1. INTRODUCTION
The environment contains trace metals due to both natural and man-made processes. The primary ways that they enter the environment are through garbage dumping, smelter stacks, atmospheric deposition, pesticide and fertilizer use, and sewage sludge application on agricultural land [1,2]. A rapidly developing field of science and technology called phytoremediation is used to clean up contaminated soil, water, or air [3]. It can be summed up as eliminating, destroying, or sequestering toxic substances from the environment using green plants. Phytoremediation is an affordable, environmental friendly, and long-lasting option for the removal of contaminated sites [4,5]. The different types of phytoremediation process are shown in (Fig. 1). Phytoextraction is defined as the process by which soil borne contaminants are taken up by plants through their roots and transfer them to shoots or any other harvestable part of the plant where they accumulate. Phytostimulation is a type of phytoremediation where the microbial activity around the roots of plants accelerates the breakdown of organic pollutants in the soil. Phytostabilization aims to lower the mobility and bioavailability of heavy metals in the environment. Phytodegradation is a process that turns contaminants into non-hazardous compounds by phytoenzymes. Phytovolatilization is the process by which plants absorb heavy metal pollution and expel them through transpiration from stem and leaf. The uptake of heavy metal from aqueous medium followed by metabolization inside the root is known as phytofiltration [5].
 | Figure 1. Diagram showing different types of phytoremediation process. [Click here to view] |
Excess concentration of heavy metals in agricultural soils can cause phytotoxicity, which could pose a severe health risk to living beings including microorganisms, animals, and plants [6,7]. As a result, it is crucial to understand the levels of metals in soil and food cultivated for human consumption. The amount of water, nutrients, soil composition, species selectivity, metal permissibility, and absorption capacity all affect the concentration of heavy metals in various foods [8]. Metals may enter the edible portions of crops grown on polluted soil, posing a risk to human health as they enter the food chain [9,10,11]. In contaminated areas, consuming food crops is thought to be one of the main ways that people are exposed to heavy metals; however, the exact role that food crops play is yet unknown [12]. The The Food and Agriculture Organization, World Health Organization, and other regulatory authorities worldwide closely control the highest allowable levels of harmful metals in food [13]. Cd and Pb are considered non-essential heavy metals as they are toxic at specific concentrations, whereas Co, Cr, Cu, Ni, and Zn are elements needed by plants [14]. Excessive accumulation of heavy metals can have adverse effects on essential organs such as the kidneys, bones, liver, and blood, as well as pose significant health risks [15–17].
Red amaranth (Amaranthus tricolor Linn) is a member of the Amaranthaceae family. Amaranth stems and leaves contain a good amount of carotenoids and proteins, including the essential amino acids methionine and lysine, fat, carbohydrates, dietary fiber, and minerals, such as magnesium, calcium, potassium, copper, phosphorus, zinc, iron, and manganese [18]. Moreover, it has been found that red amaranth leaves contain a variety of phytochemicals, including tannins, alkaloids, glycosides, phenolic acids, flavonoids, and amaranthine [19–21]. Numerous research has shown that this plant possesses a wide range of therapeutic qualities, including antibacterial, antiradical, hepatoprotective, anti-inflammatory, and antidepressant effects [20–22]. Red amaranth is an economically significant food crop and have a critical role in the health condition of developing nations since they are an excellent and affordable source of nutrients.
Cowpea (Vigna unguiculata L. Walp) is a member of the Fabaceae family, subtribe Phaeseolinae, Vigna genus, and Catjang section. It is a warm-season, vascular annual legume crop with multiple uses including green vegetables, dal for humans, and feed for farm animals. It contains 23%–32% protein, 50%–60% carbohydrates, and 1% fat [23,24]. It is high in lysine [25,26]. It contains a decent amount of dietary fiber, phytochemicals, minerals, and vitamins [27]. The ability of cowpeas to fix atmospheric nitrogen allows them to contribute to crop productivity maintenance in addition to being nutritive [28]. In addition, cowpea creates dense vegetative growth and good soil cover, which prevents soil erosion, making it an excellent source of fodder.
