1. INTRODUCTION
Polychlorinated biphenyl (PCB) congeners have been widely used since 1930s, mainly in transformers and capacitors. Varying quantities of the PCBs were also used as plasticizers, hydraulic lubricants, flame-retardants, heat-transfer fluids, sealants, pesticide extenders, and in carbonless copy paper [1]. Although the production of PCBs is barred in many countries since 1970s, considerable amount of it is still persistent in the soil, water, and air due to its low degradation rate. The exposure of PCBs to the living system is continuous because of its resistance to biological and chemical degradation and thereby accumulation in the food chain. The widespread use for decades along with improper disposal of these compounds resulted into significant damage to the ecosystems through contaminated soil and water. Reports on the physiological impact of PCB exposure have revealed endocrine disruption and few other physiological impairment [1-4]. Since the PCBs are highly lipophilic and less biodegradable, they bioaccumulate in the lipid-rich tissues of the living organisms, ultimately reaching to human [3,5-7]. PCB exposure was reportedly responsible for a wide range of adverse effects on the endocrine,neurological, metabolic, and reproductive systems of mammals [8]. Experimental reports are available on few of these effects such as hepatotoxicity, endocrine disorders, neurotoxicity, and impaired lipid metabolism [9-14]. Therefore, various information on the cellular and tissue responses of PCBs exposure to different organ systems are very much useful for characterizing the toxicity level as well as the overall health status [15-16]. In general, xenobiotics are known to induce oxidative stress by facilitating free radicals or reactive oxygen species (ROS) [17-19]. Experimental evidences indicated that PCB exposure generates a high amount of ROS in almost all kind of mammalian tissues [20]. Mammalian systems have well-developed physiological antioxidant system involving various antioxidant enzymes such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) [21]. This system is supported by scavengers of free radical such as reduced glutathione (GSH) and vitamins. All these antioxidant systems work together to counter the imposed oxidative stress generated from the excessive production of ROS [22]. Excess ROS is an important toxicological consequence of Aroclor 1254 that can subsequently mediate cytoskeletal disruption and apoptosis of hepatocytes [23]. These free radicals and intermediate products of peroxidation can damage the structural integrity of the cellular membrane systems and thereby alter their functions creating pathological lesions [24]. In the present study, we investigated the toxic effects of a sublethal oral dose of Aroclor 1254 on the antioxidative system and the induced pathological changes in the liver tissue of the male Swiss albino mice.
2. MATERIALS AND METHODS
2.1. Experimental Animal
Male Swiss albino mice of about 30–40 g were used for the experiments, which were conducted as per the ethical norms of CPCSEA (Regd. No. CP6EA/CH/RF/ACK-2003, 29-07-2003).
2.2. Chemicals and Reagents
Aroclor 1254 (CAS No. 11097-69-1) and other fine chemicals and enzyme substrates were procured from Sigma Chemical Company Inc. All other chemicals used were of AR grade from reputed companies.
2.3. Experimental Design
For each experiment, four groups of animals were used. Experiments were conducted with two sub-lethal doses (0.1 and 1 mg/kg/d) and four different exposure durations 7, 14, 21, and 28 days. Each experiment was repeated at least thrice.
2.4. Homogenate Preparation
For the experiments, 10% homogenate of liver tissue was prepared as per Bhor et al. [25]. The obtained supernatant was used for all the assays.
2.5. GPx Activity
The GPx activity was measured as per Rotruck et al. [26] and expressed as μg of GSH consumed/min/mg protein. GSH level was determined as per Ellman [27] and expressed as mg/100 g of the tissue concerned.
2.6. GST Activity
The GST activity was determined as per Habig et al. [28] and expressed as μmoles of GSH-CDNB conjugate formed/min/mg protein using an extinction coefficient of 9.6/mM/cm.
2.7. SOD
The activity of SOD was determined using the specific kit procured from Sigma-Aldrich Ltd. and read the absorbance at 450 nm using microplate reader.
2.8. Protein
Protein was measured by the method of Lowry et al. [29]. The protein content in the tissue extract was used only to calculate the specific activity of the enzymes studied.
2.9. Statistical Analysis
The obtained data from this study were subjected to ANOVA tests as per Sokal and Rohlf [30].
