1. INTRODUCTION
The batik industry has significantly contributed to the Malaysian economy, particularly through job creation and the development of cultural tourism, fashion, and exports [1]. Despite its cultural and economic benefits, the batik industry faces significant environmental challenges, particularly in managing wastewater generated during the production process. Batik dyeing involves the use of various synthetic dyes, waxes, and chemicals that, if not properly treated, can lead to serious water pollution [2]. The discharge of untreated batik wastewater into water bodies can result in detrimental effects on aquatic ecosystems, including reduced oxygen levels, disruption of aquatic life, and contamination of drinking water sources [3].
Physical treatment techniques for batik wastewater, such as filtration, sedimentation, and adsorption, are mostly successful at removing suspended particles and particulate matter but are less effective at removing dissolved contaminants and colors [4]. These methods often require significant maintenance and space and can be costly. Chemical methods, including coagulation and flocculation, oxidation, and chemical precipitation, use chemicals to neutralize, break down, or precipitate pollutants, but they generate hazardous by-products, require precise control, and can be expensive [5].
In contrast, biological treatment leverages microorganisms to break down organic pollutants and dyes, offering a cost-effective and environmentally friendly solution. Biological technologies are a more sustainable and effective way to manage batik wastewater because they create less harmful by-products, generate less sludge and effectively remove a wide spectrum of contaminants [6]. Dye remediation is often prioritized over other parameters because of their high visibility, which can cause significant aesthetic and environmental damage even at low concentrations [7]. Moreover, they require specific and targeted remediation strategies due to their complex chemical structures and some resistance to biodegradation.
Bacteria are excellent for dye decolorization due to their metabolic diversity and adaptability, allowing them to degrade a wide range of synthetic dyes in various environments [8]. They use enzymes like laccases and azoreductases to break down complex dye molecules into less toxic forms. Bacterial laccases have shown significant potential for dye decolorization in various studies. Wang et al. [9] and Sharma and Leung [10] both identified bacterial laccases with high decolorization capabilities, with Wang et al. [9] specifically highlighting the potential of Anoxybacillus ayderensis SK3-4 laccase for industrial applications. Differences in substrate specificities, stability, optimal pH, and temperature of different bacterial laccases can result in an expanded spectrum of dyes that can be treated. Therefore, the present study aims to discover, optimize, and use extracellular laccase derived from an indigenous isolate sourced from batik wastewater for the decolorization of various textile dyes. Additionally, the laccase will be employed to bioremediate actual batik wastewater and a phytotoxicity study will be conducted to determine the safety of the treated batik wastewater.
2. MATERIALS AND METHODS
2.1. Chemicals
The experiment utilized commercially available analar-grade chemicals supplied by R&M Chemicals (UK) and Vivantis Technologies Sdn Bhd (Malaysia). These materials required no further purification prior to use.
2.2. Microorganism
The bacterial strains, identified as SK1 and SK2, were isolated from wastewater from the Malaysian batik industry. The wastewater sample was collected from Jadi Batek, a local manufacturing plant in Kuala Lumpur, Malaysia. These strains were preserved at −80°C in the Culture Collection Unit of the Institute of Bio-IT, Universiti Selangor, located in Selangor, Malaysia. To repurpose the strains, one bead was removed and immediately inoculated onto a nutrient agar plate. Molecular characterization of these strains is being carried out by another team, and once identified, they will be deposited in GenBank® (NCBI).
2.3. Growth and Laccase Activity Profiling
A modified 10X M9 salts solution containing 0.5 g/l glucose, 50 g/l KH2PO4, 50 g/l K2HPO4, and 5 g/l NaCl served as the experiment’s medium. The medium was inoculated separately with a loopful of strains SK1 and SK2. The cultures were incubated at 30°C with constant shaking at 150 rpm (Jeio Tech SI-600R, Korea) and a pH of 7. The incubation continued until the exponential phase was reached: 6 hours for strain SK1 and 24 hours for strain SK2. A 2% v/v inoculum of the exponentially grown cultures were then transferred into a fresh medium and incubated for 48 hours under the same conditions (30°C, 150 rpm, and pH 7) in triplicate. A control experiment, with no inoculation of strains, was also conducted. Growth and laccase activity were measured at 6-hour intervals throughout the incubation period.
2.4. Optimization Study
The default incubation parameters for the experiments included a 0.5 g/l carbon source, 30°C temperature, 150 rpm agitation speed, 2% v/v inoculum size, a 24-hour incubation period, and a pH of 7. All experiments were conducted in triplicate, with a control set that lacked inoculum. As optimized values were determined, the default parameters were sequentially adjusted for carbon source, pH, temperature, agitation, and inoculum size.
Various parameters were tested to optimize the conditions. Fruit wastes such as banana peel, coconut husk, orange peel, sugarcane bagasse, and pineapple waste were sourced from the local market, washed with distilled water, air-dried, and ground into powder. These were used as glucose substitutes at a concentration of 0.5 g/l. Additionally, temperature (25°C to 55°C), pH (4 to 10), agitation speeds (0 rpm to 200 rpm), and inoculum sizes (2% to 10% v/v) were varied accordingly. The broth was collected after incubation and centrifuged for 15 minutes at 4°C at 13,000 ×g. (Eppendorf 5702R, South Asia), and the supernatant was collected to determine extracellular crude laccase activity.
