1. INTRODUCTION
Cyclophilins are ubiquitous proteins found among almost all genera of bacteria, higher plants, humans, and fungi. Cyclophilins are a class of immunophilins that possess binding ability towards immunosuppressant drugs cyclosporin A, FK506, and rapamycin impeding translocation of nuclear factor of activated T-cells [1–3]. The complex blocks elicitation of mRNA of interleukin-2, interleukin-4, and interferon, thereby, stopping T-cell activation in animals [4]. Cyclophilins particularly bind to cyclosporin A and show peptidyl-propryl activity that is cis to trans inter-conversion of proline peptide bond [5]. This cis to trans inter-conversion plays an important role in protein folding suggesting the role of cyclophilin as a chaperon [6,7]. In plants, cyclophilins have different roles such as pre-mRNA splicing [8], transcription regulation [9], cell division [10], signaling [11,12], and stress tolerance [13–15]. Single-domain cyclophilins have only one CYP domain. However, multi-domain has additional domains like U-box for ubiquitination, tetratrico peptide repeats (TPR for protein-protein interactions, and assembly of multi-protein complexes, WD-40 for assembly of multi-protein complexes), RNA recognition motif (RRM) for regulation of transcription, helical bundle for signal transduction, PsbQ-like for plant-specific oxygen-evolving enhancer protein 3 and last but not least RRM + Zf for RNA splicing [16]. WD40, TPR, and F-box were reported to have protein-protein interactions [17,18].
Over the past few years, genome-wide studies in plants have proven to be a great tool aiding in the achievement of cyclophilins. The highest numbers of cyclophilins have been reported in Brassica napus (94) with 79 single-domain and 12 were multi-domain [19]. Most cyclophilins were found to be localized in cytoplasm while the least in mitochondria. Similarly, a total of 85 cyclophilins were identified from Triticum aestivum with the majority of them residing in cytoplasm. Out of which, 27 had single domains and 58 had additional domains [20]. Apart from these, 31 cyclophilins in Arabidopsis thaliana [21], 75 in Gossypium barbadense, 78 in Gossypium hirsutum, 40 in Gossypium arboreum, 38 in Gossypium raimondii [22], 35 in Solanum lycopersicum [23], 33 in Medicago truncatula [24], 30 in Medicago domestica [25], 29 in Oryza sativa [21], and 62 in Glycine max [26] have been reported till now.
In this study, we have focused on three important plant species Cucumis sativus, Phaseolus vulgaris, and Vitis vinifera. Phaseolus vulgaris (common bean), a member of the Fabaceae family is an economic source of protein for a vegetarian staple diet in America, Africa, India, Brazil, and China holding 17%–30% of dry weight protein. Phaseolus vulgaris faces approximately 60% yield loss due to drought stress solely. Apart from this high saline concentration results in imbalanced ions and photograph inhibition further contributing to major yield loss [27–29]. According to a 2020 report to the New York Agricultural Experiment Station, common bean crops suffer significant losses from bacterial diseases like common bacterial blight and halo blight, caused by Xanthomonas axonopodis and Pseudomonas syringae pv. Phaseolicola, respectively. Additionally, common rust, caused by Uromyces appendiculatus, and viruses such as bean common mosaic virus and bean yellow mosaic virus also contribute substantially to yield reductions in common beans [30–32].
Cucumis sativus is a creeper plant from the Cucurbitaceae family known for its edible fruit and is native to India. Apart from being consumed as fruit is also possesses ayurvedic and cosmetic properties [33,34]. Cucumber faces oxygen depletion and reduced yield in water logging conditions. High salt stress causes ionic imbalance leading to chlorosis, hindering growth, and affecting fruit quality whereas high temperature degrades protein and seedlings in early stages. Biotic stresses from powdery mildew, downy mildew, anthracnose, fusarium wilt, and bacterial wilt also add to significant production loss in cucumber [35–41].
Problems arising due to various biotic and abiotic stresses are also significant in V. vinifera. Grapevine is widely used for the production of wine, and juices and consumed in fresh and dried forms [42]. Drought and saline stress causing leaf shedding and retarded photosynthetic rates leads to poor berry quality with compromised taste, size, and color [43,44]. Apart from this grey molds, powdery mildew, and downy mildew also add to huge yield and quality loss [45–47].