2.10. Histopathological Studies
Samples of the liver tissue from the exposed mice were cleaned, dehydrated in graded serial of alcohol, and embedded in paraffin wax to make tissue blocks [31]. 8–10 µm thick sections were cut and stained in standard hematoxylin and eosin. Properly stained sections were examined for histopathological changes using a Carl Zeiss Axioscop 2 compound microscope. Microphotography was done in the same microscope fitted with CCD color vision camera (DONPISHA XC-003) with Carl Zeiss Axiovision image analyses software.
3. RESULTS
3.1. Effects on Antioxidant Enzymes
GSH levels were significantly declined after the exposure of 0.1 and 1 mg/kg/d dose of Aroclor 1254 for 7, 14, 21, and 28 days, except in 0.1 mg/kg/d dose of Aroclor 1254 exposure for 28 days [Figure 1a]. However, the GST activity was significantly increased after the exposure in 0.1 and 1 mg/kg/d Aroclor1254 for 7, 14, 21, and 28 days (P < 0.05 and P < 0.01) [Figure 1b]. On the other hand, the GPx activity in 0.1 mg/kg/d was increased with increasing exposure duration reaching the maximum after 14 and 21 days exposure (P < 0.05). This was followed by a significant increase in the GPx activity after 28 days exposure (P < 0.01) while, GPx activity in the 1 mg/kg/d group was significantly increased after 7, 14, 21, and 28 days of Aroclor 1254 exposure [Figure 1c]. SOD activity in the 0.1 and 1 mg/kg/d group was significantly increased after 7, 14, 21, and 28 days exposure of Aroclor 1254 (P < 0.05 and P < 0.01) [Figure 1d].
3.2. Hepatic Histopathology
In the present study, mortality of the experimental mice was observed in all the treated groups during the experiment [Figure 2]. Slight pathological changes were observed with the increase in exposure durations after 0.1 mg/kg/ddoses of Aroclor 1254 in the liver tissue of mice as compared to control. However, notable histopathological lesions were visible in mice liver after 1 mg/kg/d dose of Aroclor 1254 with increasing exposure durations [Figure 3]. The details of histopathological changes at various exposure durations after 0.1 and 1 mg/kg/d dose of Aroclor 1254 are given in Table 1.
4. DISCUSSION
Our earlier studies report that Aroclor 1254 was induced significant alterations in the specific activities of few membrane ATPases by indirect way after a relatively short-term exposure in mice liver [32,33]. In the present study, we have examined the way the oxidative stress was induced by oral dosing of Aroclor 1254 leading to structural alterations in the mice liver cells. The important outcome of the present study was that even the sub-lethal doses and very short-term exposures of Aroclor 1254 also induced oxidative stress in the liver tissue of mice that ultimately caused histopathological alterations and necrosis [Figures 2 and 3]. Previous reports have indicated that the PCB generated oxidative stress played a significant role in the onset of the pathological lesions [34]. The higher degree of the ROS generation might have induced oxidative stress and disturbed the normal redox state of the mice [35]. It was reported that the PCBs normally utilize their oxidative effects by oxidation using 1st phase metabolism by oxidases and quinone cycle of the metabolites [36]. It can also be done indirectly by releasing metabolic enzymes induced by the toxic exposure [37]. Plenty of evidences were reported which indicate that the oxidative stress was becoming an important concern in the toxicological effects [38-40]. It was also reported earlier that the production of excess ROS triggered the alteration of the phosphorylation state of hepatocytes as a responses of the antioxidant system [39]. It may also be possible that the antioxidant system reacted to the ROS thereby reducing the oxidative stress using the antioxidant enzymes. SOD catalyzed the conversion of superoxide to H2O2 and the CAT could have reduced the produced H2O2 to H2O [41]. On the other hand, tGpx protects the cell from oxidative damage [42]. The results of the present study showed significant alterations of these antioxidant enzymes which might have relieved the imposed oxidative stress in the liver tissue [Figure 1c and d] to eliminate the over-production of ROS induced by 0.1 and 1 mg/kg/ddose of Aroclor 1254 [43].