Moth bean (Vigna aconitifolia) belongs to the genus Vigna and sub-family Papilionoideae which comes under the family Fabaceae. Moth bean seeds are valued for their high protein and carbohydrate content, as well as their sufficient amount of unsaturated fatty acids, minerals (calcium, iron, magnesium, and zinc), vitamins, and amino acids [29]. The moth bean is a well-known and recognised source of several bioactive phytoconstituents, including macronutrients and micronutrients, with antidiabetic, antineoplastic, antimicrobial, antihypertensive, and antioxidant properties [30]. It is a versatile legume crop that may be utilised to provide feed, fodder, green manure, and food. Basella alba also known as Malabar spinach belongs to the Basellaceae family. It is high in iron, calcium, and vitamins A and C [31]. Malabar spinach has been utilised since ancient times to treat a wide range of illnesses, including wound healing, androgenic, anticancer, antiviral, antioxidant, anti-inflammatory, anti-cholesterol, anti-ulcer, antimicrobial, and anti-hypoglycemic [32]. The presence of betacyanin, carotenoids, bioflavonoids, β-sitosterol, and lupeol in basella plants results in their antioxidant, antiproliferative, antibacterial, and anti-inflammatory properties [33].
These four plants are selected because they are widely cultivated in the studied areas and are practicable in terms of phytoremediation. However, no investigations have been made on the screening of different plant parts for heavy metal remediation in agricultural soils near industrial areas. Therefore, the present study aimed to assess the potential of two legume plant vegetables and two leafy plant vegetables to remediate or bioaccumulate heavy metals in soil collected from five different agricultural lands near industrial sites in Jamshedpur, Jharkhand, India. Additionally, data on the concentrations of trace metals (Cd, Co, Cr, Cu, Ni, Pb, and Zn) in the soil as well as the carcinogenic risk associated with these metals in adult diets were also evaluated, if applicable.
2. MATERIALS AND METHODS
2.1. Site Description
The soil samples used for this study were collected from five agricultural land sites near industrial areas of Jamshedpur (East Singhbhum) in Jharkhand, India. Jamshedpur has a total area of 224 km2 and is also known as Steel City. Industrial growth, on a large and local scale, has occurred randomly throughout the area.
2.2. Experimental Design
Approximately 10–15 kg of soil was collected from each site up to 15 cm depth, using a stainless-steel auger. The study focused on two legume plants: Cowpea (Vigna unguiculata L. Walp.) and Moth bean (Vigna acontifolia), and two leafy plants: Malabar spinach (Basella alba) and Red amaranth (Amaranthus tricolor L). Seeds of the plants were collected from the local market. Local seeds were chosen for their ability to withstand harsh conditions. The experiment was conducted at the University campus using pots filled with 10 kg soil and labeled from 1 to 5. No chemical fertilizers were used to avoid external heavy metal contamination, and plants were watered daily with tap water. All plants were cultivated in the same manner in the natural environment. Metal uptake was examined after harvesting the vegetables for 12 weeks for legume plants and leafy plants.
2.3. Analysis of Metals in Soil
Soil samples were sieved with a 2 mm mesh to remove stones, pebbles, and plant roots. The soil sample was taken onto a porcelain plate and dried in a hot air oven at 105°C for 24 hours. To achieve a homogeneous particle size, the samples were crushed in a dry iron mortar and sieved through a standard 200 mesh sieve [34]. Sample digestion involves adding HNO3 and H2O2 repeatedly to a representative 1 g (dry weight) sample. The volume of the digestate is lowered by heating and then diluted to a final volume of 100 ml. HCl was added to the initial digestate to increase the solubility of some metals. After filtering this digestate, hot distilled water and hot HCl are used to rinse the filter paper and residues. The residue and filter paper are put back into the digestion flask, refluxed with more HCl, and then filtered again. The digestate is subsequently diluted to a 100 ml final volume [35].
Cationic concentrations of Cd, Co, Cr, Cu, Ni, Pb, and Zn were analyzed directly using an Atomic Absorption Spectrometer (AAS) (Make: GBC Scientific, Australia; Model: SavantAA). The instrument uses the technique of atomic absorption for elemental analysis. A light source (hollow cathode lamp) emitting a narrow spectral line of the characteristic energy was used to excite the free atoms formed in the flame. The wavelengths of 228.8, 240.7, 357.9, 324.7, 232.0, 217.0, and 213.9, nm were used from the light source for the analysis of Cd, Co, Cr, Cu, Ni, Pb, and Zn, respectively. A D2 lamp was used in the instrument for automatic background correction. The decrease in energy (absorption) in the light beam was measured after passing the flame, which contains elements in atomic states. The absorption is proportional to the concentration of free atoms in the flame, which follows the Lambert-Beer law. The individual metal’s limits of detection at the above-mentioned wavelengths were 0.2, 2.5, 2, 1, 1.8, 2.5, and 0.4 μg/ml for Cd, Co, Cr, Cu, Ni, Pb, and Zn, respectively. The AAS was calibrated using NIST traceable certified reference standards for quality assurance and control purposes during the analysis.