Previous studies on cyclophilins from A. thaliana have revealed the roles of AtCYP21-2, AtCyP18-1, and AtCYP20-2 in drought stress, temperature stress, and light stress tolerance. OsCYP20-2, OsCyp2 (Os02g0121300), and OsCYP18-2 from O. sativa showed upregulated levels on light, saline, and drought stress [16,48]. The role of Arabidopsis cyclophilin ROC1/AtCYP18-3 and GmCYP1 cyclophilin of soybean also provides defense against P. syringae and Phytophthora sojae infection, proving its multi diverse role in both abiotic and biotic stress tolerance [49,50]. However, only a handful of genome-wide analysis have been conducted so far on various plants in an attempt to identify this important class of proteins.
In view of the above, it is important to study the evolution, expression, and functional role of cyclophilins in P. vulgaris, C. sativus, and V. vinifera. Hence, we have identified cyclophilins from P. vulgaris, C. sativus, and V. vinifera through genome-wide analysis. We hypothesize that our identification and characterization of cyclophilins from these three important plant species could provide crucial information regarding their distribution, expression, and evolution along with their putative role in biotic/abiotic stresses and other physiological traits.
2. MATERIALS AND METHODS
2.1 Protein Sequence Retrieval
Protein sequences of C. sativus, P. vulgaris, and V. vinifera were obtained from the NCBI database by using At1g01940 as a query sequence against default parameters [51].
2.2 Location, Molecular Weight (MW), Isoelectric Point (PI), and Sub Cellular Localization
Chromosome location, exon, and intron of PhvCYPs and GsvCYPs were obtained from the phytozome database [52], where parameters like proteome as target type, BLASTP as program type, −1 is selected as threshold, and BLOSUM62 as comparison matrix is used. In the case of CucCYPs Ensembl Plants using parameters like TBLASTN, 1e-1 E-value threshold, and BLOSUM62 search matrix were used [53]. The pictorial presentation was carried out using Mapchart software for individual chromosomes [54]. For the prediction of sub-cellular localization of protein sequences, the Wolfsport online tool was used [55]. MW and PI analyses were estimated through Prot pi [56].
2.3 Synonymous and Non-Synonymous Substitution Rates (Ka-Ks)
For synonymous and non-synonymous substitution rate analyses, KaKs_Calculator 2.0 was used utilizing genomic sequences of individual cyclophilin [57]. Genomic sequences were retrieved from the phytozome database [52].
2.4 Multiple Sequence Alignment and Phylogenetic Analysis
All the cyclophilin protein sequences (Supplementary Fig. S1) were aligned using BioEdit 7.2 [58]. The aligned sequences were used to generate a phylogenetic tree with neighbor-joining clustering and iTOL: Interactive tree of life to study the ancestral history of all the identified cyclophilins [59]. A cladogram was also constructed to study sequence similarity between homologous and previously reported cyclophilins with known tolerance against abiotic stresses. Chord diagrams were constructed among the cyclophilins from C. sativus, P. vulgaris, and V. vinifera with their previously reported counterparts from A. thaliana, G. max, and M. truncatula. This was performed in two steps. Initially, the data was structured using python (3.10.11) to generate a worksheet where all protein sequences were compared against each other and the matched sequences between the proteins were counted. Post formatting, Chord diagrams were created using the Python package d3blocks (https://d3blocks.github.io/d3blocks, Ver. 1.1.5).
2.5 Domain Search
For domain search, a batch-CD tool of NCBI was used to obtain the presence of single and multiple domains in each cyclophilin by searching amino acid sequence in CDD v3.1-62456 PSSMs version utilizing default parameters [60]. A pictorial presentation was made using GSDS 2.0 [61].
2.6 Co-Expression Analysis
Gene expression data of P. vulgaris were obtained from phytozome [52], while in case of C. sativus, cucurbit genome database (CuGenDB) from bioproject PRJNA80169 was used [62]. Expression data of V. vinifera was retrieved from the EMBL-EBI expression atlas [63]. Representation of expression data in heatmap was performed using TB tools software [64].
3. RESULTS
3.1 Genome-Wide Distribution of Cyclophilin Proteins
Genome-wide search of cyclophilins from C. sativus, P. vulgaris, and V. vinifera were conducted for the identification of putative cyclophilin proteins. Genome-wide analysis revealed the presence of a total of 21, 26, and 22 cyclophilins in C. sativus, P. vulgaris, and V. vinifera, respectively (Tables 1–3).