2.5. Decolorization Study
Seven commonly used dyes in the textile industry were selected for this study: Methylene Blue (λmax = 664 nm), Crystal Violet (λmax = 590 nm), Congo Red (λmax = 498 nm), Methyl Red (λmax = 435 nm), Methyl Orange (λmax = 465 nm), Malachite Green (λmax = 614 nm), and Alizarin Yellow (λmax = 389 nm), each at concentrations of 100 and 250 mg/l. One ml each of laccase, dye solution, and phosphate buffer (pH 8) formulated the reaction mixture. The mixtures were incubated at 35°C for 1 hour, after which absorbance readings were taken at each dye’s maximum absorbance wavelength. A control set without laccase was also prepared for comparison.
2.6. Phytotoxicity Study
Batik wastewater with a pH of 10.5 and a temperature of 37°C was collected from a local batik facility. At a wavelength of 300 nm, the wastewater showed the highest absorption and had a deep blue color. In accordance with APHA Standard Methods for the Examination of Water and Wastewater, all research parameters, including temperature, pH, total dissolved solids (TDSs), total suspended solids (TSSs), biological oxygen demand (BOD), chemical oxygen demand (COD), and dissolved oxygen (DO), were measured before and after treatment with 1 ml of bacterial laccase for 24 hours. In the experiment, 1 ml of laccase and 1 ml of wastewater from the batik facility were combined, and the mixture was incubated for 1 hour. At the beginning and end of the incubation period, a triplicate absorbance reading was obtained. For comparison, a control set devoid of laccase was also generated. With a few minor adjustments, a phytotoxicity investigation was conducted using the approach described by Maniyam et al. [11].
2.7. Analytical Methods
To determine bacterial growth, 1 ml of bacterial broth was aseptically pipetted into cuvettes. After thorough homogenization, absorbance was measured at 600 nm (Biomate 3 Thermo Scientific, USA) in triplicate at regular intervals, using distilled water as a blank.
To determine the laccase activity, the methodology described by Sasmaz et al. [12] was adopted. Using spectrophotometry, the oxidation of guaiacol by laccase was tracked by variations in the absorbance measurement at 465 nm. According to the definition, one activity unit (U/ml) was the quantity of enzyme that oxidized and increased absorbance by 0.1 absorbance units per minute at 465 nm. The culture supernatant exhibiting elevated laccase activity was administered with catalase in order to test the hypothesis that laccase was the cause of the decolorization [12]. In addition, peroxidases that are involved in dye decolorization and degradation require the presence of hydrogen peroxide to get activated.
The percentage of decolorization was calculated using the formula shown in Equation 1:
2.8. Statistical Analysis
The groups were compared using IBM SPSS version 23’s one-way analysis of variance. For post hoc analysis, the Duncan test, represented by different letters, was selected. A p-value of less than 0.05 indicated statistical significance.
3. RESULTS AND DISCUSSION
3.1. Growth and Laccase Activity Profiling
As seen in Figure 1, the laccase synthesis of two bacterial strains, strains SK1 and SK2, was investigated in relation to time at various stages of bacterial growth. The SK1 strain’s growth and laccase production profile showed that extracellular laccase synthesis began after 6 hours of incubation and continued to rise as the bacteria proliferated into the exponential phase up to 24 hours. A comparable pattern was noted for strain SK2, however a longer incubation of 24 hours was required.
 | Figure 1. Growth and laccase activity profiling of strains SK1 and SK2. The data presented are the means of triplicate samples ± standard errors. [Click here to view] |
After 24 hours of incubation which corresponded to an early stationary phase, the maximum laccase synthesis of 321.5 U/ml by strain SK1 was reached and an additional 24 hours of incubation resulted in a subsequent decline in enzyme production. Similar findings were published by Javadzadeh and Asoodeh [13] about the symbiotic bacteria of Bacillus sp. CF96. They found that the stationary phase started after 25 hours of inoculation and the declining phase happened after 35 hours. At 26 hours of incubation, which corresponded to the early stationary phase, the maximum amount of enzyme was produced. Additionally, extracellular laccase appeared to be a secondary metabolite, based on the growth patterns of the Kabatiella bupleuri G3 [14]. On day 20, it achieved a value of 35.21 ± 0.64 U/l, having reached its maximal concentration in the supernatant during the strain’s stationary growth phase. Conversely, Gogotya et al. [15]’s study showed that the mid exponential phase, which happened at 84 hours of incubation, was when laccase production was at its highest yielding a titre of 40.58 ± 2.35 U/ml. With an extended incubation period, the synthesis of enzymes declined significantly beyond the optimal duration of 84 hours. This indicated that laccase synthesis at its peak during growth phases was reliant on the type of microorganism.
Strain SK2 exhibited delayed expression of the laccase enzyme, requiring 36 hours of incubation to achieve an optimum yield of 169.0 U/ml. Similarly, a downward trend in laccase production was observed with a prolonged incubation period. At this point in its growth, the bacterium’s own secreted metabolites in the medium may be have an inhibitory influence on laccase formation [16].
Similar to the present study, extracellular bacterial laccase generated by Serratia proteamaculans AORB19 [17] and Lysinibacillus macroides LSO [18] have been reported in previous studies. Because they are simpler to produce and purify on a large scale than intracellular and spore-bound laccases, extracellular bacterial laccases are well suited for industrial applications [19]. Therefore, further investigations will be carried out using strain SK1 since this strain was able to produce a high titer of laccase by 90% in comparison to strain SK2.