2.4. Geo-Accumulation Index (Igeo)
Müller’s 1969 method [36] for assessing pollution levels in bottom sediments was used to determine the trace metal contamination in soil. This has been widely used to evaluate the level of metal pollution in the water and land environments. [37]. Igeo is calculated by the following equation [38]:
where Ci is the metal concentration in the soil and Bi is the metal’s background concentration in the earth’s crust. The 1.5 factor is included to reduce the impact of potential fluctuations in background values caused by lithogenic differences in the sediment [39]. The level of metal pollution is classified into seven contamination classifications according to the increasing value of the index [40], shown in (Table 1).
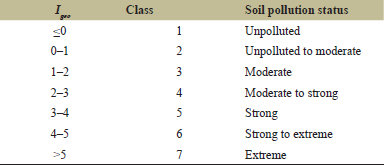 | Table 1. Igeo classes and corresponding pollution status. [Click here to view] |
The fundamental component of this technique is the determination of trace element background concentrations in soil samples. Although the equation used to calculate Igeo includes a factor that accounts for the background content of lithogenic effects, erroneous heavy metal background contents will result in incorrect results. Because there is no indication of background metal values in Jamshedpur, it was derived from UCC or upper continental crust values. UCC is a well-acknowledged and commonly utilised geochemical background. Background metal values from UCC are 0.10, 11.6, 35, 14.3, 18.6, 17, and 52 mg/kg for Cd, Co, Cr, Cu, Ni, Pb, and Zn, respectively [41].
2.5. Analysis of Metals in Plants
After 12 weeks of growing, all the plants were harvested separately according to the soil sample, separated into four compartments, roots, stem, leaves, and seed for legume plants, while three compartments, roots, stem, and leaves for leafy plants. The plants were washed in water to eliminate soil, dirt, possible parasites, or their eggs, and finally with deionized water. Then the samples were cut into small pieces with a sharp stainless-steel knife. The cut pieces were completely oven-dried at 105°C in a hot air oven for 24 hours until all moisture was removed then it was crushed, sieved, and stored in airtight labelled plastic containers. In a 250 ml beaker, about 1 g of the oven-dried ground sample was added to a 10 ml mixture of concentrated HNO3 –concentrated HClO4 which was prepared in a ratio of 10:1 [42]. Then, the content was mixed and heated slowly on a hotplate for 45 minutes continuously till brown fumes disappeared and dense white fumes appeared, leaving behind a clear solution. Finally, it was heated strongly for about 20 minutes and then it was allowed to cool down. Using Whatman 42 filter paper, the cooled sample was filtered into a 50 ml volumetric flask, and the necessary amount of deionized water was added and made up to the mark. The filtrate was then transferred from the 50 ml flask into a 50 ml HDPE bottle and sealed to prevent spillage or wastage, leaving the sample ready for atomic absorption spectrophotometric analysis.
2.6. Carcinogenic Risk
The International Agency for Research on Cancer (IARC) has assigned probable carcinogenic metal designations to the following metals: Ni, Pb, Cd, Co, and Cr. Meanwhile, zinc and copper are designated as non-carcinogenic metals [43,44]. The carcinogenic risk was calculated for metals exceeding the permissible limit in the edible part of the plants. The following formula is used to determine lifelong cancer risk, or the carcinogenic risk [45,46]:
where Ck is the carcinogenic risk or lifelong risk of cancer, Ea is the annual exposure which is 365 days/year, the exposure duration (D) is 70 years, the average time for carcinogens is T which is (Ea × D), W is the body weight, FC is the food consumption rate in g/person/day, CSF is the oral carcinogenic slope factor, and Cm is the heavy metal concentration in foods in mg/kg. For adults, the daily intake of leafy vegetables was taken at 100 g, and for pulses, 75 g, this is needed as a bare minimum to have a healthy diet [47]. Adults were considered to weigh an average of 55 kg [48].
3. RESULTS AND DISCUSSION
3.1. Metal Concentration in the Soil
Trace element concentration in soil samples of five agricultural sites varied from 12.40 to 19.76, 51.58 to 89, 27.86 to 79.51, 448.78 to 487.65, 32.59 to 46.21, and 50.55 to 514.02 mg/kg for Co, Cr, Cu, Ni, Pb, and Zn, respectively. The mean values were 16.84 (Co), 74.12 (Cr), 48.99 (Cu), 471.33 (Ni), 38.22 (Pb), and 172.07 (Zn) mg/kg. Cd was not detected in the soil samples except for site one which is 0.28 mg/kg. The average heavy metal content in the soil samples showed a falling order pattern, which was discovered in the following order: Ni > Zn > Cr > Cu > Pb > Co > Cd. The elemental concentrations in soil samples are shown in (Table 2). Relatively low concentrations of Co, Cu, Ni, Pb, Zn, and similar concentrations of Cd were found in the area of Ghatsila, Jharkhand, India [49]. A study in the soil of Khunti district in South Chotanagpur, Jharkhand, India, showed lower levels of Cd, Co, Cu, Ni, Pb, and Zn [50]. A lower range of Cu, Pb, Ni, and Cr and a similar range of Cd were reported from 40 districts of Madhya Pradesh, India [51]. A study in the coastal soil of the Puducherry region, Pondicherry, India showed a similar content range of Cr and a low content range of Co, Cu, Ni, Pb, and Zn [52].