3.2 Cucumis sativus
In C. sativus, out of 21 cyclophilins, five cyclophilins are present on chromosomes 2 and chromosome 7 followed by three cyclophilins each on chromosomes 1 and chromosome 6, respectively (Fig. 1a). Two cyclophilins are present on chromosomes 3 and chromosome 5, whereas only one cyclophilin is present on chromosome 4 (Fig. 1a). In C. sativus, cyclophilin CucCYP21 is the largest protein with 799 amino acids while CucCYP7 with 111 amino acids is the smallest (Table 1). Out of 21 cyclophilins, 15 cyclophilins are single-domain while six are multi-domain cyclophilins (Supplementary Fig. S2). Cyclophilins viz. CucCYP1, CucCYP4, CucCYP6, CucCYP7, CucCYP8, CucCYP9, CucCYP10, CucCYP11, CucCYP12, CucCYP13, CucCYP14, CucCYP15, CucCYP18, CucCYP19, and CucCYP20 has Cyclophilin-like domain (CLD), which is responsible for cis to trans PPIase activity. The WD40 domain was found in CucCYP2 while TPRs and TPR-1 domains were observed in CucCYP3 and CucCYP16. CucCYP3 has a mitochondrial precursor protein import receptor domain (3a0801s09). Cyclophilin CucCYP5 is multi-domain protein and has RRM, polyadenylate binding protein (PABP_1234), glycine-rich RNA-binding protein (PLAN03134), transcription termination factor Rho (PRK12678), U2 small nuclear RNA auxiliary factor (U2AF_Ig), and zinc finger domain (Zf_CCHC). Ring_Ubox domain was observed in CucCYP17 while splicing factor CC1-like family (SF_CC1) domain was observed in CucCYP21 along with transcription regulator ICP4 (PHA03307) (Fig. 2a). Seven CucCYPs are localized in cytosol followed by six in chloroplast and only one was observed in vacuole (Table 1).
3.3 Phaseolus vulgaris
A total of 26 cyclophilins were identified on nine P. vulgaris chromosomes (Fig. 1b). Seven cyclophilins were found on chromosome 1, followed by four and three cyclophilins on chromosome 3 and chromosome 4, respectively. Chromosome 7 had two cyclophilins whereas chromosomes 5 and 6 have only one cyclophilin. In P. vulgaris, cyclophilin PhvCYP5 is the longest with 933 amino acids whereas PhvCYP14 is the shortest with 136 amino acids (Table 2). Out of 26 cyclophilins, 19 cyclophilins have a single CLD domain while seven cyclophilins are multiple domain proteins (Supplementary Fig. S2). Cyclophilins viz. PhvCYP1, PhvCYP2, PhvCYP3, PhvCYP4, PhvCYP6, PhvCYP7, PhvCYP9, PhvCYP10, PhvCYP11, PhvCYP13, PhvCYP14, PhvCYP16, PhvCYP17, PhvCYP19, PhvCYP20, PhvCYP21, PhvCYP23, PhvCYP25, and PhvCYP26 are single-domain cyclophilins. PhvCYP5 had PHA3307 as an additional domain, while PhvCYP8 hasRAM signaling pathway protein (SOG2) and104 kDa microneme (PTZ00449) along with PHA3307 domain. Cyclophilin PhvCYP12 has two pairs of ring_Ubox as extra domains. Cyclophilins PhvCYP15 and PhvCYP18 both have TPR_1 and TPR_19 domains. In addition, two more domains namely cytochrome c-type biogenesis protein (NrfG) domain and type IV pilus biogenesis/stability protein (TypeIV_pilW) domain were identified in PhvCYP18. WD40 domain was identified only in PhvCYP22. Two domains from super-family RRM and RRM_SF, polyadenylate binding protein (PABP_1234), and glycine-rich RNA-binding protein (PLAN03134) were observed in PhvCY24 (Fig. 2b). Localization of cyclophilins indicated that eight cyclophilins are localized in cytosol followed by seven in chloroplast and only one was localized in vacuole and mitochondria each (Table 2).
