoot tips. A mean of 9.5 flowers blossomed under in vitro condition from a single shoot tip when cultured onto a medium supplemented with 3.5 mg/L and 2 mg/L of AgNO3 and GA3 respectively whereas a highest of 8.6 fruits (mean value) was obtained at moderately higher concentrations of AgNO3 and GA3 (4 mg/L each). A sharp reduction in the number of flowers (2.1 ± 0.59) and fruits (2.5 ± 0.86) was seen with the removal of AgNO3 from medium. This clearly reflected the importance of AgNO3 on reproductive stages. AgNO3 has been reported to inhibit ethylene action [15,31]. The exact mechanism of AgNO3 mediated ethylene production and its activity regulation is unclear but it has been explained by an interference of ethylene perception or stress exerted by silver ion [15]. Silver nitrate is an ethylene action inhibitor and ethylene inhibits S-adenosyl methionine decarboxylase, which in turn promotes polyamine levels, which are implicated in flowering [15,32]. There have been reports confirming the significance of AgNO3 on in vitro processes in C.Annuum [27], C. frutescens [15] and C. chinense [33]. Alongside AgNO3, role of glutamine was also analyzed. Glutamine, although was found to have played a significant role in vegetative phase, its effect on reproductive phase (flowering and fruiting) was not satisfactory in our case. It is well known that nitrogen is required for initial growth and development of plant. At maturity, its requirement is not very much needed. In our study, addition/withdrawal of glutamine did not make any significant difference.
 | Table 3: Effects of various media composition on in vitro flower and fruit formation of seed explants of Lota Bhot. [Click here to view] |
3.3. In Vitro Root Induction and Acclimatization
In vitroroot induction experiment was conducted keeping BAP concentration unaltered at 2.5 mg/L. Unlike the previous experiments where only one type of auxin was used, multiple root induction was achieved in the presence of two auxins; NAA and IBA. Data obtained from both seed and shoot tips followed quite a similar pattern of regeneration [Fig. 1(f)]. As per figures shown in Table 5 and Table 6, maximum in vitro root initiation occurred in medium fortified with AgNO3 (2.5 mg/L) and glutamine (1 mg/L). Unlike in shoot multiplication and elongation, glutamine failed to show any profound effect on root development. While shoot tips generated 67.3 roots/explants, plantlets obtained from seed explants produced 63.3 roots within 14 days after being transferred to rooting medium. Percentage of root induction was fairly high in both the experiments. Shoot tip explants showed better response than seed explants. Regeneration percentage of shoot tips was 98.6% whereas it was slightly less (96.6%) in plantlets derived from seeds. Our study revealed positive impact of AgNO3 on root induction. However, our findings were not in accordance with the work done by Anantasaran and Kanchanapoom [29] where the authors reported that addition of AgNO3 reduced the percentage of rooting in Zinnia cultivars. The results obtained from the said investigation complied with that of Ebida and Hu [34] where the authors confirmed in their report that incorporation of AgNO3 into MS medium enhanced regeneration in micropropaged plantlets of C. annuum cv. Early California Wonder. Such variations in results might be due to differences in genotypes and probably explants. As far the auxins were concerned, both NAA and IBA proved to be almost equally considerable for root development. While some researchers preferred IBA over NAA and Indole 3-Acetic acid (IAA) [1,22,35,36,37,38], others reported to have received better response from NAA and IAA [39,40].