3.2. Fruit Waste as Low-Cost Carbon Source
With an estimated value of RM1.46 billion (USD 347 million) in 2020, tropical fruit exports from Malaysia rank among the top exports from the country’s agricultural industry [20]. This substantial export activity results in the generation of large quantities of fruit waste and remnants, particularly peels, due to the high consumption and industrial processing of the edible sections of fruits [21]. Consequently, Malaysia generates a variety of fruit leftovers that might potentially serve as a valuable commercial supply of carbon for the synthesis of enzymes. The current study, therefore, aims to explore the feasibility of utilizing materials with minimal or no financial benefits as an alternative carbon source for laccase synthesis, addressing the challenge of fruit waste disposal.
All five different forms of fruit waste that were used to increase laccase output were efficiently hydrolyzed by strain SK1, as shown in Table 1. Among the various substrates tested, banana peels emerged as the most effective nutrient source, yielding an optimal laccase production of 501.54a ± 23.11 U/ml. Strain SK1 exhibited no significant variation (p > 0.05) in laccase activity when cultivated on coconut husks, orange peels, sugarcane bagasse, and pineapple peels. This finding indicated that strain SK1 had broad substrate selectivity across various types of fruit waste, suggesting its potential for effective commercial-scale laccase synthesis. The culture containing glucose exhibited the lowest laccase activity, measuring 321.50c ± 21.50 U/ml. Despite being a conventional carbon source, glucose was found to be less effective than fruit waste in the synthesis of laccase in this study. Similarly, the white-rot fungus Coriolopsis gallica NCULAC F1 demonstrated the lowest laccase production in response to glucose supplementation, as observed by Cen et al. [22].
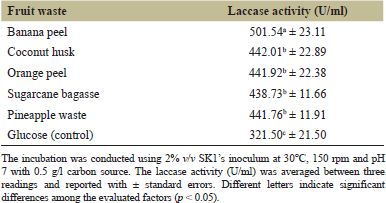 | Table 1. Utilization of fruit waste as substrate for the production of laccase from strain SK1. [Click here to view] |
Banana peels have been identified as an effective fermentation substrate due to their significant content of lignin (6%–12%), pectin (10%–21%), cellulose (7%–10%) and hemicelluloses (6%–9.4%) [23]. Additionally, the polysaccharides and phenolic compounds present in banana peels can enhance bacterial growth and subsequently stimulate laccase production [24]. When banana peels were present, strain SK1 secreted more laccase, indicating that this substrate was ideally suited for the strain to penetrate and break down plant cell walls and their constituent components [25]. Kumar et al. [26] reported the use of banana peel as an effective precursor for the production of laccase by Bacillus sp. strain AKRC01. Similarly, Enterobacter sp. AI1 efficiently utilized banana peel, resulting in an optimal laccase yield of 18.87 U/ml [25]. Alternatively, a study by Omeje et al. [27] found that using groundnut husk as a carbon source resulted in the highest laccase activity (336 ± 11.31 U/l) by the isolate Aspergillus species among the agricultural wastes used in the fermentation process. This suggested that the characteristics of agro-waste residues that enhanced laccase production were strain-specific. The study by Thakkar and Bhatt [28] demonstrated that laccase from Alternaria alternata exhibited significant activity with 1% sucrose, suggesting that leftover industrial molasses could be further explored as a substrate for industrial laccase production. Therefore, from both environmental and economic perspectives, synthesizing laccase from fruit waste is highly appealing and represents a significant step towards sustainable development.
3.3. Optimization Study
Due to their ability to function well in harsh environments and their potential for production in industrial prokaryotic expression systems like Escherichia coli, laccase enzymes originating from bacteria have become a growing priority [29]. Our investigation showed that the bacterial laccase was active across a pH range of 5.0 to 10.0, with a maximum activity of 583.03a ± 17.81 U/ml observed at pH 8.0, suggesting that this is the ideal pH for the enzyme (Table 2). This laccase’s alkaline character was demonstrated by its greater activity in the pH range of 7.0 to 9.0, making it suitable for textile wastewater remediation [30]. These findings align with similar studies on laccases from Alcaligenes faecalis, which showed 100% relative activity [16], and from Chitinophaga sp., which exhibited the most activity at slightly basic pH values [31]. Specifically, after 16 hours of incubation at 37°C with guaiacol, Chitinophaga sp. laccase achieved 100% activity in CAPS buffer at pH 10.5 [31]. Both studies highlight the optimal activity of these laccases in alkaline pH ranges, underscoring a common trait among bacterial laccases that often show better performance at higher pH levels. It is well-known that the optimum pH for laccase activity can vary depending on the bacterial source and the substrate used. Our findings are consistent with the literature for phenolic substrates like guaiacol, where laccase activity is reported to be greater at more basic pH readings. Sondhi et al. [32] reported that when syringaldazine, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), DMP (2,6-Dimethoxyphenol), and guaiacol were used as substrates, the optimal pH values for the MSKLAC laccase from Bacillus sp. MSK-01 were recorded as 7.0, 4.5, 8.0, and 8.0, respectively.