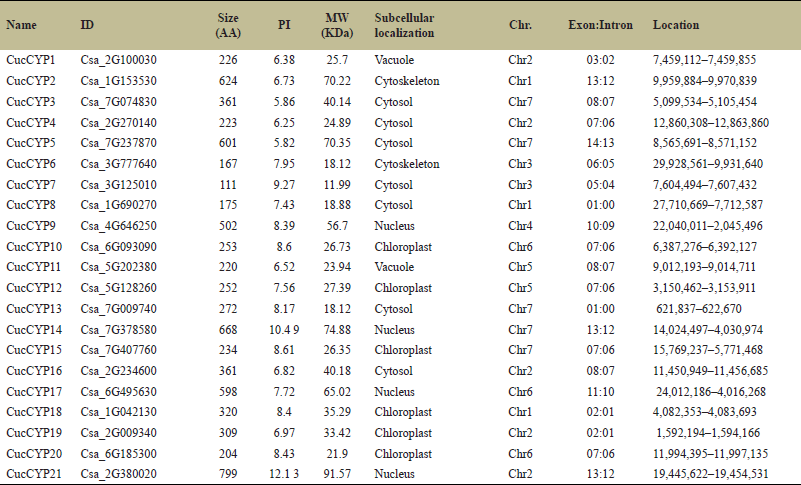 | Table 1. There are 21 cyclophilins identified in C. sativus, and for each of them, comprehensive information is available. This includes their ID, amino acid size, PI, MW, sub-cellular localization within the cell, chromosome location, exon-intron count, and specific positions on the chromosome. The gathered data offers a detailed overview of these cyclophilins and their characteristics in the context of C. sativus. [Click here to view] |
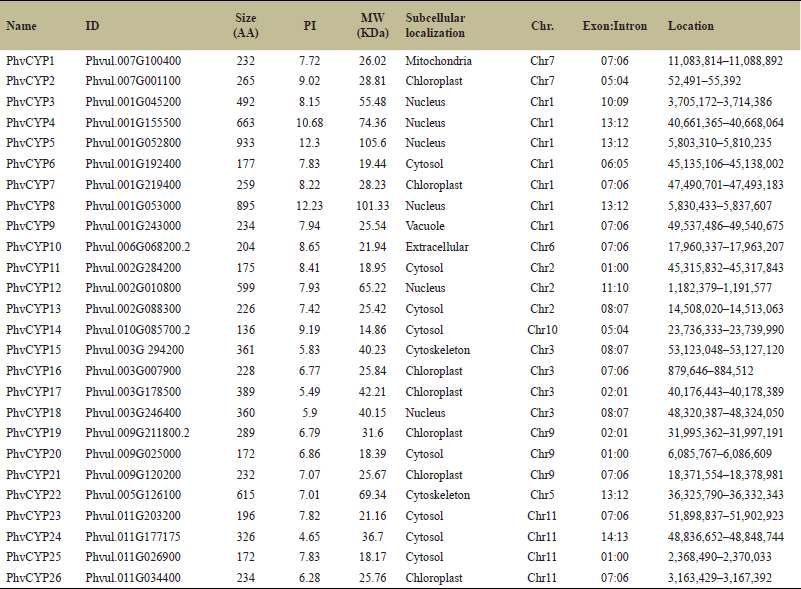 | Table 2. In total, there are 26 cyclophilins from P. vulgaris, each providing details such as ID, size (in amino acids), PI, MW, sub-cellular localization, chromosome location, exon-intron count, and their respective positions on the chromosome. [Click here to view] |
3.4 Vitis vinifera
In V. vinifera, 22 cyclophilins are distributed on 14 different chromosomes (Fig. 1c). Chromosomes 1, 3, 4, 6, 7, and 13 have two cyclophilins each, while chromosomes 5, 8, 11, 14, 15, 17, 18 and 19 have only one cyclophilin. The remaining two cyclophilins are located on two different scaffolds. In V. vinifera, cyclophilin GsvCYP16 is the largest with 700 amino acids while cyclophilin GsvCYP19 is the smallest with 66 amino acids (Table 3). Out of 22 V. vinifera cyclophilins, 16 are single-domain while 6 are multi-domain cyclophilins (Supplementary Fig. S2). The 16 single-domain cyclophilins are viz. GsvCYP1, GsvCYP2, GsvCYP3, GsvCYP4, GsvCYP7, GsvCYP8, GsvCYP9, GsvCYP10, GsvCYP12, GsvCYP13, GsvCYP15, GsvCYP17, GsvCYP18, GsvCYP19, GsvCYP20, and GsvCYP21. WD40 domain is present in cyclophilin GsvCYP5 while centriole, cilia, and spindle-associated domain is present in GsvCYP11. Domains TPR, TPR_1, TPR_19, 3a0801s09, PEP_TPR_LIPO, and PLN03088 are present in cyclophilin GsvCYP6. Two copies of ring_Ubox domain are in cyclophilin GsvCYP14. GsvCYP22 had the highest number of seven domains consisting of RRM, RRM_SF, PABP_1234, PLAN03134, SF_CC1 and two pair of PRK12678 domains (Fig. 2c). Localization of cyclophilins in V. vinifera indicated that eight cyclophilins were localized in chloroplast while only one is in mitochondria (Table 3).