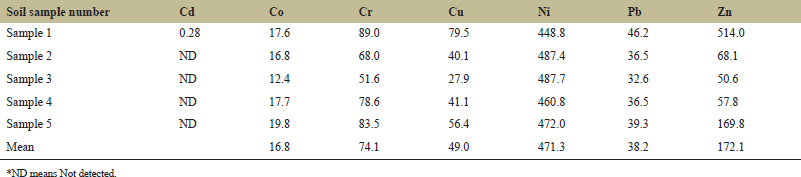 | Table 2. Heavy metal concentration (mg/kg) in soil samples. [Click here to view] |
3.2. Assessment According to Geoaccumulation Index
According to Geoaccumulation index, all the soil sample sites are unpolluted to moderately polluted for Co, Cd, Pb, and Cr. Sites 2, 3, 4 and 5 are unpolluted to moderately polluted, and site one is moderately polluted with Cu and Zn. Sites 1 and 4 are strong to highly polluted and sites 2, 3, and 5 are highly polluted for Ni. Igeo values of the trace metal in the soil are shown in (Table 3).
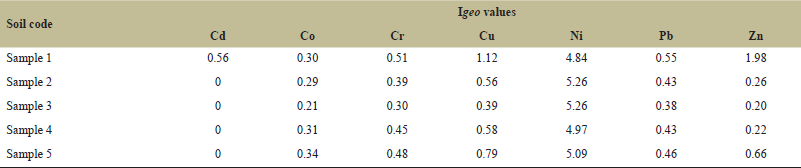 | Table 3. Igeo values of heavy metals in soil samples. [Click here to view] |
Anthropogenic sources of nickel in soil include waste from metal manufacturing, commercial waste, sludge, coal fly ash, and agricultural and industrial activities. In locations close to cities, industrial regions or even waste-affected agricultural areas, nickel contamination could be a serious issue.
3.3. Metals in Plants
Variation in heavy metal accumulation was observed within all the plant parts. Heavy metal content in various parts of the legume plants (Moth bean and Cowpea) was found in descending order as roots > stem > seeds > leaves. Malabar spinach also showed a similar trend of metal accumulation, except for chromium. Cr concentrations showed the descending order of roots > leaves > stem in both the leafy plants. In red amaranth, zinc followed the same general trend as the legumes, whereas copper and lead showed distinctive trends of leaves > stem > roots and stem > roots > leaves, respectively.
Heavy metals are transferred by the transpiration stream present in the xylem from the roots to the transpiring shoot parts (such as photosynthesizing leaves) [53–55]. After being released into the root xylem, free or chelated ions of heavy metal flow upwards with the xylem sap. If there is no further redistribution, the heavy metals would mainly accumulate in the photosynthetically active transpiring leaves. The redistribution of heavy metals from senescing leaves to sinks (such as growing vegetative parts, mature fruits, and seeds) inside the shoot is facilitated by phloem transport. Studies showed that elevated concentrations of Cd, Co, Ni, and Zn caused slightly higher contents in wheat grains, but leaves and glumes were significantly more affected, indicating control of heavy metal distribution to the grains via the phloem [56–58]. In the present study, the seeds of legume plants showed higher contents than leaves.
3.3.1. Metal in plant roots
The average Cr content in the roots of every plant sample was 2.26 mg/kg (range, 0.97−4.80 mg/kg, n = 20). The average Cu content in the roots of every plant sample was 0.72 mg/kg (range, 0.01−2.48 mg/kg, n = 20). The average Ni content in the roots of every plant sample was 1.54 mg/kg (range, 0.79−3.21 mg/kg, n = 20). The average Pb content in the roots of every plant sample was 3.72 mg/kg (range, 1.39−7.37 mg/kg, n = 20). The average Zn concentration in the roots of every plant sample was 20.76 mg/kg (range, 3.13−49.50 mg/kg, n =20) (Table 4). The root samples of none of the plants contained Cd and Co.
