1. INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) are one of the essential microbial communities thriving in the rhizosphere [1]. AMF colonization starts with the spores, they start the catabolism of stored lipids and then feed the byproducts to the pre-developmental hyphae for germination [2]. The mycelium that grows outside of the root is referred to as extraradical mycelium. The location of fungal structures within roots has led to the classification of Arbuscular mycorrhizal (AM) fungal colonization into three types: Arum, Paris, and Intermediate. The linear fungal hyphae of the Arum-type, which is common in field crops, propagate intracellularly and develop into arbuscules on brief lateral branches [3]. The fungal hyphae in the Paris-type, which is more prevalent in naturally developing plants, spread intracellularly across cells to create hyphal or arbusculate coils [4,5].
AMF–phylum Glomeromycota are known to play a significant role in terrestrial ecosystems because they help to improve plant nutrition, particularly through the absorption of low mobility nutrients from soil solutions such as P, Zn, and ultimately Cu, to increase plant ability to withstand drought, salinity, and pathogen tolerance, and to improve soil quality framework [6–8]. AM symbiosis can be expressed as the relationship between soil-borne fungi and the roots of higher plants, which corresponds to an advantageous relationship without jeopardizing the plant [9].
A significant portion of the plant approximately 72% of plant species on land that these ancient groups of fungi establish connections with has obligatory symbionts as an important part. They form their relationship with soil and plants by extending their extra radical mycelium in the soil beyond the depletion zone, they improve the uptake of water and nutrients, especially immobile nutrients like phosphorus [P], which is important for agroecosystems [10–13]. According to DNA sequence evaluations and fossilized evidence, the relationship between fungi and the roots of plants has existed for at least 450 million years [14,15]. To date, 334 AM fungi have currently been identified worldwide [16].
The functions of AMF in ecosystems have not received much attention; however, a number of functions related to plant growth responses to AM colonization, beyond just phosphorus uptake, have been reported [17,18]. Moreover, there is evidence suggesting that variations in the composition of AMF communities may have distinct effects on plants, and may contribute to plant diversity, ecosystem variability, and productivity.
Although the distribution of AMF is worldwide but the diversity of AMF is more in tropical regions. Tropical forests, play an important role in the proliferation of AMF in the biodiverse ecosystems. The distribution of AM fungi in tropical forests is influenced by various factors including soil type, climate, vegetation, and land-use practices.
Invasive plants are a new challenge for the growth and development of native plant species. Natural activities and human activities both are responsible for the introduction and invasion of alien plant species in the natural forest [19].
Invasive species known as weeds invade controlled and natural settings, in which they supposedly fight with other plants for resources including water, nutrients, sunshine, and space [20]. Enemy release, resource utilization efficiency, and the new weapons hypothesis are a few of the highly complicated mechanisms and processes underlying plant invasion in non-native locations [17–23]. Climate change, enemy release, and several other theories have been the subject of numerous studies. Still, little is known about biotic resistance and how it influences how some soils become more prone to plant invasion than others [23,24]. It has been demonstrated that invasive plants benefit from these typically common beneficial fungi during invasion [25,26].
The role of below-ground diversity is very important and has a positive relation with above-ground diversity, which has been poorly studied in relation to the expansion and invasion of invasive species in tropical forests. AMF is one of the major components of below-ground diversity which have a unique role in plant growth and development. In view of the role of AM fungi in the regeneration, growth, and development of native and invasive plants, the present study aimed to study the colonization pattern of AMF in the root of common invasive plants and dominant tree species of the tropical forest to understand the possible mechanism of sustainable invasion of invasive plant species.
2. MATERIALS AND METHODS
2.1. Study Area
Tropical Dry Deciduous Forest and Tropical Moist Deciduous Forests are the two main types of forests found in Chhattisgarh. The study site is situated at 22° 30′32″ N, 82° 4′6″ E, in Bilaspur Forest Division, Chhattisgarh, India. This location is 55 km away from Bilaspur district. This study site is dominated by Teak trees. Along with teak and other forest tree, many invasive plant species also thrive in Belgahna forest. The samples were collected from 10 common invasive plants and the teak plant from the teak-dominated tropical dry deciduous forest area of Belgahna.
2.2. Selection and Identification of Plants
The plants have been selected on the basis of their presence near the forest tree species. Plants were identified on the basis of morphological traits. Ten common herbaceous plants were selected on the basis of availability near the forest tree (Table 1).