3.5 Conserved Domain Analysis
Multiple sequence alignments of P. vulgaris, C. sativus, and V. vinifera cyclophilins showed the presence of conserved regions (Supplementary Fig. S3). The FHR motif is conserved in all cyclophilins of P. vulgaris. However, in V. vinifera, it is conserved in all cyclophilins except for GsvCYP5 and GsvCYP9 (Fig. 3). In C. sativus, the FHR motif is conserved in all CucCYPs except for CucCYP5, CucCYP17, CucCYP18, and CucCYP19. The ENF motif is conserved in most cyclophilins, with exceptions including CucCYP5, CucCYP6, CucCYP9, CucCYP14, CucCYP17, CucCYP18, CucCYP19, and CucCYP21 in C. sativus, and PhvCYP3, PhvCYP6, PhvCYP12, and PhvCYP14 in P. vulgaris. Additionally, ENF is not conserved in GsvCYP5, GsvCYP8, GsvCYP9, and GsvCYP10 of V. vinifera. The SI and DE motifs are other majorly conserved domains found in all cyclophilins of P. vulgaris, but they are missing in CucCYP17, CucCYP18, CucCYP19, and CucCYP21 of C. sativus, as well as GsvCYP9 of V. vinifera. The GSQFFI motif is conserved in most cyclophilins of C. sativus, except for CucCYP5, CucCYP17, CucCYP18, and CucCYP19. However, it is not conserved in PhvCYP5, PhvCY8, and PhvCYP23. GsvCYP9 and GscCYP17 also showed no conservation of the GSQFFI motif, whereas it is observed in all other GsvCYPs. The QGGD motif is conserved in most cyclophilins, except for CucCYP5, CucCYP17, CucCYP18, CucCYP19, and CucCYP21 of C. sativus, as well as PhvCYP3, PhvCYP5, PhvCYP8, PhvCYP22, and PhvCYP23 of P. vulgaris. Additionally, GsvCYP5, GsvCYP9, and GsvCYP13 do not exhibit conservation of the QGGD motif, while it is conserved in all other GsvCYPs. The SMAN motif is also largely conserved, with exceptions including CucCYP5, CucCYP6, CucCYP9, CucCYP17, CucCYP18, CucCYP19, and CucCYP21 of C. sativus, PhvCYP3, PhvCYP5, PhvCYP8, PhvCYP14, and PhvCYP21 of P. vulgaris, as well as GsvCYP1, GsvCYP5, GsvCYP8, GsvCYP9, and GsvCYP10 of V. vinifera.
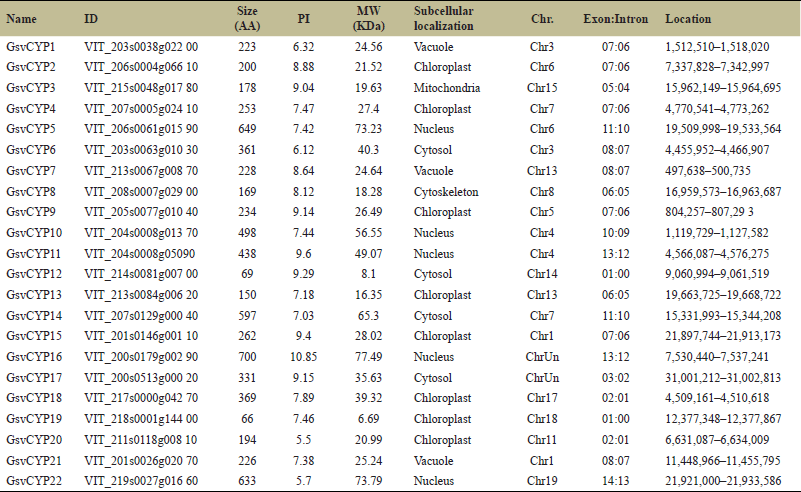 | Table 3. In total, there are 22 cyclophilins from V. vinifera, each providing details such as ID, size (in amino acids), PI, MW, sub-cellular localization, chromosome location, exon-intron count, and their respective positions on the chromosome. [Click here to view] |
Multiple sequence alignment of cyclophilins from P. vulgaris, C. sativus, V. vinifera, A. thaliana, and G. max showed conservation of WD40 domain in CucCYP2, PhvCYP22, GsvCYP5, GmCYP2, GmCYP35, and Arabidopsis CYP 71 (AT3g44600) (Supplementary Fig. S4). Conserved domains were also observed between CucCYP7, PhvCYP14, and GsvCYP3 cyclophilins and AT1G01940 (ATCYP18-1) (Supplementary Fig. S5A). Similarly, CucCYP6, PhvCYP6 and GsvCYP8 showed conserved regions with AT2G36130 (ATCYP18-2) (Supplementary Fig. S5B).