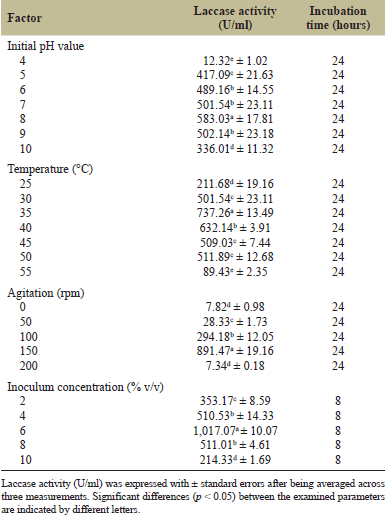 | Table 2. Optimization of different culture conditions for the optimum production of laccase by strain SK1 using one-factor-at-a-time (OFAT) approach. [Click here to view] |
The investigation into the temperature dependence of laccase activity revealed that the enzyme exhibits substantial catalytic function within the range of 30°C to 50°C. Optimal laccase activity of 737.26a ± 13.49 U/ml was observed at 35°C, suggesting this temperature was the most favorable for maximum enzyme efficiency (Table 2). This aligns with the known behavior of laccase from Pleurotus sajor-caju, reported by Rampinelli et al. [33], which typically exhibited peak activity within a similar temperature range. In addition, the current study’s findings on the optimal activity of the laccase enzyme closely matched those reported by Umar and Ahmed [34], who studied laccase from Ganoderma leucocontextum. They documented an optimal laccase activity of 855 U/l at 40°C. This corroborates our data, further validating the optimal temperature range for laccase activity observed in this study. However, a decline in laccase activity was observed when the temperature exceeded 50°C. Prolonged exposure to elevated temperatures likely results in denaturation or structural alterations of the enzyme, thereby reducing its catalytic efficacy. Additionally, high temperatures may impair the stability of the microorganism, leading to lower overall metabolic rates and, hence, a decrease in substrate-to-product conversion.
Laccase activity increased progressively with agitation rates from 0 to 150 rpm (Table 2). This trend suggested that moderate agitation enhanced enzyme production. At 150 rpm, the optimal laccase activity of 891.47a ± 19.16 U/ml was observed, indicating that this agitation rate provides sufficient oxygen transfer and mixing, promoting microbial growth and enzyme synthesis [35]. However, at an agitation rate of 200 rpm, a notable decline in laccase production was recorded, with the activity dropping to 7.34d ± 0.18 U/ml. This sharp decrease suggested that excessive agitation adversely affected enzyme production. The reduction in laccase activity at 200 rpm can be attributed to the increased shear forces and potential cell damage caused by high agitation [36]. Excessive agitation may lead to a higher diffusion rate of oxygen, which, paradoxically, can harm the bacterial cells, thereby reducing their ability to produce the enzyme. These findings align with those reported by Geethanjali et al. [37] who observed that Mucor circinelloides GL1 exhibited maximum laccase activity under shaking conditions at 150 rpm, while a lower activity was recorded at 0 rpm after an 8-d incubation period. Additionally, in the case of the newly discovered Ganoderma multistipitatum sp. nov., maximum laccase production of 19.44 × 105 ± 0.28 U/l was attained at an agitation speed of 150 rpm, which provided ideal DO levels for laccase synthesis [38]. This consistency supports the notion that moderate agitation is beneficial for laccase production, while both low and excessively high agitation rates are suboptimal.
It is necessary to employ the right cell inoculum level in order to produce industrial enzymes in large quantities. An optimal inoculum size is essential to control the initial lag phase of microbial growth [39]. In this study, it was observed that smaller inoculum sizes extended the lag phase, necessitating a longer incubation period. Conversely, increasing the inoculum size shortened the lag phase, thereby reducing the overall incubation period to just 8 hours. Laccase production was found to increase incrementally with an increase in inoculum size from 2% to 6% (v/v) as shown in Table 2. However, the synthesis of laccase decreased when the inoculum size was increased beyond 6% (v/v). This reduction in enzyme yield at higher inoculum sizes was likely due to the rapid depletion of nutrients, which stunted bacterial growth and subsequently lowered enzyme production [40]. Furthermore, the synthesis of laccase may be further inhibited by the build-up of hazardous metabolites at larger inoculum levels [40]. Our findings are consistent with previous research. For instance, Sharma et al. [41] reported that an inoculum size of 1% (v/v) of Bacillus marisflavi Strain BB4 was sufficient for maximum laccase production. In another study, a 2 ml inoculum size of Aspergillus niger yielded the highest laccase activity, measuring 3.206 U/ml [42]. However, increasing the inoculum size to 7 ml, while keeping other parameters constant, resulted in a 59% decrease in laccase activity.
3.4. Dye Decolorization
In many different sectors, synthetic dyes are widely employed, especially in the manufacturing of textiles. The two biggest groups of these dyes are azo and triphenylmethane [43]. Because of their intricate makeup, these dyes are not biodegradable and can cause major health and environmental. The application of enzymes particularly laccase can be presented as an economical, ecologically responsible, and successful method of breaking down these harmful dyes [44]. Therefore, the present study examined how effectively the laccase produced by strain SK1 decolorized seven common dyes with various structures, including triphenylmethane and azo dyes.
Although mediators significantly enhance laccase activity for dye decolorization, they are both hazardous and expensive. Asadi et al. [45] have revealed that some laccases exhibit mediator-insensitivity, which presents a benefit for industrial applications. Therefore, the ability of laccase to decolorize all tested dyes without mediators in the current study is highly favorable for industrial use. The bacterial laccase demonstrates its capacity to decolorize a range of dyes, as illustrated in Table 3. The rate of decolorization was observed to increase in the following order: Congo Red > Alizarin Yellow > Methyl Orange and Methyl Red > Methylene Blue, Crystal Violet, and Malachite Green, with respective removal efficiencies of 32%d ± 1%, 43%c ± 0%, 66%b ± 1%, and 64%b ± 1% (p > 0.05) and 99%a ± 1%, 99%a ± 0% and 99%a ± 0% (p > 0.05) within 30 minutes of incubation with dye concentration of 100 mg/l. When the incubation was extended to 60 minutes, almost complete decolorization of all dyes was observed except for Alizarin Yellow and Congo Red yielding 81%b ± 0% and 68%c ± 0% removal efficiency, respectively.