2.3. Sample Collection
The investigation was carried out in the teak-dominated forest area of Belgahna, Forest Area. Root samples were collected from the rhizosphere of the 10 selected invasive plants and the dominant tree Tectona grandis. Root samples were collected in triplicate. Samples were brought to the laboratory for processing of root samples. Root samples were processed immediately.
2.4. Root Clearing and Staining Process
The “Rapid Clearing and Staining Method” was used to assess AM colonization in the roots [23,27]. Roots were gently washed 2–3 times with distilled water and chopped into small segments approximately 1 cm long. These root segments underwent a 24-hour cleaning procedure at 90°C in 10% KOH for 1 hour, then a 20-minute 1% HCl acidification. After acidification roots were stained with Lactophenol cotton blue stain for 24 hours, following this, stained root segments were placed in Lactophenol for a day to remove extra stain and then put into a glass tube for further process and study.
2.5. Assessment of AM Fungal Root Colonization
AM colonization was assessed based on the presence of AM fungal structure inside the root. Approximately 100 segments of the root of each plant species were studied for the assessment of AM colonization. Stained roots were placed on the slide and were mounted in a lactic phenol solution. Each slide has 10 root pieces, observed under a microscope for the presence of AM colonizing structures, i.e., hyphal coil, arbuscules, vesicles, intraradical spore, and colonization pattern of AM fungi. The root slide technique was used to evaluate the root colonization [28].
 | Table 1. Invasive plants selected for AMF colonization pattern from the tropical dry deciduous forest of Belgahna. [Click here to view] |
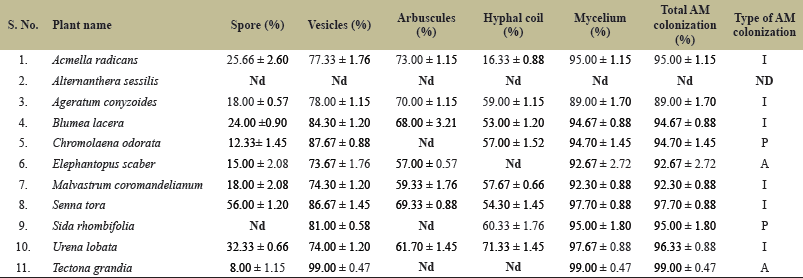 | Table 2. Different attributes of AMF colonization.Nd = Not detected, I = Intermediate type, A = Arum type, P = Paris type. [Click here to view] |
2.6. Micrography and Data Analysis
The micrographs of AM colonization were taken by magnus MX21Iledfs11 microscope and micrographs were analyzed by using HP Wide Vision HD Camera. The mean value of AMF colonization, number of vesicles, hyphal coil, arbuscules, and spores were recorded manually. Mean value, standard deviation, and standard error have been calculated by using MS Excel.
3. RESULT
The colonization percentages of various plant species by AMF across different colonization parameters, including, vesicles, arbuscules, hyphal coils, mycelium, and total colonization percentage in the cortical cells of plant root.
The result of the present study has shown AMF colonization in all invasive plants except Alternanthera sessilis and the native tree species T. grandis plant species from Belgahna Teak Forest. The roots have shown three morphotypes of AMF which are Arum-type, Paris-type, and Intermediate-type. Arum-type, characterized by arbuscules, and Paris-type, characterized by coils; nevertheless, data indicates that, depending on the host plant and the fungus, there may be a continuum between both. Intercellular or intracellular linear hyphae in intracellular hyphal coils or arbuscules indicate intermediate-type AM morphology. No detectable AM fungal structures were observed in A. sessilis indicating a lack of association with AMF, hence, a non-mycorrhizal plant (Table 2; Fig. 1 ALT 1-3).