3.6 Phylogenetic and Evolutionary Studies of Cyclophilins
Phylogenetic analysis was performed with cyclophilins from P. vulgaris, C. sativus, and V. vinifera which clustered them into 5 different subgroups (Fig. 4a). Seven cyclophilins viz. CucCYP3, PhvCYP15, GsvCYP6, GsvCYP13, CucCYP13, CucCYP16, and PhvCYP18 grouped together on clade 1 and has TPR domains and CLD domain. Clade 2 had 26 cyclophilins while Clade 3 had 17 cyclophilins. Three cyclophilins such as PhvCYP24, CucCYP5, and GsvCYP22 grouped together on clade 5 and have glycine-rich RNA-binding protein (PLAN03134), polyadenylate binding protein (PABP_1234), and (RRM) domains. Similarly, three cyclophilins viz. GsvCYP14, CucCYP17, and PhvCYP12 with ring Ubox domain grouped on clade 4. Three cyclophilins (GsvCYP10, CucCYP9, and PhvCYP3) with nucleus-directed signal domain CYPs clubbed together on clade 5 and chloroplast-directed domains (CucCYP18, PhvCYP17, GsvCYP18, PhvCYP19, CucCYP19, and GsvCYP20) were on clade 3. A correlation of phylogenetic analysis with the sub-cellular localization of cyclophilins was observed.
A cladogram was constructed using cyclophilins from P. vulgaris, C. sativus, and V. vinifera cyclophilins and their homologs from other species and four major clades were observed (Fig. 4b). Clade 1 has OsCYP25 (rice) showed 87% protein sequence similarity with CucCYP8 and 93% similarity with PhvCYP11. PhvCYP11 has 91% similarity with AtCYP18-1 (Roc2) of Arabidopsis. Clade 2 with PhvCYP25, PhvCYP20, and CucCYP13 has 96.5%, 92.5% and 97.7%, similarity to GmCYP1. CucCYP13 has 90.8% similarity with AtCYP18-3 and 86.1% similarity with OsCYP2. Clade3 with CucCYP10 has 79.5% similarity to AtCYP20-2 while GsvCYP6 has 76.6% similar with OsCYp20-2. Phylogenetic analysis revealed that PhvCYP25 and GmCYP1 are present in the same clade and have 96.5% amino acid sequence similarity. Similarly, PhvCYP20 and CucCYP13 have 92.5% and 97.7%, protein sequence similarity with GmCYP1, respectively. Cyclophilin GsvCYP6 has 76.6% protein similarity with thylakoid luminal cyclophilin of rice OsCYp20-2.
 | Figure 1. Distribution of the identified cyclophilins on different chromosomes (no. marked on top) with an uneven distribution. (a) Total 21 C. sativus cyclophilins (CucCYPs) were on 7 chromosomes, (b) Twenty-six P. vulgaris cyclophilins (PhvCYPs) distributed on 9 chromosomes while no cyclophilins detected on chromosomes 4 and 8, (c) Twenty-two V. vinifera cyclophilins (GsvCYPs) were on 14 chromosomes while 2 were present on scaffold. [Click here to view] |
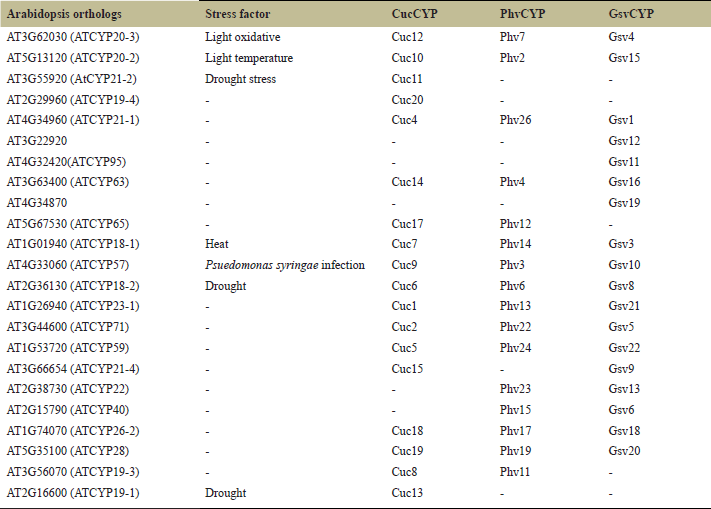 | Table 4. The information is organized in a table format, displaying the CucCYPs, GsvCYPs, and PhvCYPs, alongside their corresponding A. thaliana orthologs. Additionally, the table provides a detailed summary of the roles these cyclophilins play in various abiotic and biotic stresses. [Click here to view] |