 | Table 4. Heavy metal uptake by plant roots. [Click here to view] |
3.3.2. Metal in plant stem
The average Cr content in stem of every plant sample was 0.59 mg/kg (range, 0.12−1.29 mg/kg, n = 20). The average Cu content in stem of every plant sample was 0.44 mg/kg (range, 0.03−1.44 mg/kg, n = 20). The average Ni content in stem of every plant sample was 0.71 mg/kg (range, 0.12−1.63 mg/kg, n = 20). The average Pb content in stem of every plant sample was 2.53 mg/kg (range, 0.47−6.55 mg/kg, n = 20). The average Zn concentration in stem of every plant sample was 9.67 mg/kg (range, 2.39−43.51 mg/kg, n = 20) (Table 5). The stem samples of none of the plants contained Cd and Co.
 | Table 5. Heavy metal uptake by plant stem. [Click here to view] |
3.3.3. Metal in plant leaves
The average Cr content in leaves of every plant sample was 0.64 mg/kg (range, 0.05−1.20 mg/kg, n = 20). The average Cu content in leaves of every plant sample was 0.48 mg/kg (range, 0.03−1.91 mg/kg, n = 20). The average Ni content in leaves of every plant sample was 0.75 mg/kg (range, 0.29−1.63 mg/kg, n = 20). The average Pb content in leaves of every plant sample was 1.12 mg/kg (range, 0.39−2.72 mg/kg, n = 20). The average Zn concentration in leaves of every plant sample was 6.31 mg/kg (range, 0.88−35.08 mg/kg, n = 20) (Table 6). The leaf samples of none of the plants contained Cd and Co.
 | Table 6. Heavy metal uptake by plant leaves. [Click here to view] |
3.3.4. Metal in plant seed
The average Cr content in seed of legume plant sample was 0.54 mg/kg (range, 0.16−0.99 mg/kg). The average Cu content in seed of legume plant sample was 0.37 mg/kg (range, 0−1.58 mg/kg). The average Ni content in seed of legume plant sample was 0.58 mg/kg (range, 0.26−0.99 mg/kg). The average Pb content in seed of legume plant sample was 1.30 mg/kg (range, 0.35−2.86 mg/kg). The average Zn content in seed of legume plant sample was 6.64 mg/kg (range, 2.21−21.62 mg/kg) (Fig. 2). The seed samples of none of the moth bean and cowpea plants contained Cd and Co.
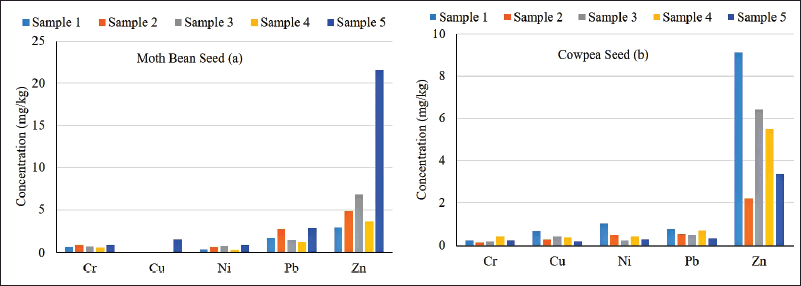 | Figure 2. Heavy metal uptake by Moth bean (a) and Cowpea (b) seeds. [Click here to view] |
Comparatively much higher contents of Cu, Pb, Ni, Zn, Co, and Cd were found in the edible parts of vegetables collected from the soil of Ghatsila Jharkhand, India [49]. Another study showed similar concentrations of Cd, Co, Cu, Ni, Pb, and Zn, in the consumable vegetable parts collected from the soil of South Chotanagpur, Jharkhand, India [50].
3.3.5. Heavy metal limit
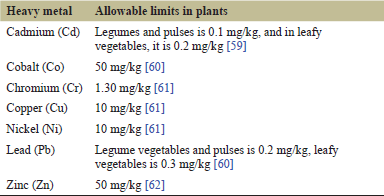
Heavy metal limit in plants is given in (Table 7). Cobalt and cadmium were not detected in any of the collected plant samples. The content of copper and nickel in every plant sample was found to be below the allowable range.
 | Table 7. The allowable limits of heavy metals in plants. [Click here to view] |
Chromium (Cr): In Cowpea plant, the chromium content was below the allowable limit in every plant sample. In the Moth bean plant, the chromium content was above the allowable limit in the roots, but below it in the stem, leaf, and seed. In the Malabar spinach and red amaranth, the chromium content was above the allowable limit in the root, but below it in the leaf and stem.
Lead (Pb): In every plant sample, the lead content was found to be above the allowable limit.
Zinc (Zn): In every plant sample, zinc content was the highest but within the allowable range. Zinc is an essential element necessary for plant growth, reproduction, and signaling [63]. For most crops, the average zinc concentration required for healthy crop growth ranges from 15 to 20 mg/kg dry weight [64].