 | Figure 1. Different attributes of AM fungi in invasive plants. ACM, Acmella radicans; ALT, Alternanthera sessilis; AZE, Ageratum conyzoides; BLU, Blumea lacera; CHR, Chromolaena odorata; V, Vesicles; ARB, Arbuscules; HC, Hyphal Coil; S, Spore; and Bar-100 μm. [Click here to view] |
The root pieces of all eight invasive species and one native tree species T. grandis were observed under the microscope representing a variety of AMF colonization patterns. The percentage root segment colonization of AM fungi was more than 89% in invasive as well as in native plant roots. All three types of AM colonization patterns were observed in the study area. Among the three morphotypes of AM fungi, intermediate types of AM colonization were dominant with the roots of six invasive plant species namely Acmella radicans, Ageratum conyzoides, Blumea lacera, Malvastrum coromandelianum, Senna tora, and Urena lobata plant species (Table 2; Fig. 1, ACM-1-3, AZE-1-3, BLU-1-3; Fig. 2. MAL 1-3, SEN-1-3, URE 1-3). Paris-type of AM colonization was observed in Chromolaena odorata (Fig. 1 CHR 1-3) and Sida rhombifolia (Fig. 2. SID 1-3). Arum-type had fungal hyphae in the intercellular space and arbuscules emerged from intercellular hyphae present in the cortical cells of Elephantopus scaber (Fig. 2, ELE-1-3). Arum-type od AM colonization was predominantly detected in the dominant tree species T. grandis (Fig. 3 TEC 1-3) with the highest degree of AM colonization in the study area.
 | Figure 2. Different attributes of AM fungi in invasive plants. ELE, Elephantopus scaber; MAL, Malva spp; SEN, Senna tora; SID, Sida rhombifolia; URE, Urena lobata; Tectona grandis, L., V, Vesicles, ARB, Arbuscules; HC, Hyphal Coil; S, Spore; and Bar-100 μm. [Click here to view] |
 | Figure 3. Different attributes of AM fungi in dominant native plant Tectona grandis. V, Vesicles; S, Spore; Bar-100 μm. [Click here to view] |
Arbuscules are the site of nutrient transfer from the host cell to the hyphae of AMF and vice versa. In the rest of the 10 plant species, arbuscules were observed in seven invasive species. The presence of arbuscules percentage ranged from 57.00 ± 0.57 to 73.00 ± 1.15 root. Arbuscules were not detected in the roots of A. sessilis, C. odorata, and S. rhombifolia. Another important structure of AMF fungi is the hyphal coil which was present in eight invasive plant species and T. grandis (Table 2; Fig. 3 TEC 1-3).
A maximum number (87.67 ± 0.88) and minimum number (73.67 ± 1.76) of vesicle percentage was found in C. odorata and E. scaber, respectively. Different shapes and sizes of vesicles were observed in the host plants. Oblong and spherical vesicles were the most common followed by elliptical rectangular and irregular vesicles. Some vesicles had hyphal attachments in the base (Figs. 1 and 2). Oval and elliptical vesicles between the cortical cells can be considered Glomus species (Figs. 1 and 2, BLU-1; CHR1-2; SID-2,3; SEN-1). Irregularly shaped vesicles or rectangular vesicles can be an indication of Acaulospora species. giant oblong vesicles were also observed but could not be linked to any particular genus (Fig. 1, ACM-1).
Some of the host plants had spores in the cortical cells (Fig. 1 CHR-2). One of the host plants had sclerotium (Fig. 1, CHR-3). Though various spores were seen during the microscopic study in different host plants, a thin-walled small spore was captured in the roots of C. odorata (Fig. 1, CHR-2). There was no correlation observed between AM colonization and intraradical spores. The higher degree of spores was observed inside root cells was in S. tora, and the minimum number of spores was in T. grandis, irrespective of the highest AM colonization (Table 2).
4. DISCUSSION
AMFs are known to be multifunctional in natural ecosystems as well as in agricultural land and improve soil by improving the physical, chemical, and biological properties of soil [29,30]. The level of mycorrhizal association is more or less similar in invasive plant species and native tree species. The degree of AMF association can affect nutrient competition and absorption rates in an invaded environment [31]. Out of 10 invasive plants, 9 have shown great AM colonization which can suggest higher AMF dependency of plants [32]. Some previous studies also suggest that some invasive plants can be non-mycorrhizal [33,34]. Invasive plant species unveiled noteworthy differences in structures of vesicles, hyphae, hyphal coil, arbuscules, and AMF percent colonization rates some previous studies also suggested that the symbiosis of AMF may vary in native and invasive plants [35,36]. Some of the plants use alternate methods for disrupting AMF colonization in native plants which is reported in previous studies that invasive plants may release some bio-chemicals in soil which affects native plants’ growth and creates significant competitiveness [18,34,37]. The presence of AMF in all the examined host plants suggests the widespread association of AMF in invasive plants. Some previous studies have also reported the AMF association with invasive plants and their description [38–41]. AMF morphotype identification was earlier reported in the plants of the family Solanaceae [29]. The vesicle is known as one of the typical organs of mycorrhizal fungi that store nutrients, and it plays a role in the host plants’ survival [31,42]. For the host plant to withstand unfavorable environmental circumstances, the creation of a vesicle structure can protect its limited supply of carbohydrates [31,43]. The variations in the structures of vesicles show how they alter different plants and their development in identical surroundings. In addition, with recent findings [31,44,45], hyphal development and penetration can be the primary method for AMF infection in invasive species. One of the most important aspects of an AM fungus’s life cycle is the production of storage organs such as spores and vesicles [46]. Oval and elliptical vesicles in intercellular space are significant for Glomus species and have been reported previously in different plants [47,48]. Irregular, oblong-lobed vesicles were linked to Genus-Acaulospora in this study similarly some previous studies also reported that irregular-lobed vesicles are a characteristic feature of the Genus Acaulospora [47–49]. A comparative study of AM colonization in invasive plant species and the dominant tree species shows a similar level of AM colonization. This may be one of the reasons that invasive plants very well thrive in the tropical forest.