3.7 Cyclophilin Grouping: Conserved Domains and Common Sub-Cellular Localization
A similar pattern of correlation between sub-cellular localization and domains was observed by constructing phylogenetic tree with all novel cyclophilins and previously reported ones from other plants (such as A. thaliana, O. sativa, M. truncatula, and G. max) (Fig. 5). Eight cyclophilins (GsvCYP5, GmCYPs20, GmCYPs35, AT3G44600, PhvCYP22, CucCYP2, LOC_ Os08 g44330 and Medtr2g085075-MtCYP71) with WD40 domain grouped together on clade 5. ii. Cyclophilins with common ring_UBox domain and nucleus location (CucCYP17, PhvCYP12, GmCYP18, GmCYP19, AT5G67530, and Medtr5g015500) grouped together on clade5.vi, while cyclophilins with ring_UBox domain and cytosol localization (GsvCYP17, GsvCYP14, and LOC_Os03g10400) grouped on another branch of the same clade. All cyclophilins with TPR domain (Medtr8g079690, AT2G15790, PhvCYP18, GmCYP9, GmCYP8, CucCYP3, CucCYP16, GsvCYP6, Medtr4g086760, PhvCYP15, GmCYP17, and GmCYP16) grouped together on clade 1. Similarly, RRM domain containing cyclophilins (Medtr8g442420, Medtr6g084140, GsvCYP22, CucCYP5, PhvCYP24, GmCYP59, GmCYP56, AT1G44478, LOC_Os06 g45900, LOC_Os06 g45910, Medtr8g442420, and AT1G53720) formed separate subgroup on clade 5.i.
In addition, sequence similarity amongst cyclophilins was observed in C. sativus, P. vulgaris, and V. vinifera sequences (Fig. 6). Multiple shared sequences were observed among cyclophilins of C. sativus, P. vulgaris, and V. vinifera sequences when compared with previously reported cyclophilins from A. thaliana, M. truncatula, and G. max. GsvCYP16 and PhvCYP20 showed most repeated sequences (Fig. 6a). Family-wise sequence similarity was observed among cyclophilins of leguminosae viz. P. vulgaris, G. max, and M. truncatula (Fig. 6b). High similarities were also observed between cyclophilins from C. sativus A. thaliana (Fig. 6c).
3.8 Comparative Analysis of Cyclophilins With Arabidopsis Orthologous
The cyclophilins CucCYP12, PhvCYP7, and GsvCYP4, which share a common single CLD domain, are orthologous to AT3G62030 (ATCYP20-3) in A. thaliana. This gene has been associated with a role in light-induced oxidative stress [65] (Table 4). Additionally, AT5G13120 (ATCYP20-2), which plays a similar role in light stress, was found to be orthologous to CucCYP10, PhvCYP2, and GsvCYP15 [66]. AT4G33060 (ATCYP57), known for its role in P. syringae infection, is orthologous to CucCYP9, PhvCYP3, and GsvCYP10 [67]. Furthermore, three A. thaliana cyclophilins, AT2G16600 (ATCYP19-1), AT2G36130 (ATCYP18-2), and AT3G55920 (ATCYP21-2) which are up regulated multiple folds, enhance stress tolerance against drought. They were orthologous to CucCYP13, CucCYP6, PhvCYP6, GsvCYP8, and CucCYP11 [68–70]. Another important cyclophilin in A. thaliana, AT1G01940 (ATCYP18-1), known to confer tolerance against heat stress, is orthologous to CucCYP7, PhvCYP14, and GsvCYP3 [71].