3.4. Carcinogenic Risk
Among all the metals, only the content of Pb was higher than the allowable range in every plant sample. So, the Ck of Pb were calculated for the edible parts of the four plants. CSF for Pb was 8.5 × 10–3 mg/ kg/day according to the Integrated Risk Information System of USEPA [65]. In all the edible parts of the four plants, the Ck values of Pb was above the USEPA threshold limit (1 × 10–6) for causing cancer, which indicates carcinogenic concerns for all people in the study area [65]. The carcinogenic risk values of Pb in the four plants are shown in (Fig. 3).
 | Figure 3. Carcinogenic risk (Ck) of Pb in plants. [Click here to view] |
4. CONCLUSION
The results showed that the soils around industrial areas contained all the heavy metals except cadmium, which was only detected in one soil sample. According to the geoaccumulation index, all the soil samples are highly contaminated with Nickel. This study demonstrated the potential of Red amaranth, Moth bean, Malabar spinach, and Cowpea to remediate contaminated soil. This study found that Red amaranth is efficient in phytoremediating heavy metals like Cu and Pb. The experimental result reflected that the heavy metal concentration in the edible portions of the four plants was found to be low and below the maximum permissible limit, except for Pb. All the plant vegetables studied had Pb concentrations 2–10 times higher than the maximum permissible limit. So, planting these four plants in soil polluted with Pb could pose a significant risk to a population that harvests these plants for consumption because they were found to accumulate substantial quantities of Pb in their edible parts. In terms of carcinogenic risk, the Ck values of Pb exceeded the USEPA’s threshold limit (10-6) in the edible part of all four plants. To ensure safe food, the sources of metal contamination should be managed because the health hazards connected to food consumption are not negligible.
5. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
6. FUNDING
There is no funding to report.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 2008;152(3):686–92.
2. Rahman SH, Khanam D, Adyel TM, Islam MS, Ahsan MA, Akbor MA. Assessment of heavy metal contamination of agricultural soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: implication of seasonal variation and indices. Appl Sci 2012;2(3):584–601.
3. Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 2000;3(2):153–62.
4. Madhav S, Mishra R, Kumari A, Srivastav AL, Ahamad A, Singh P, et al. A review on sources identification of heavy metals in soil and remediation measures by phytoremediation-induced methods. Int J Environ Sci Technol 2024;21(1):1099–120.
5. Sharma JK, Kumar N, Singh NP, Santal AR. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: an approach for a sustainable environment. Front Plant Sci 2023;14:1076876.
6. Ullah S, Depar N, Khan D, Memon AA, Ali A, Naeem A. Selenate and selenite induced differential morphophysiological modifications to mitigate arsenic toxicity and uptake by wheat. Soil Sediment Contam Int J 2024;33(3):331–52.
7. Ullah S, Naeem A, Calkaite I, Hosney A, Depar N, Barcauskaite K. Zinc (Zn) mitigates copper (Cu) toxicity and retrieves yield and quality of lettuce irrigated with Cu and Zn-contaminated simulated wastewater. Environ Sci Pollut Res 2023;m30(19):54800–12.
8. Ahmad JU, Goni MA. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ Monit Assess 2010;166:347–57.
9. Tariq Y, Ehsan N, Riaz U, Nasir R, Khan WA, Iqbal R, et al. Assessment of heavy metal (oid) s accumulation in eggplant and soil under different irrigation systems. Water 2023;15(6):1049.
10. Yadav P, Singh RP, Gupta RK, Pradhan T, Raj A, Singh SK, et al. Contamination of soil and food chain through wastewater application. In advances in chemical pollution, environmental management and protection. Amsterdam, The Netherlands: Elsevier; 2023. Vol. 9, pp. 109–32.
11. Yadav R, Singh G, Santal AR, Singh NP. Omics approaches in effective selection and generation of potential plants for phytoremediation of heavy metal from contaminated resources. J Environ Manag 2023;336:117730.
12. Kachenko AG, Singh B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut 2006;169:101–23.
13. US Environmental Protection Agency (USEPA). Risk Assessment Guidance for Superfund, Human Health Evaluation Manual (Part A). Washington, DC: United States Environmental Protection Agency; 1989.
14. World Health Organization. Evaluation of certain food additives and contaminants fifty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives; WHO technical report series 909. Geneva, Switzerland: World Health Organization; 2002.
15. Haidar Z, Fatema K, Shoily SS, Sajib AA. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol Rep 2023;10:554–70.
16. Obasi PN, Chibuike A, Immaculate N. Contamination levels and health risk assessment of heavy metals in food crops in Ishiagu area, lower Benue trough South-eastern Nigeria. Int J Environ Sci Technol 2023;20(11):12069–88.