 | Figure 1: Effects of oral dosage of 0.1 and 1 mg/kg/dAroclor 1254 on various enzyme activities in the liver of mice. (a) Reduced glutathione, (b) glutathioneS-transferase, (c) glutathione peroxidase, and (d) superoxide dismutase. Data denoted as a mean ± standard error (n = 3). Differences relative to control were statistically significant at P < 0.05 (*) and P Ë‚ 0.01 (**) [Click here to view] |
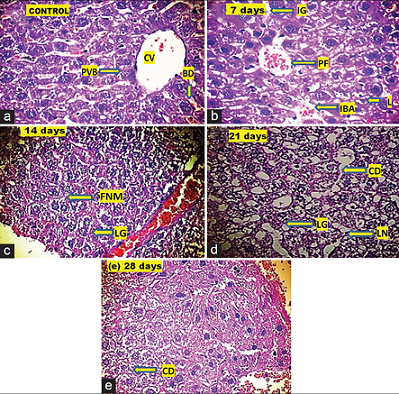 | Figure 2: Photomicrographs (×400) of mice liver tissue from control and treated animals. The photomicrographs are showing the histopathological alterations in the liver of mice after the exposure of 0.1 mg/kg/ddose of Aroclor 1254 for 7, 14, 21, and 28 days as a compared control. (a) Control revealed the normal liver parenchyma with central vein (CV), portal vein branch (PVB), bile duct (BD); (b) after 7 days exposure: Lesions (L), Intravascular blood corpuscles accumulation (IBA), interstitial gap between two adjacent cells (IG), and portal fibrosis (PF); (c) after 14 days exposure: Fragmentation of nuclear material (FNM) and lipid granulomas (LG); (d) after 21 days exposure: Cellular destruction (CD), lysis of nucleus (LN), and lipid granulomas (LG); and (e) after 28 days exposure: Cellular destruction (CD) [Click here to view] |
 | Figure 3: Photomicrographs (×400) of mice liver tissue from control and treated animals. The photomicrographs are showing the histopathological alterations in the liver of mice after the exposure of 1 mg/kg/ddose of Aroclor 1254 for 7, 14, 21, and 28 days as a compared control.(a) Control revealed the normal liver parenchyma with central vein (CV) and portal vein branch (PVB); (b) after 7 days exposure: Intravascular blood corpuscles accumulation (IBA), lipid granulomas (LG), and cellular fibrosis (CF); (c) after 14 days exposure: Nuclear fragmentation associated with intra- and inter-cellular lipid accumulation (LA), and sinusoidal degeneration (SD); (d) after 21 days exposure: Maximum lipid deposition (LD) and nuclear material fragmentation (NMF); and (e) after 28 days exposure: Massive cellular destruction (MCD), complete cellular destruction (CCD), and cellular aggregation (CA) [Click here to view] |
As observed in the present study, an increase in the GST activity [Figure 1b] was perhaps related to the detoxification of ROS produced by the xenobiotics, such as MCs, PCBs, and GSH in the liver cells. These findings are consistent with previously available reports [21,39,44,45]. Further, the Aroclor 1254 exposure might have caused a significant decrease in the glutathione level in the hepatocyte cells [Figure 1a]. However, the GSH, which was probably involved in the toxication of PCBs, perhaps initiated the conjugation reaction to GSH non-enzymatically catalyzed by GST [21]. This GSH, on the other hand, conjugates to aid the excretion of PCBs [36]. It is well supported by the report that xenobiotics caused GSH efflux which, in turn, damages the membranes of the hepatocytes [46]. From the histopathological studies, the observed cytological changes in mice liver appeared to coincide with the significant changes in the intracellular antioxidant system. Therefore, the alterations in the intracellular antioxidant system might have a close relationship with alterations in the tissue morphology in the mice liver to certain extent. It was earlier reported that exposure to microcystins might have caused histopathological changes in the liver, kidney, spleen, heart, and gills tissues of fish [47-49]. Liver is the site of all major metabolic cycles, which produce energy to keep the organism alive. It has been reported that the entry of lipophilic toxicant can cause asphyxia, which, in turn, damage hepatocyte cells [50]. In the present study, a dose and duration dependent histopathological changes in the liver tissue of mice were observed. Most severe hepatic lesions were observed at 21 and 28 days exposure of 1 mg/kg/d dose of Aroclor 1254 [Table 1]. Therefore, the results of the present study revealed an increased severity of the damage with increasing exposure duration, which, possibly indicative of both, direct and indirect toxic effect of Aroclor 1254 to the liver of mice.
 | Table 1: Histopathological observations of the liver of mice after Aroclor 1254 exposure [Click here to view] |
From the results, it can be concluded that the PCB induced dose and duration dependent oxidative stress severely affected the liver tissue and generated histopathological lesions. The results provided evidence of oxidative stress by marked changes in the GSH content and specific activities of SOD, GST, and GPx. Excessive generation of ROS and depletion of GSH might have caused series of severe histopathological alteration in mice liver cells after the exposure of 1 mg/kg/d dose of Aroclor 1254 for 21 and 28 days.