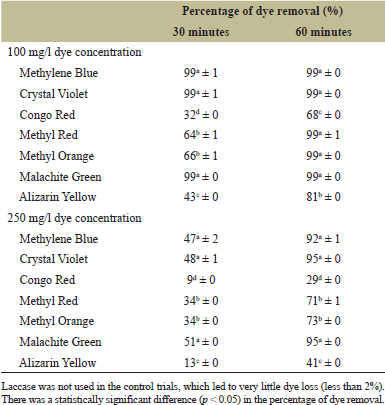 | Table 3. Decolourization of selected dyes by laccase derived from strain SK1. [Click here to view] |
When the concentration was increased to 250 mg/l representing the typical dye concentration in actual textile wastewater samples [46], a decreasing trend of dye removal efficiencies was observed after 30 minutes of incubation. Following a 60-minute incubation, Malachite Green, Crystal Violet, and Methylene Blue exhibited nearly complete decolorization. The removal efficiencies for Methyl Red and Methyl Orange on the other hand escalated to 71%b ± 0% and 73%b ± 0%, respectively. Congo Red and Alizarin Yellow demonstrated resilience to decolorization, showing reduced dye removal at higher concentrations post 60 minutes of incubation. Even when the incubation period was extended to 2 hours, there was no discernible increase in the removal efficiency. This resistance may be attributed to metabolites formed during the enzyme’s decolorization process and the dyes’ inherent toxicity [47]. In addition, due to their intricate structures, azo dyes namely Congo Red and Alizarin Yellow are resistant to most laccases, resulting in slow oxidation. In contrast, triphenylmethane dyes are readily oxidized by the majority of laccases [16]. In comparison to the laccase used in this study, other published laccases demonstrated less than 50% decolorization of known azo and triphenylmethane dyes at lower concentrations and/or longer reaction times [29,48–52]. This study, therefore, highlights the regional biodiversity and encourages further research into local strains with enhanced dye decolorization capabilities. Furthermore, future studies will be carried out using Fourier Transform Infrared Spectroscopy (FTIR) to analyze dye decolorization processes. FTIR can identify chemical changes in dyes and degradation products, providing insights into the decolorization mechanism and efficiency, which could enhance the optimization and understanding of dye removal strategies [53].
3.5. Phytotoxicity Study
Laccase derived from strain SK1 demonstrated the ability to decolorize a variety of dyes. Therefore, the utilization of this enzyme to decolorize real Malaysian batik (textile) wastewater was attempted. The batik wastewater was collected from a local facility and possessed the following characteristics: a temperature of 37°C, a pH level of 10.5, and a maximum absorbance at a wavelength of 300 nm, representing a deep blue hue. After an incubation period of 30 minutes at 160 rpm and 37°C, a removal efficiency of 99% ± 0% was achieved, recording a complete disappearance of the deep blue color. In addition, all parameters namely pH, temperature, TDS, TSS, BOD, and COD of the batik wastewater exceeded the limits set by Malaysian regulations (Table 4). Treatment with strain SK1’s laccase improved the quality of the batik wastewater, reducing the pH from 10.50 ± 0.31 to 7.81 ± 0.02 and the temperature from 37.00°C ± 0.04°C to 36.00°C ± 0.11°C, bringing both into compliance with standard values. The removal efficiencies for TDS, TSS, BOD, and COD were 67.5%, 93.5%, 99.7%, and 97.0%, respectively, indicating the active role of laccase in reducing these pollutants, as supported by the study conducted by Iqbal et al. [54]. These findings signify the potential of bacterial laccase for treating actual textile wastewater.
 | Table 4. Characterization of Malaysian batik wastewater. [Click here to view] |
Significant amounts of highly contaminated wastewater are produced by textile manufacturing, which is one of the main causes of ecological damage [55]. Hazardous contaminants are present in this effluent, with dye being one of the main concerns [56]. Since textile wastewater can potentially be used for irrigation, it is crucial to remove dyes from it before reuse. Thus, this study examined the relative susceptibility of Vigna radiata seeds to Malaysian batik wastewater, comparing the effects of laccase-treated and untreated wastewater, and the findings are presented in Figure 2.
 | Figure 2. Impact of batik wastewater and its decolorization by-products on V. radiata growth. A 7-day investigation of phytotoxicity was conducted on batik wastewater and its decolorization metabolites (0.2 g/l) at room temperature. By averaging three repetitions, the results for germination and seedling length were determined and shown with +/- standard errors. Among the parameters under investigation, statistically significant differences (p < 0.05) were noted. [Click here to view] |
The phytotoxicity study indicated that soaking V. radiata seeds in laccase-untreated batik wastewater inhibited germination by approximately 67%. In contrast, laccase-treated batik wastewater resulted in only a 2% suppression of V. radiata seed germination compared to the control. Additionally, the study demonstrated that the lengths of seedlings germinated in the untreated batik wastewater were approximately 11.56 times shorter than those germinated in the decolorization metabolites (p < 0.05). Studies have consistently shown that treated textile wastewater can lead to higher germination rates and seedling lengths compared to untreated counterparts. For example, Selim et al. [57] discovered comparable seedling lengths with the control system (dH2O) when textile effluent treated by a consortium of Aspergillus flavus and Fusarium oxysporum was used to germinate Vicia faba. Similarly, when comparing the toxicity of all treated textile wastewaters using Citrobacter sp. M41 in a bioaugmented packed bed column bioreactor with granulated corncob and its biochar to untreated textile wastewater, the phytotoxicity study using V. radiata seeds showed a significant decrease in toxicity [58]. Therefore, treated batik wastewater, after the effective removal of harmful dyes using laccase treatment, can be repurposed for irrigation, supporting water recycling and promoting water sustainability.