17. Verma N, Rachamalla M, Kumar PS, Dua K. Assessment and impact of metal toxicity on wildlife and human health. In Metals in water. Amsterdam, The Netherlands: Elsevier; 2023. pp. 93–110.
18. Sarker U, Oba S. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS One. 2019;14(12):e0222517.
19. Dasgupta N, De B. Antioxidant activity of some leafy vegetables of India: a comparative study. Food Chem 2007;101(2):471–4.
20. Srivastava R. An updated review on phyto-pharmacological and pharmacognostical profile of Amaranthus tricolor: a herb of nutraceutical potentials. Pharm Innov 2017;6(6, Part B):124.
21. Pulipati S, Babu PS, Naveena U, Parveen SR, Nausheen SS, Sai MT. Determination of total phenolic, tannin, flavonoid contents and evaluation of antioxidant property of Amaranthus tricolor (L). Int J Pharmacogn Phytochem Res 2017;9(6):814–9.
22. Baraniak J, Kania-Dobrowolska M. The dual nature of amaranth—functional food and potential medicine. Foods. 2022;11(4):618.
23. Cruz FJ, de Almeida HJ, dos Santos DM. Growth, nutritional status and nitrogen metabolism in’Vigna unguiculata’(L.) Walp is affected by aluminum. Aust J Crop Sci 2014;8(7):1132–9.
24. Kirse A, Karklina D. Integrated evaluation of cowpea (Vigna unguiculata (L.) Walp.) and maple pea (Pisum sativum var. arvense L.) spreads. Agron Res 2015;13(4):956–68.
25. Trehan I, Benzoni NS, Wang AZ, Bollinger LB, Ngoma TN, Chimimba UK, et al. Common beans and cowpeas as complementary foods to reduce environmental enteric dysfunction and stunting in Malawian children: study protocol for two randomized controlled trials. Trials 2015;16:1–2.
26. Gonçalves A, Goufo P, Barros A, Domínguez-Perles R, Trindade H, Rosa EA, et al. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: nutritional advantages and constraints. J Sci Food Agri 2016;96(9):2941–51.
27. Liyanage R, Perera OS, Weththasinghe P, Jayawardana BC, Vidanaarachchi JK, Sivakanesan R. Nutritional properties and antioxidant content of commonly consumed cowpea cultivars in Sri Lanka. J Food Legumes 2014;27(3):215–7.
28. Dhanasekar P, Souframanien J, Suprasanna P. Breeding cowpea for quality traits: a genetic biofortification perspective. In: Breeding for enhanced nutrition and bio-active compounds in food legumes. Springer, New York, UK, pp. 157–79, 2021.
29. Edeoga HO, Gomina A. The medicinal values and chemical composition of some bean seeds. J Food Sci 2000;66:288–90.
30. Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res Int 2010;43(2):432–42.
31. Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, et al. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutri 2004;80(3):705–14.
32. Shade A, Jacques MA, Barret M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr Opin Microbiol 2017;37:15–22.
33. Moutusi S, Parivallal BP, Prasannakumar MK, Kiranmayee P. Morphological and molecular characterization of culturable leaf endophytic fungi from Malabar Spinach, The first report. Studies Fungi 2019;4(1):192–204.
34. International Atomic Energy Agency. Soil sampling for environmental contaminants TECDOC-1415. Vienna, Austria: International Atomic Energy Agency; 2004.
35. US Environmental Protection Agency. Acid digestion of sediments, sludges and soils, Method 3050B, Rev 2. Washington, DC: Environmental Protection Agency; 1996. p. 3–5.
36. Muller G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969;2:108–18.
37. Tijani MN, Onodera S. Hydrogeochemical assessment of metals contamination in an urban drainage system: a case study of Osogbo township, SW-Nigeria. J Water Res Protect 2009;1(3):164.
38. Khuzestani RB, Souri B. Evaluation of heavy metal contamination hazards in nuisance dust particles, in Kurdistan Province, western Iran. J Environ Sci 2013;25(7):1346–54.
39. Mediolla LL, Domingues MCD, Sandoval MRG. Environmental assessment of and active tailings pile in the State of Mexico (Central Mexico). Res J Environ Sci 2008;2:197–208.
40. Huu HH, Rudy S, Van Damme A. Distribution and contamination status of heavy metals in estuarine sediments near CauOng harbor, Ha Long Bay, Vietnam. Geol Belg 2010;13:37–47.
41. Wedepohl, KH. The composition of the continental crust. Geochim Et Cosmochim Acta 1995;59:1217–32.
42. Hseu ZY. Evaluating heavy metal contents in nine composts using four digestion methods. Biores Technol 2004;95(1):53–9.