5. CONCLUSION
AMF are mutualistic fungi, ubiquitous in nature. AM fungi play an important role in the plant’s growth and distribution. Invasive plants grow well in adverse conditions and compete for nutrition and space with the seedlings of forest trees. A similar type of AM fungal colonization pattern was detected in the roots of invasive and native plants. Although these invasive plants compete with native plants; on the other hand, these invasive plants are a good source for the proliferation of AM fungi in the forest soil. These annual invasive plants’ roots may provide a good source of inoculum for the regeneration of native and invasive plants in favorable seasons.
6. ACKNOWLEDGMENTS
The authors are thankful to the Head of the Department of Rural Technology and Social Development, Guru Ghasi Vishwavidyalaya, Koni, Bilaspur, Chhattisgarh, for providing laboratory facilities.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. FUNDING
There is no funding to report.
9. AUTHOR CONTRIBUTIONS
All authors are contributed equally to the plan and execution of this study made noteworthy contributions to plan and design, and acquisition of data; took part in framing the article or rechecking it critically for essential intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be account for all aspects of the work.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. PUBLISHER’S NOTE:
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
13. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Juntahum S, Kuyper TW, Boonlue S. Distribution of arbuscular mycorrhizal fungi in sugarcane rhizosphere from various agricultural management practices in Northeast, Thailand. Curr Res Environ Appl Mycol 2022;12(1):44–55; http://doi.org/10.5943/CREAM/12/1/4’
2. Akiyama K, Matsuzaki Ki, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005;435:824–7; http://doi.org/10.1038/nature03608
3. Smith SE, Read DJ. Mycorrhizal symbiosis. Academic Press, Cambridge, MA, 2008.
4. Smith FA, Smith, SE. Tansley review no. 96 structural diversity in (vesicular)–arbuscular mycorrhizal symbioses. New Phytol 1997;137(3):373–88.
5. Dickson S, Smith FA, Smith SE. Structural differences in arbuscular mycorrhizal symbioses: more than 100 years after Gallaud, where next?. Mycorrhiza 2007;17:375–93.
6. Adejuwon JO, Ekanade O. Edaphic component of the environmental degradation resulting from the replacement of tropical rain forest by field and tree crops in SW Nigeria. Int Tree Crops J 1987;4(4):269–82; http://doi.org/10.1080/01435698.1987.9752828
7. Jasper D, Abbott L, Robson A. Soil disturbance in native ecosystems-the decline and recovery of infectivity of VA mycorrhizal fungi. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds.). Mycorrhizas in ecosystems, CAB International, Wallingford, UK, pp 151–5, 1992.
8. Baral K, Giri A, Shah PK, Kemmelmeier K, Stürmer SL, Gyawali S, et al. Diversity of arbuscular mycorrhizal fungi (Glomeromycota) in adjacent areas of different land use in Nepal. GSCBPS 2021;15(1):141–50; http://doi.org/10.30574/gscbps.2021.15.1.0098
9. Rautela I, Auravindam R, Semwal A, Sharma J, Kunwar S, Sharma A, et al. Isolation of VAM from Dehradun and a study for determining bio synergistic effects of VAM strengthening possible future strategy design for eradicating soil pollution. Plant Arch 2020;20(2):3701–6. Available via https://www.plantarchives.org/20-2/3701-3706%20(6353)
10. Aroca R, Porcel R, Ruiz-Lozano, JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold, or salinity stresses? New Phytol 2007;173(4):808–16; http://doi.org/ 10.1111/j.1469-8137.2006.01961.x
11. Smith SE, Read DJ. Mycorrhizal symbiosis. Academic Press, Cambridge, MA, 2010.
12. Sharif M, Claassen N. Action mechanisms of arbuscular mycorrhizal fungi in phosphorus uptake by Capsicum annuum L. Pedosphere 2011;21(4):502–11.
13. Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key players in sustainable agriculture by improving soil fertility, plant tolerance, and crop productivity. Microb Cell Fact 2014;13:1–10; http://doi.org/10.1186/1475-2859-13-66
14. Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013;23:515–31; http://doi.org/10.1007/s00572-013-0486-y
15. Delaux PM. Comparative phylogenomics of symbiotic associations. New Phytol 2017;213(1):89–94; http://doi.org/10.1111/nph.14161
16. AMF – Phylogeny. Glomeromycota TAXONOMY. 2019. Available via http://www.amfphylogeny.com/amphylo_taxonomy.html17.
17. Callaway RM, Thelen GC, Rodriguez A, Holben WE. Soil biota and exotic plant invasion. Nature 2004;427(6976):731–3; http://doi.org/10.1038/nature02322
18. Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, et al. Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008;89:1043–55; http://doi.org/10.1890/07-0370.1
19. Richardson DM, Holmes PM, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, et al. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers Distrib 2007;13(1):126–39; http://doi.org/10.1111/j.1366-9516.2006.00314.x
20. El Omari B, El Ghachtouli N. Arbuscular mycorrhizal fungi-weeds interaction in cropping and unmanaged ecosystems: a review. Symbiosis 2021;83(3):279–92; http://doi.org/10.1007/s13199-021-00753-9
21. Rai PK. Plant invasion ecology: impacts and sustainable management. Nova Science Publishers, Incorporated, New York, NY, 2013.
22. Dawkins K, Esiobu N. Emerging insights on Brazilian pepper tree (Schinus terebinthifolius) invasion: the potential role of soil microorganisms. Front Plant Sci 2016;7:712; http://doi.org/10.3389/fpls.2016.00712
23. Dawkins K, Esiobu N. Arbuscular and ectomycorrhizal fungi associated with the invasive Brazilian pepper tree (Schinus terebinthifolius) and two native plants in South Florida. Front Microbiol 2017;8:665; http://doi.org/10.3389/fmicb.2017.00665
24. Levine JM, Adler PB, Yelenik SG. A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 2004;7:975–89; http://doi.org/10.1111/j.1461- 0248.2004.00657.x
25. Richardson DM, Williams PA, Hobbs RJ. Pine invasions in the Southern Hemisphere- determinants of spread and invadability. J Biogeogr 1994;21:511–27.
26. Lekberg Y, Gibbons SM, Rosendahl S, Ramsey PW. Severe plant invasions can increase mycorrhizal fungal abundance and diversity. ISME J 2013;7(7):1424–33; http://doi.org/10.1038/ismej.2013.41
27. Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 1970;55:118–58.
28. Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 1980;84:489–500 http://doi.org/10.1111/J.1469-8137.1980.Tb04556.X
29. Muthukumar T, Sathya R. Endomycorrhizal fungal association and colonization patterns in Solanaceae. Pol Bot J 2017;62(2):287–99; http://doi.org/10.1515/pbj-2017-0016
30. Cardoso IM, Kuyper TW. Mycorrhizas and tropical soil fertility. Agric Ecosyst Environ 2006;116(1-2):72–84; http://doi.org/10.1016/j.agee.2006.03.011
31. Zeng K, Huang D, Zhang X, Liu S, Huang X, Xin G. Fern species and seasonal variation alter arbuscular mycorrhizal fungal colonization and co-occurrence patterns in the Heishiding Natural Reserve, South China. Appl Soil Ecol 2024;193:105172; http://doi.org/10.1016/j.apsoil.2023.105172
32. Fu W, Chen B, Rillig MC, Jansa J, Ma W, Xu C, et al. Soil disturbance in native ecosystems-the decline and recovery of infectivity of VA mycorrhizal fungi. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds.). Mycorrhizas in ecosystems, CAB International, Wallingford, UK, pp 151–5, 1992; http://doi.org/10.1007/s11258-021-01160-2
33. Pringle A, Bever JD, Gardes M, Parrent JL, Rillig MC, Klironomos JN. Mycorrhizal symbioses and plant invasions. Annu Rev Ecol Evol Syst 2009;40:699–715.