 | Table 4: Effects of various media composition on in vitro flower and fruit formation of shoot tip explants of Lota Bhot. [Click here to view] |
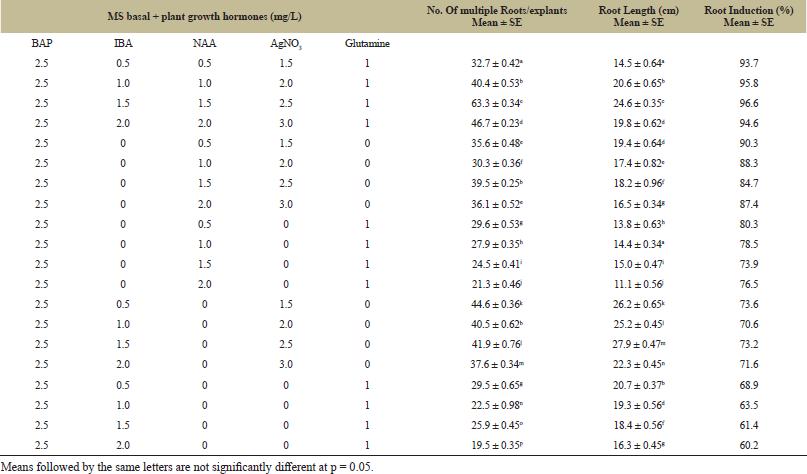 | Table 5: Effects of various media composition on the percentage of regenerated roots, in vitro root induction and root length of seed explants of Lota Bhot. [Click here to view] |
After successful root induction, in vitro rooted plantlets were transferred to the poly house for hardening and acclimatization. Substrates containing sterile soil and vermicompost generated best results [Fig. 1(g)]. Percentage of plant survival from seed was 78% while it was slightly higher (82%) in the case of plantlets derived from shoot tips. According to Azad et al. [41], organic farming with the inclusion of biofertilizers in the long-run can be considered an important contributor to food security. Ponti et al. [42] further stated that the difference in crop yields under organic and conventional production systems is 20% depending on the crops and regions. As per the reports of Bhat et al. [43], use of good quality compost and biofertilizers stimulate the activity of heterotrophic microbes present in the rhizosphere region where it mineralizes nutrients, particularly nitrogen in the incorporated organic fertilizers, thus making them available to the plants. Additionally, it improves soil texture, reduces bulk density and increases the available water content.
 | Table 6: Effects of various media composition on the percentage of regenerated roots, in vitro root induction and root length of shoot tip explants of Lota Bhot. [Click here to view] |
 | Figure 2: Response of explants (seed and shoot tip) of Lota Bhot towards various in vitro processes. (a) Shoot induction (b) flower induction, (c) fruit induction (d) root induction (e) acclimatization. [Click here to view] |
In the entire investigation, the results achieved from shoot tip explants were better than seed explants [Fig. 2]. Shoot tips, because of the presence of actively dividing meristematic zone, were found to be highly responsive towards various in vitro processes. Moreover, the chances of pathogenic contamination were less in the cultures derived from shoot meristems. Although the seed explants used in the investigation possessed good germination potential and high multiplication rate, the evidence of fungal contamination was most prevalent in seed cultures.
4. CONCLUSIONS
An attempt was made through our investigation to elucidate the importance of AgNO3 and glutamine on in vitro regeneration of Lota Bhot, an indigenous fiery chilli pepper of Assam. In the entire course of micropropagation, effects of AgNO3 were found influential. However, the same could not be said in the case of glutamine. Although glutamine did improve in vitro shooting, it failed to generate a positive influence on flower induction, fruit development and in vitro root induction. The response of meristems was found to be better than seed explants. Lota Bhot, being one of the hottest chillies, has tremendous economic and research potential as food components, medicines, and pharmaceuticals. Unfortunately, a tissue culture of this chilli is still in its infancy. The present investigation opens a way to get into the insights of micropropagation techniques in this particular chilli pepper.
5. ACKNOWLEDGMENTS
We thank the Director, Defence Research Laboratory (DRL), Tezpur, Assam for showing a keen interest in the work and providing a DRDO fellowship. The DRL, Tezpur, and Department of Biotechnology, Gauhati University were duly acknowledged for providing the necessary facilities and permission to conduct the investigation.
6. REFERENCES
1. Sanatombi K, Sharma GJ. Micropropagation of Capsicum annumL. using axillary shoot explants. Sci Hort 2007; 113:96-99.
2. Bosland PW. 1996. Capsicums: Innovative uses of an ancient crop. In: Janick J (ed.), Progress in new crops. Arlington; 479-487.
3. Odland ML, Porter AM. A study of natural crossing in pepper (Capsicum frutescens L.). J. Am Soc Hort Sci 1941; 38:585-588.