ACKNOWLEDGMENTS
The UGC, Government of India, New Delhi is thankfully acknowledged for supporting this study through its CAS programme sanctioned at the Department of Biosciences, Saurashtra University, Rajkot, Gujarat, India. There are no conflicts of interest.
REFERENCES
1. Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 2001;159:11-21. Crossref
2. Safe SH. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 1994;24:87-149. Crossref
3. Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health 1984;74:378-9. Crossref
4. Berghuis SA, Soechitram SD, Sauer PJ, Bos AF. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with neurological functioning in 3-month-old infants. Toxicol Sci 2014;142:455-62. Crossref
5. Bush B, Snow J, Koblintz R. Polychlorobiphenyl (PCB) congeners, p,p’-DDE, and hexachlorobenzene in maternal and fetal cord blood from mothers in upstate new york. Arch Environ Contam Toxicol 1984;13:517-27. Crossref
6. Lanting CI, Huisman M, Muskiet FA, van der Paauw CG, Essed CE, Boersma ER, et al. Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatr Res 1998;44:222-5. Crossref
7. Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, WeisglasKuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr 1999;134:33-41. Crossref
8. World Health Organization. WHO Polychlorinated Biphenyls: Human health Aspects, Geneva, Switzerland: World Health Organization; 2003. p. 1-64.
9. Foekema EM, Deerenberg CM, Murk AJ. Prolonged ELS test with the marine flatfish sole (Solea solea) shows delayed toxic effects of previous exposure to PCB 126. Aquat Toxicol 2008;90:197-203. Crossref
10. Monosson E. Reproductive and developmental effects of PCBs in fish: A summary of laboratory and field studies. Rev Tox 1999/2000;3:25-75.
11. Ross G. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Saf 2004;59:275-91. Crossref
12. Safe S. Clinical correlates of environmental endocrine disruptors. Trends Endocrinol Metab 2005;16:139-44. Crossref
13. Schell LM, Gallo MV. Relationships of putative endocrine disruptors to human sexual maturation and thyroid activity in youth. Physiol Behav 2010;99:246-53. Crossref
14. van Ginneken V, Palstra A, Leonards P, Nieveen M, van den Berg H, Flik G, et al. PCBs and the energy cost of migration in the European eel (Anguilla anguilla L.). Aquat Toxicol 2009;92:213-20. Crossref
15. Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A, et al. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci Total Environ 2000;247:295-311. Crossref
16. Sarasquete C, Segner H. Cytochrome P450 1A (CYP1A) in teleost fishes. A review of immune histochemical studies. In towards an integrative approach in environmental contamination and toxicology. Sci Total Environ 2000;247:313-32. Crossref
17. Tabrez S, Ahmad M. Oxidative stress-mediated genotoxicity of wastewaters collected from two different stations in northern India. Mutat Res 2011;726:15-20. Crossref
18. Tabrez S, Ahmad M. Mutagen city of industrial wastewaters collected from two different stations in northern India. J App Toxi 2011b;31:783-9.
19. Siddiqui AH, Tabrez S, Ahmad M. Validation of plant based bioassays for the toxicity testing of Indian waters. Environ Monit Assess 2011;179:241-53. Crossref
20. Pérez-López M, Nóvoa-Valiñas MC, Melgar-Riol MJ. Glutathione S-transferase cytosolic isoforms as biomarkers of polychlorinated biphenyl (Arochlor-1254) experimental contamination in rainbow trout. Toxicol Lett 2002;136:97-106. Crossref
21. Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, et al. Acute toxicity of 3, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB 126) in male sprague-dawley rats: Effects on hepatic oxidative stress, glutathione and metals status. Environ Int 2010;36:918-23. Crossref
22. Supriyo De, Somiranjan Ghosh, Raghunath Chatterjee, Y-Q Chen, Linda Moses, Akanchha Kesari, Eric Hoffman, Sisir KD. PCB Congener Specific Oxidative Stress Response by Microarray Analysis using Human Liver Cell Line. Environ. Int 2010; 36: 907–917. Crossref
23. Jiang J, Shan Z, Xu W, Wang X, Zhou J, Kong D, et al. MicrocystinLR induced reactive oxygen species mediate cytoskeletal disruption and apoptosis of hepatocytes in Cyprinus carpio L. PLoS One 2013;8:e84768. Crossref
24. Gutteridge JM. Free radicals in disease processes: A compilation of cause and consequence. Free Radic Res Commun 1993;19:141-58. Crossref
25. Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol 2004;36:89-97. Crossref
26. Ahrens RA. Glutathione peroxidase: A role for selenium (Rotruck 1972). J Nutr 1997;127:1052S-1053S.
27. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-7. Crossref
28. Habig WH, Pabst MJ, Jako WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Sci 1974;249:7130-9.
29. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-75.