4. CONCLUSION
This study presents the first report on tropical laccase derived from an indigenous bacterial strain isolated from batik wastewater. The successful utilization of fruit waste as a substrate for laccase production by strain SK1 offers a dual advantage: it provides an economical solution and helps mitigate fruit waste disposal. Upon optimization of key parameters—temperature (35°C), pH (8), agitation (150 rpm), and inoculum size (5% v/v) using banana peel as the substrate—the laccase yield improved significantly from 321.5 to 1,017.07 U/ml, and the incubation time was reduced from 24 to 8 hours. The optimized laccase was effective in decolorizing various dyes commonly found in textile wastewater, including Congo Red, Alizarin Yellow, Methyl Orange, Methyl Red, Methylene Blue, Crystal Violet, and Malachite Green, at a concentration of 250 mg/l. This demonstrates the enzyme’s viability as a biocatalyst for actual bioremediation of textile wastewater. When applied to real batik wastewater, the laccase achieved a 99% dye removal rate, showcasing its potential in industrial applications. Moreover, the treated batik wastewater did not hinder the germination of V. radiata seeds, with results comparable to the use of distilled water as a control. This indicates that the treated wastewater is safe and can be repurposed for irrigation, contributing to sustainable water management practices. Future studies will focus on immobilizing the laccase to enhance its biocatalytic performance for treating batik wastewater. This biological method presents a green solution for mitigating textile wastewater pollution and the disposal of fruit waste, aligning with environmental sustainability goals.
5. ACKNOWLEDGMENT
This study was funded by the Malaysian Ministry of Higher Education (MOHE) under the Fundamental Research Grant Scheme (FRGS), project code [FRGS/1/2021/WAB11/UNISEL/02/1].
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Syed Shaharuddin SI, Shamsuddin MS, Drahman MH, Hasan Z, Mohd Asri NA, Nordin AA, et al. A review on the Malaysian and Indonesian batik production, challenges, and innovations in the 21st century. SAGE Open 2021;11(3):21582440211040128; CrossRef
2. Martuti NKT, Hidayah I, Margunani M, Alafima RB. Organic material for clean production in the batik industry: a case study of natural batik Semarang, Indonesia. Recycling 2020;5(4):28; CrossRef
3. Zakaria N, Rohani R, Wan Mohtar WHM, Purwadi R, Sumampouw GA, Indarto A. Batik effluent treatment and decolorization—a review. Water 2023;15(7):1339; CrossRef
4. Wei F, Shahid MJ, Alnusairi GS, Afzal M, Khan A, El-Esawi MA, et al. Implementation of floating treatment wetlands for textile wastewater management: a review. Sustainability 2020;12(14):5801; CrossRef
5. Kordbacheh F, Heidari G. Water pollutants and approaches for their removal. Mater Chem Horizons 2023;2(2):139–53.
6. Singh A, Pal DB, Mohammad A, Alhazmi A, Haque S, Yoon T, et al. Biological remediation technologies for dyes and heavy metals in wastewater treatment: new insight. Bioresour Technol 2022;343:126154; CrossRef
7. Zakaria Z, Othman MR, Hasan SZ, Ahmad WW. Electrochemical degradation of reactive orange 16 by using charcoal-based metallic composite electrodes. Sains Malays 2019;48(4):791–801; CrossRef
8. Maniyam M, Azman H, Abdullah H, Yaacob NS. Process optimization for efficacious biodecolorization of crystal violet by Malaysian Rhodococcus pyridinivorans using monothetic analysis. J Appl Biol Biotechnol 2022;10(2):107–13; CrossRef
9. Wang J, Chang F, Tang X, Li W, Yin Q, Yang Y, et al. Bacterial laccase of Anoxybacillus ayderensis SK3-4 from hot springs showing potential for industrial dye decolorization. Ann Microbiol 2020;70:1–9; CrossRef
10. Sharma N, Leung IK. Characterisation and optimisation of a novel laccase from Sulfitobacter indolifex for the decolourisation of organic dyes. Int J Biol Macromol 2021;190:574–84; CrossRef
11. Maniyam MN, Azman HH, Abdullah H, Yaacob NS. Response surface methodology as an optimization tool to achieve an effective decolourization of crystal violet by the Malaysian Rhodococcus pyridinivorans strain. Biomass Convers Biorefin 2024;14(10):11023–34; CrossRef
12. ?a?maz S, Gedikli S, Aytar P, Güngörmedi G, Çabuk A, Hür E, et al. Decolorization potential of some reactive dyes with crude laccase and laccase-mediated system. Appl Biochem Biotechnol 2011;163:346–61; CrossRef
13. Javadzadeh SG, Asoodeh A. A novel textile dye degrading extracellular laccase from symbiotic bacterium of Bacillus sp. CF96 isolated from gut termite (Anacanthotermes). Int J Biol Macromol 2020;145:355–63.