4. Franceschetti U. Natural cross pollination in pepper (Capsicum annuum L.). Proc. Eucarpia Meeting on Genetic and Breeding of Capsicum. Turin, Italy; 1971; 346-353.
5. Tanksley SD. High rates of cross-pollination in chile pepper. Hort Sci 1984; 19:580-582.
6. Bosland PW, Votava EJ. Peppers: vegetables and spice Capsicums. Crop Production Science in Horticulture. England; 2000; 12:204.
7. De AK. Capsicum: The Genus Capsicum. Medicinal and aromatic plants. Industrial Profiles Taylor & Francis, London and New York; 2003; 33:275.
8. Ramage CM, Leung DWM. Influence of BA and sucrose on the competence and determination of Pepper (Capsicum annumL var. Sweet Banana) hypocotyl cultures during shoot formation. Plant Cell Rep1996; 15:974-979.
9. Franck-Duchenne M, Wang Y, Tahar SF, Beachy RN. In vitro stem elongation of sweet pepper in media containing 24-epi-brassinolide. Plant Cell Tiss Org Cult 1998; 53:79-84.
10. Mihalka V, Fari M, Szasz A, Balazs E. Optimized protocols for efficient regeneration and gene transfer in pepper (Capsicum annuum L.). J Plant Biotechnol2000; 2:143-149.
11. Mohiuddin AKM, Chowdhury MKU, Zaliha C, Abdullah, Napis S. Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tiss Org Cult 1997; 51:75-78.
12. Williams J, Pink DAC, Biddington NL. Effect of silver nitrate on long-term culture and regeneration of callus from Brassica oleracea var. gemmifera. Plant Cell Tiss Org Cult 1990; 21:61-66.
13. Wua LM, Weia YM, Zhenga YL. Effects of silver nitrate on the tissue culture of immature wheat embryos. Rus J Plant Physiol 2006; 53:530-534.
14. Sandra AT, Maira O. Effect of culture medium consistence and silver nitrate on micropropagation of two potato (Solanum tuberosum) cultivars. Rev Colomb Biotechnol 2013; 2:55-62.
15. Sharma A, Kumar V, Giridhar P, Ravishankar GA. Induction of in vitroflowering in Capsicum frutescensunder the influence of silver nitrate and cobalt chloride and pollen transformation.Electr J Biotech 2008; 11:1-6.
16. Pawar B, Kale P, Bahurupe J, Jadhav A, Kale A, Pawar S. Proline and glutamine improve in vitrocallus induction and subsequent shooting in rice. Sci Dir Rice Sci 2015; 22:283-289.
17. Chowdhry CN, Tyagi AK, Maheshwari N, Maheshwari SC. Effect of L-proline and L-tryptophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativaL. cv. Pusa 169). Plant Cell Tiss Org Cult 1993; 32:357-36.
18. Ge XJ, Chu ZH, Lin YJ, Wang SP. A tissue culture system for different germplasms of indica rice. Plant Cell Rep 2006; 25:392-402.
19. Shahsavari E. Impact of tryptophan and glutamine on the tissue culture of upland rice. Plant Soil Environ 2011; 57:7-10.
20. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962; 15:473-97.
21. Song JY, Sivanesan I, An CG, Jeong BR. Adventitious shoot regeneration from leaf explants of miniature paprika (Capsicum annuum) ‘Hivita Red’ and ‘Hivita Yellow’. Afr J Biotechnol 2010; 9:2768-2773.
22. Otroshy M, Moradi K, Nekouei MK. The effect of different cytokenins in propagation of Capsicum annuumby in vitronodal cutting. Trakia J Sci 2011; 9:21-30.
23. Buzzy NS, Flick AC, Perez FB, Peniche MCM, Castillo PYZ, Ruiz AS, Colli AZ, Alonso OG, Ham MLM. Regeneration of Hebenero pepper (Capsicum chinense J.) via organogenesis. HortSci 2005; 40:1829-1831.
24. Kehie M, Kumaria S, Tandon P. In vitro plantlet regeneration from nodal segments and shoot tips of Capsicum chinense Jacq. cv. Naga King Chili. Biotech 2012; 2:31–35.