34. Soti PG, Jayachandran K, Purcell M, Volin JC, Kitajima K. Mycorrhizal symbiosis and Lygodium microphyllum invasion in South Florida-a biogeographic comparison. Symbiosis 2014;62:81–90; http://doi.org/10.1007/s13199-014-0272-4
35. Fei S, Kivlin SN, Domke GM, Jo I, LaRue EA, Phillips RP. Coupling of plant and mycorrhizal fungal diversity: its occurrence, relevance, and possible implications under global change. New Phytol 2022;234:1960–6; http://doi.org/10.1111/nph.17954
36. Zubek S, Kapusta P, Rozek K, Blaszkowski J, Gielas I, Nobis M, et al. Fungal root colonization and arbuscular mycorrhizal fungi diversity in soils of grasslands with different mowing intensities. Appl Soil Ecol 2022;172:104358; http://doi.org/10.1016/j.apsoil.2021.10435837.
37. Callaway R, Aschehoug E. Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 2000;290:521–3; 10.1126/science.290.5491.521
38. Adholeya A, Tiwari P, Singh R. Large-scale inoculum production of arbuscular mycorrhizal fungi on root organs and inoculation strategies. In: Declerck S, Fortin JA, Strullu DG (eds.). In vitro culture of mycorrhizas, Springer, Berlin, Germany, pp 315–38, 2005; http://doi.org/10.1007/3-540-27331-X_17
39. Khade SW, Rodrigues BF, Applications of arbuscular mycorrhizal fungi in agroecosystems. TAEC 2009;10(3):337–54.
40. Chen M, Arato M, Borghi L, Nouri E, Reinhardt D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front Plant Sci 2018;9:408113; http://doi.org/10.3389/fpls.2018.01270
41. Chen E, Liao H, Chen B, Peng S. Arbuscular mycorrhizal fungi are a double-edged sword in plant invasion controlled by phosphorus concentration. New Phytol 2020;226(2):295–300; http://doi.org/10.1111/nph.16359
42. Schmid E, Oberwinkler F. A light- and electron-microscopic study on a vesicular-arbuscular host-fungus interaction in gametophytes and young sporophytes of the Gleicheniaceae (Filicales). New Phytol 1995;129:317–24; http://doi.org/10.1111/j.1469-8137.1995.tb04302.x
43. Wang Y, Li Y, Li S, Rosendahl S. Ignored diversity of arbuscular mycorrhizal fungi in co-occurring mycotrophic and non-mycotrophic plants. Mycorrhiza 2021;31:93–102. Available via https://link.springer.com/content/pdf/10.1007/s00572-020-00997-1.pdf
44. Bautista-Cruz AA, Montaño NM, Camargo-Ricalde SL, Pacheco L. Arbuscular mycorrhizal fungi and soil nutrients associated with four fern species in two ecosystems in Oaxaca, Mexico. Rev Chapingo Ser Cie For Ambient 2014;20(3):199–212; http://doi.org/10.5154/r.rchscfa.2014.02.007
45. Krzyzanski H, Milaneze-Gutierre MA, Carrenho R. Arbuscular mycorrhizal and dark septate fungi are not common in roots of epiphytic pteridophytes of a transitional forest area in South Brazil. Acta Bot Bras 2021;35:569–76; http://doi.org/10.1590/0102-33062020abb0396
46. Müller A, Ngwene B, Peiter E, George E. Quantity and distribution of arbuscular mycorrhizal fungal storage organs within dead roots. Mycorrhiza 2017;27:201–10; http://doi.org/10.1007/s00572-016-0741-0
47. Dodd JC, Boddington CL, Rodriguez A, Gonzalez-Chavez C, Mansur I. Mycelium of arbuscular mycorrhizal fungi (AMF) from different genera: form, function and detection. Plant Soil 2000;226:131–51; http://doi.org/10.1023/A:1026574828169
48. Beck A, Haug I, Oberwinkler F, Kottke I. Structural characterization and molecular identification of arbuscular mycorrhiza morphotypes of Alzatea verticillata (Alzateaceae), a prominent tree in the tropical mountain rain forest of South Ecuador. Mycorrhiza 2007;17:607–25; http://doi.org/10.1007/s00572-007-0139-0
49. Morton JB, Redecker D. Two new families of Glomales, Archaeosporaceae and Paraglomeraceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 2001;93:181–95; http://doi.org/10.1080/00275514.2001.12063147