14. Wi?niewska KM, Twarda-Clapa A, Bia?kowska AM. Screening of novel laccase producers—isolation and characterization of cold-adapted laccase from Kabatiella bupleuri G3 capable of synthetic dye decolorization. Biomolecules 2021;11(6):828; CrossRef
15. Gogotya A, Nnolim NE, Digban TO, Okoh AI, Nwodo UU. Characterization of a thermostable and solvent-tolerant laccase produced by Streptomyces sp. LAO. Biotechnol Lett 2021;43:1429–42; CrossRef
16. Mehandia S, Sharma SC, Arya SK. Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol Rep 2020;25:e00413; CrossRef
17. Ali NS, Huang F, Qin W, Yang TC. Identification and characterization of a new Serratia proteamaculans strain that naturally produces significant amount of extracellular laccase. Front Microbiol 2022;13:878360; CrossRef
18. Abdelgalil SA, Soliman NA, Abo-Zaid GA, Abdel-Fattah YR. Bioprocessing strategies for cost-effective large-scale production of bacterial laccase from Lysinibacillus macroides LSO using bio-waste. Int J Environ Sci Technol 2021;19:1633–52; CrossRef
19. Maia LF, Hoyos SLM, Watanabe AK, Moralez ATP, Furlaneto MC, de Souza Daniel JF. Extra and intracellular laccase titers of xylophagic bacteria isolated from adult termite. Acta Sci Technol 2021;43:e51805; CrossRef
20. Mohd Israfi NA, Mohd Ali MIA, Manickam S, Sun X, Goh BH, Tang SY, et al. Essential oils and plant extracts for tropical fruits protection: from farm to table. Front Plant Sci 2022;13:999270; CrossRef
21. Ibrahim UK, Kamarrudin N, Suzihaque MUH, Abd Hashib S. Local fruit wastes as a potential source of natural antioxidant: an overview. IOP Conf Ser Mater Sci Eng 2017;206(1):012040; CrossRef
22. Cen Q, Wu X, Cao L, Lu Y, Lu X, Chen J, et al. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Express 2022;12(1):96; CrossRef
23. Zehra M, Syed MN, Sohai M. Banana peels: a promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Pol J Microbiol 2020;69(1):19–26; CrossRef
24. Vipotnik Z, Michelin M, Tavares T. Biodegradation of chrysene and benzo [a] pyrene and removal of metals from naturally contaminated soil by isolated Trametes versicolor strain and laccase produced thereof. Environ Technol Innov 2022;28:102737; CrossRef
25. Edoamodu CE, Nwodo UU. Enterobacter sp. AI1 produced a thermo-acidic-tolerant laccase with a high potential for textile dyes degradation. Biocatal Agric Biotechnol 2021;38:102206; CrossRef
26. Kumar A, Singh AK, Ahmad S, Chandra R. Optimization of laccase production by Bacillus sp. strain AKRC01 in presence of agro-waste as effective substrate using response surface methodology. J Pure Appl Microbiol 2020;14(1):351–62; CrossRef
27. Omeje KO, Nnolim NE, Ezema BO, Ozioko JN, Eze SO. Synthetic dyes decolorization potential of agroindustrial waste-derived thermo-active laccase from Aspergillus species. Biocatal Agric Biotechnol 2020;29:101800; CrossRef
28. Thakkar AT, Bhatt SA. Isolation, identification and optimization of laccase from Alternaria alternata. J Appl Biol Biotechnol 2020;8(3):64–9; CrossRef
29. Navas LE, Carballo R, Levin L, Berretta MF. Fast decolorization of azo dyes in alkaline solutions by a thermostable metal-tolerant bacterial laccase and proposed degradation pathways. Extremophiles 2020;24:705–19; CrossRef
30. Coria-Oriundo LL, Battaglini F, Wirth SA. Efficient decolorization of recalcitrant dyes at neutral/alkaline pH by a new bacterial laccase-mediator system. Ecotoxicol Environ Saf 2021;217:112237; CrossRef
31. Buzzo BB, Lima NSM, Pereira PAM, Gomes-Pepe ES, Sartini CCF, Lemos EGDM. Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp. Microbiol Spectr 2024;12(6):e04013–23; CrossRef
32. Sondhi S, Kaur R, Madan J. Purification and characterization of a novel white highly thermo stable laccase from a novel Bacillus sp. MSK-01 having potential to be used as anticancer agent. Int J Biol Macromol 2021;170:232–8; CrossRef
33. Rampinelli JR, Melo MPD, Arbigaus A, Silveira MLD, Wagner TM, Gern RM, et al. Production of Pleurotus sajor-caju crude enzyme broth and its applicability for the removal of bisphenol A. An Acad Bras Ciênci 2021;93(1):e20191153; CrossRef
34. Umar A, Ahmed S. Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci Rep 2022;12(1):2416; CrossRef
35. Albulaihed Y, Adnan M, Jamal A, Snoussi M, Patel K, Patel M. Optimization of laccase from Stenotrophomonas maltophilia E1 by submerge fermentation using coconut husk with its detoxification and biodecolorization ability of synthetic dyes. Bioresour Bioprocess 2023;10(1):80; CrossRef
36. Pinheiro VE, Michelin M, Vici AC, de Almeida PZ, Teixeira de Moraes Polizeli MDL. Trametes versicolor laccase production using agricultural wastes: a comparative study in Erlenmeyer flasks, bioreactor and tray. Bioprocess Biosyst Eng 2020;43:507–14; CrossRef
37. Geethanjali PA, Gowtham HG, Jayashankar M. Optimization of culture conditions for hyper-production of laccase from an indigenous litter dwelling fungus Mucor circinelloides GL1. Environ Sustain 2020;3:481–95; CrossRef
38. Umar A, Abid I, Elshikh MS, Dufossé L, Abdel-Azeem AM, Ali I. Agitation role (dissolved oxygen) in production of laccase from newly identified Ganoderma multistipitatum sp. nov. and its effect on mycelium morphology. BMC Microbiol 2023;23:280; CrossRef
39. Hamill PG, Stevenson A, McMullan PE, Williams JP, Lewis AD, Stevenson KE. Microbial lag phase can be indicative of, or independent from, cellular stress. Sci Rep 2020;10(1):5948; CrossRef
40. Rajeswari M, Bhuvaneswari V. Production of extracellular laccase from the newly isolated Bacillus sp. PK4. Afr J Biotechnol 2016;15(34):1813–26.