25. Bhutia KL, Meetei NGT, Khanna VK. In vitro regeneration of Dalle Khursani, an important chilli cultivar of Sikkim, using various exlants. Agrotechnol 2016; 5. DOI: 10.4172/2168-9881.1000142.
26. Hyde CL, Phillips GC. Silver nitrate promotes shoot development and plant regeneration of chili pepper (Capsicum annuumL) via organogenesis. In Vitro Cell Dev Biol Plant 1996; 32:72-80.
27. Ashrafuzzaman M, Hossain MM, Ismail MR, Haque MS, Shahidullah SM, Zaman S. Regeneration potential of seedling explants of chilli (Capsicum annuum). Afr J Biotechnol 2009; 8:591-596.
28. Sheeja TE, Mandal AB. In vitro flowering and fruiting in tomato (Lycopersicon esculentum Mill.). AsPac J Mol Biol Biotechnol 2003; 11:37-42.
29. Anantasaran J, Songklanakarin KK. Influence of medium formula and silver nitrate on in vitroplant regeneration of Zinniacultivars. J Sci Technol 2008; 30:1-6.
30. Shahsavari E. Impact of tryptophan and glutamine on the tissue culture of upland rice. Plant Soil Environ 2011; 57:7-10.
31. Beyer EM. Silver ion: A potent anti-ethylene agent in cucumber and tomato. Hort Sci 1976; 11:175-196.
32. Bais HP, Sudha GS, Ravishankar GA. Putrescine and AgNO3 influences shoot multiplication, in vitroflowering and endogenous titers of polyamines in Cichorium intybus L. cv. Lucknow Local. J Plant Growth Regul 2000; 19:238-248.
33. Bora G, Gogoi HK, Handique PJ. Micropropagation of Capsicumchinensejacq. cv. Lota bhot via indirect organogenesis. Intl J Agri Sci 2014; 6:384-387.
34. Ebida AIA, Hu C. In vitromorphogenic responses and plant regeneration from pepper (Capsicum annumL. cv Early California Wonder) seedling explants. Plant Cell Rep 1993; 13:107-110.
35. Kumari M, Patade VY, Ahmed Z. Establishment of high regeneration potential in sweet pepper (Capsicum annuumL) cultivars Yolo Wonder and California Wonder. World J Sci Technol 2012; 2:26-35.
36. Sanatombi K, Sharma GJ. In vitro propagation of Capsicum chinense Jacq. Biol Plantar 2008; 52:517–520.
37. Gayathri N, Gopalakrishnan M, Sekar T. In vitromicropropagation of Capsicum ChinenseJacq. (Naga King Chili). Pelagia Research Lib 2015; 5:13-18.
38. Hegde V, Partap PS, Yadav RC. Plant regeneration from hypocotyl explants in Capsicum(Capsicum annuumL.). Int J Curr Microbiol App Sci 2017; 6:545-557.
39. Siddique I, Anis M. Thidiazuron induced high frequency shoot bud formation and plant regeneration from cotyledonary node explants of Capsicum annuumL.Ind J Biotechnol 2006; 5:303–308.
40. Bodhipadma K, Leung DWM. In vitrofruiting and seed set of Capsicum annuumL. cv. Sweet banana. In VitroCell Dev Biol –Plant 2003; 39:536-539.
41. Azad H, Schoonbeek S, Mahmoudi H, Derudder B, Maeyer PD, Witlox F. Organic agriculture and sustainable food production system: Main potentials. Agric Ecosyst Environ 2011; 144:92-94.
42. Ponti TD, Rijk B, Martin K, Ittersum V. The crop yield gap between organic and conventional agriculture. Agril Sys 2012; 108:1-9.
43. Bhat TA, Gupta M, Ganai MA, Ahanger RA, Bhat HA. Yield, soil health and nutrient utilization of field pea (Pisum sativum L.) as affected by phosphorus and biofertilizers under subtropical conditions of Jammu. Intl J of Mod Plant Ani Sci 2013; 1:1-8.