43. Environmental Protection Agency. Risk assessment guidance for superfund, Volume I: Human Health Evaluation Manual (Part A), Washington, DC: Environmental Protection Agency; 2004.
44. International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Bristol, UK: IOP Publishing Physics Web; 2012.
45. United States Environmental Protection Agency Office of Emergency, & Remedial Response. Risk Assessment Guidance for Superfund (Vol. I) Human health evaluation manual (Part A), Office of Emergency and Remedial Response. Washington, DC: US Environmental Protection Agency; 1989.
46. Islam MS, Ahmed MK, Habibullah-Al-Mamun M. Heavy metals in cereals and pulses: health implications in Bangladesh. J Agri Food Chem 2014;62(44):10828–35.
47. National Institute of Nutrition. Dietary guidelines for Indians—a manual. Hyderabad, India: National Institute of Nutrition; 2011 [cited 2024 May 06]. Available from: https://wwwninresin/downloads/DietaryGuidelinesforNINwebsitepdf
48. Indian Council of Medical Research. Nutrient requirements and recommended dietary allowances for Indians National Institute of Nutrition. Hyderabad, India: Indian Council of Medical Research; 2010 [cited 2024 May 06]. https://wwwenacnetworkcom/files/pdf/ICMR_RDA_BOOK_2010pdf
49. Kumar A, Kumar K, Singh MK, Denre M, Sarkar AK. Trace metal content in plants and potential risk for human health in subarnarekha command area of ghatsila (East Singhbhum), Jharkhand. J Indian Soc Soil Sci 2016;64(2):193–7.
50. Kumar A, Manas D, Ruplal P. Concentration of trace metals and potential health risk assessment via consumption of food crops in the South Chotanagpur of Jharkhand, India. India Pharm Innov J 2017;6(9):159–67.
51. Dwivedi AP, Tripathi IP. Diffuse heavy metals pollution in central India. Int J Sci Technol Eng 2016;2(08):291–9.
52. Sudhakaran M, Ramamoorthy D, Savitha V, Balamurugan S. Assessment of trace elements and its influence on physico-chemical and biological properties in coastal agroecosystem soil, Puducherry region. Geol Ecol Landscapes 2018;2(3):169–76.
53. Page V, Weisskopf L, Feller U. Heavy metals in white lupin: uptake, root-to-shoot transfer and redistribution within the plant. New Phytol 2006;171(2):329–41.
54. Page V, Feller UR. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann Botany 2005;96(3):425–34.
55. Page V, Blösch RM, Feller U. Regulation of shoot growth, root development and manganese allocation in wheat (Triticum aestivum) genotypes by light intensity. Plant Growth Regul 2012;67:209–15.
56. Herren T, Feller U. Transfer of zinc from xylem to phloem in the peduncle of wheat. J Plant Nutr 1994;17(9):1587–98.
57. Zeller S, Feller U. Redistribution of cobalt and nickel in detached wheat shoots: effects of steam-girdling and of cobalt and nickel supply. Biol Plant 1998;41:427–34.
58. Herren T, Feller U. Influence of increased zinc levels on phloem transport in wheat shoots. J Plant Physiol 1997;150(1-2):228–31.
59. FAO/WHO. General standards for contaminants and toxins in food and feed (CODEX STAN 193-1995). Rome, Italy: FAO; 2014.
60. World Health Organization. Permissible limits of heavy metals in soil and plants Geneva, Switzerland: World Health Organization; 1996 [cited 2024 May 06]. Available from: https://doiorg/104172/2161-05251000334
61. Hasan Z, Anwar Z, Khattak KU, Islam M, Khan RU, Khattak JZ. Civic pollution and its effect on water quality of river Toi at district Kohat, NWFP. Res J Environ Earth Sci 2012;4(3):334–9.
62. Shah A, Niaz A, Ullah N, Rehman A, Akhlaq M, Zakir M, et al. Comparative study of heavy metals in soil and selected medicinal plants. J Chem 2013;2013(1):621265.
63. Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC. Arbuscular mycorrhizal influence on zinc nutrition in crop plants–a meta-analysis. Soil Biol Biochem 2014;69:123–31.
64. Cakmak I, Öztürk L, Karanlik S, Marschner H, Ekiz H. Zinc-efficient wild grasses enhance release of phytosiderophores under zinc deficiency. J Plant Nutr 1996;19(3-4):551–63.
65. USEPA (US Environmental Protection Agency). Risk-based Concentration Table. Washington, DC: USEPA; 2010 [cited 2024 May 06]. http://wwwepagov/reg3hwmd/risk/human/indexhtm