30. Sokal RR, Rohlf FJ. Biometry. 1st ed. San Francisco: W.H. Freeman and Company; 1969. p. 260.
31. Michael JD. The Toxicologist’s Pocket Handbook. 2nd ed. New York: Informa Healthcare USA, Inc.; 2008. p. 44.
32. Pathak S, Kundu R. Effects of low concentrations of a polychlorinated biphenyl, aroclor 1254 on membrane bound ion dependent ATPases in mice liver. Ind J Exp Biol 2013;51:477-80.
33. Pathak S, Pansuria H, Kundu R. Low concentration of PCB (Aroclor 1254) alter membrane -bound ion dependent ATPases in the hepatocytes cells of mice. IOSR J Env Sci Tox Food Tech 2013d;3:86-90. Crossref
34. Lehmann WD, Levine JF, McHugh J. Polychlorinated biphenyls exposure causes gonadal atrophy and oxidative stress in Corbicula fluminea clams. Toxi Patho 2007;35:356-65. Crossref
35. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th ed. New York: Oxford University Press; 2007.
36. Twaroski TP, O’Brien ML, Larmonier N, Glauert HP, Robertson LW. Polychlorinated biphenyl-induced effects on metabolic enzymes, AP-1 binding, vitamin E, and oxidative stress in the rat liver. Toxicol Appl Pharmacol 2001;171:85-93. Crossref
37. McLean MR, Twaroski TP, Robertson LW. Redox cycling of 2-(x′-mono, -di, -trichlorophenyl)-1, 4-benzoquinones, oxidation products of polychlorinated biphenyls. Arch Biochem Biophys 2000;376:449-55. Crossref
38. Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 2001;42:656-66. Crossref
39. Amado LL, Monserrat JM. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ Int 2010;36:226-35. Crossref
40. Palipoch S, Punsawad C, Koomhin P, Suwannalert P. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complement Altern Med 2014;14:111. Crossref
41. Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem 1989;58:79-110. Crossref
42. Katar M, Ozugurlu AF, Ozyurt H, Benli I. Evaluation of glutathione peroxidase and superoxide dismutase enzyme polymorphisms in celiac disease patients. Genet Mol Res 2014;13:1030-7. Crossref
43. Shi Y, Jiang J, Shan Z, Bu Y, Deng Z, Cheng Y, et al. Oxidative stress and histopathological alterations in liver of Cyprinus carpio L. Induced by intraperitoneal injection of microcystin-LR. Ecotoxicology 2015;24:511-9. Crossref
44. Pflugmacher S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 2004;70:169-78. Crossref
45. Cazenave J, Nores ML, Miceli M, Díaz MP, Wunderlin DA, Bistoni MA, et al. Changes in the swimming activity and the glutathione S-transferase activity of Jenynsia multidentata fed with microcystin-RR. Water Res 2008;42:1299-307. Crossref
46. Ding WX, Shen HM, Ong CN. Microcystic cyanobacteria extract induces cytoskeletal disruption and intracellular glutathione alteration in hepatocytes. Environ Health Per 2000;108:605-9. Crossref
47. Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, et al. Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed sub chronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicology 2005;46:725-35. Crossref
48. Malbrouck C, Kestemont P. Effects of microcystins on fish. Environ Toxicol Chem 2006;25:72-86. Crossref
49. Jiang JL, Gu XY, Song R, Zhang Q, Geng JJ, Wang XR, et al. Time-dependent oxidative stress and histopathological alterations in Cyprinus carpio L. exposed to microcystin-LR. Ecotoxicology 2011b;20:1000-9. Crossref
50. Roth JD. Temporal variability in arctic fox diet as reflected in stable-carbon isotopes; The importance of sea ice. Oecologia 2002;133:70-7. Crossref