41. Sharma A, Muthupriya M, Raj R, Shameen Z, Veena SM, Niyonzima FN, et al. Properties of laccase of Bacillus marisflavi strain BB4 and its synthetic dyes decolorization analysis. Proc Natl Acad Sci India Sect B Biol Sci 2021;91(2):477–85; CrossRef
42. Hasan S, Anwar Z, Khalid W, Afzal F, Zafar M, Ali U, et al. Laccase production from local biomass using solid state fermentation. Fermentation 2023;9(2):179; CrossRef
43. Varjani S, Rakholiya P, Ng HY, You S, Teixeira JA. Microbial degradation of dyes: an overview. Bioresour Technol 2020;314:123728; CrossRef
44. Paraschiv G, Ferdes M, Ionescu M, Moiceanu G, Zabava BS, Dinca MN. Laccases—versatile enzymes used to reduce environmental pollution. Energies 2022;15(5):1835; CrossRef
45. Asadi E, Makhdoumi A, Asoodeh A. Laccase mediator system obtained from a marine spore exhibits decolorization potential in harsh environmental conditions. Ecotoxicol Environ Saf 2020;191:110184; CrossRef
46. Sundarajoo A, Maniyam MN, Azman HH, Abdullah H, Yaacob NS. Rhodococcus strain UCC 0010 as green biocatalyst for enhanced biodecolourization of Congo red through response surface methodology. Int J Environ Sci Technol 2022;19:3305–22; CrossRef
47.Motamedi E, Kavousi K, Motahar SFS, Ghaffari MR, Mamaghani ASA, Salekdeh GH, et al. Efficient removal of various textile dyes from wastewater by novel thermo-halotolerant laccase. Bioresour Technol 2021;337:125468; CrossRef
48. El-Bendary MA, Ezzat SM, Ewais EA, Al-Zalama MA. Optimization of spore laccase production by Bacillus amyloliquefaciens isolated from wastewater and its potential in green biodecolorization of synthetic textile dyes. Prep Biochem Biotechnol 2021;51(1):16–27; CrossRef
49. Toker SK, Evlat H, Koçyi??i?t A. Screening of newly isolated marine-derived fungi for their laccase production and decolorization of different dye types. Reg Stud Mar Sci 2021;45:101837.
50. Liu J, Chen J, Zuo K, Li H, Peng F, Ran Q, et al. Chemically induced oxidative stress improved bacterial laccase-mediated degradation and detoxification of the synthetic dyes. Ecotoxicol Environ Saf 2021;226:112823; CrossRef
51. Unuofin JO. Sustainability potentials of novel laccase tinctures from Stenotrophomonas maltophilia BIJ16 and Bordetella bronchiseptica HSO16: from dye decolourization to denim bioscouring. Biotechnol Rep 2020;25:e00409; CrossRef
52. Jiang J, Deng JL, Wang ZG, Chen XY, Wang SJ, Wang YC. Characterization of a new laccase from Vibrio sp. with pH-stability, salt-tolerance and decolorization ability. Molecules 2023;28(7):3037; CrossRef
53. Jain D, Navariya JK, Bhojiya AA, Singh A, Mohanty SR, Upadhyay SK. Bioprospecting of novel ligninolytic bacteria for effective bioremediation of agricultural by-product and synthetic pollutant dyes. Microbiol Res 2023;270:127330.
54. Iqbal K, Nadeem A, Zafar U. Biostoning of textile effluent with laccase enzyme. Bangladesh J Sci Ind Res 2021;56(2):115–24; CrossRef
55. Wang X, Jiang J, Gao W. Reviewing textile wastewater produced by industries: characteristics, environmental impacts and treatment strategies. Water Sci Technol 2022;85(7):2076–96; CrossRef
56. Uddin F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 2021;28(17):10715–39; CrossRef
57. Selim MT, Salem SS, Mohamed AA, El-Gamal MS, Awad MF, Fouda A. Biological treatment of real textile effluent using Aspergillus flavus and Fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J Fungi 2021;7(3):193; CrossRef
58. Bilal Y, Sarwar T, Mazhar E, Mahmood F, Shahzad T, Al-Farraj DA, et al. Reduction in phytotoxicity of a textile wastewater against Vigna radiata using Citrobacter sp. M41 in a bioaugmented packed bed column bioreactor. J King Saud Univ Sci 2024;36(2):103030; CrossRef