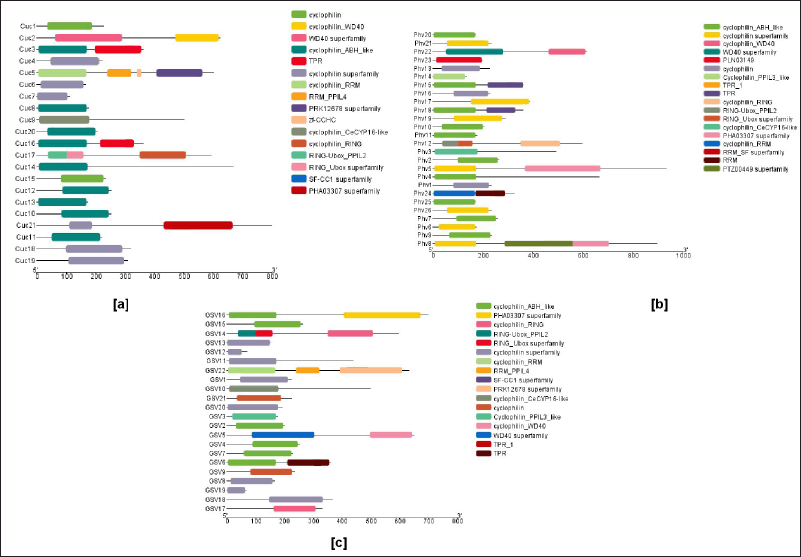 | Figure 2. Schematic representation of single and multi-domain in the identified cyclophilins. Domain analysis was done by using NCBI batch-CD tool (https:/www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) for the protein sequence of cyclophilins derived from (A) C. sativus, (B) P. vulgaris, and (C) V. vinifera. In C. sativus, out of the 21 cyclophilins identified, 15 were found to be single domain, and the remaining 6 were categorized as multi-domain cyclophilins. In the case of P. vulgaris, 19 cyclophilins were classified as single domain, and 7 were identified as multi-domain cyclophilins. Similarly, V. vinifera exhibited 16 single domain cyclophilins, with the remaining 6 being categorized as multi-domain cyclophilins. [Click here to view] |
3.9 Intron-Exon Structure in Cyclophilins
The distribution of intron-exon was analyzed among C. sativus, P. vulgaris, and V. vinifera in the present study where the number of introns varied in the range of 0–13 in all three plant species (Tables 1–3). In C. sativus, CucCYP5 had the highest number of 14 exons followed by 13 exons in CucCYP2, CucCYP14, and CucCYP21 while only one exon was present in CucCYP8. However, PhvCYP24 has 14 exons followed by 13 exons each in PhvCYP4, PhvCYP5, PhvCYP8, and PhvCYP22. Interestingly, no introns were found in PhvCYP20 and PhvCYP25. In V. vinifera, GsvCYP22 has 14 exons followed by 13 exons each in GsvCYP11 and GsvCYP16. On the other end, GsvCYP12 and GsvCYP19 have no introns.
3.10 Synonymous and Non-Synonymous Substitution Rate (Ka-Ks)
The Ks values of 21 cyclophilins from C. sativus ranged from 0.2 (for gene pair CucCYP3-CucCYP16) to 0.57 (for pair CucCYP19-CucCYP7) (Supplementary Fig. S6). Meanwhile, the Ks values of 26 cyclophilins from P. vulgaris ranged from 0.2 (for gene pair PhvCYP16-PhvCYP1) to 0.56 (for gene pair PhvCYP24-PhvCYP19). In the case of V. vinifera Ks values ranged from 0.03 (for gene pair GsvCYP17-GsvCYP14) to 0.6 (for gene pair GsvCYP22-GsvCYP9). The divergence time (in MYA) of C. sativus ranged from 59.6 to 130.9 MYA, with the lowest divergence observed in the CucCYP3-CucCYP16 gene pair and the highest in the CucCYP19-CucCYP7 gene pair. In P. vulgaris, the divergence time ranged from 12.32 to 33.59 MYA, with the lowest divergence observed in the PhvCYP21-PhvCYP26 gene pair and the highest in the PhvCYP24-PhvCYP19 gene pair. In V. vinifera, the GsvCYP22-GsvCYP9 gene pair showed the highest divergence time, while the GsvCYP17-GsvCYP14 gene pair exhibited the lowest divergence time, approximately around 7.5 MYA, indicating a recent divergence event between GsvCYP17 and GsvCYP14.
3.11 In silico Expression Analysis of Cyclophilins
Gene expression analysis indicated differential expression of key cyclophilins from various plant species. Transcriptomic data of C. sativus from different tissues (root, male, female, ovary, fertilized ovary, unfertilized ovary, stem, tendril base, tendril, and leaf) was obtained from cucurbit genome database (CuGenDB), bio project PRJNA80169 and analyzed to see the differences/similarities in gene expression pattern (Fig. 7a) (Supplementary Fig. S7). Expression analysis revealed that cyclophilins in leaves have higher gene expression as compared to the root. Gene expression of Csa1G042130, Csa2G234600, Csa1G153530, Csa1G690270, Csa2G380020, and Csa5G202380 cyclophilins increased in fertilized ovary as compared to the unfertilized one while the level stayed nearly unchanged on fertilization for Csa7G407760, Csa7G237870, Csa2G270140, and Csa2G009340. Similarly, Csa5G128260 showed higher expression of cyclophilins in female plants as compared to the male plants. Cyclophilin Csa7G009740 (CucCYP13) had overall higher expression in roots as compared to any other tissue and was also 97.7% similar with GmCYP1.
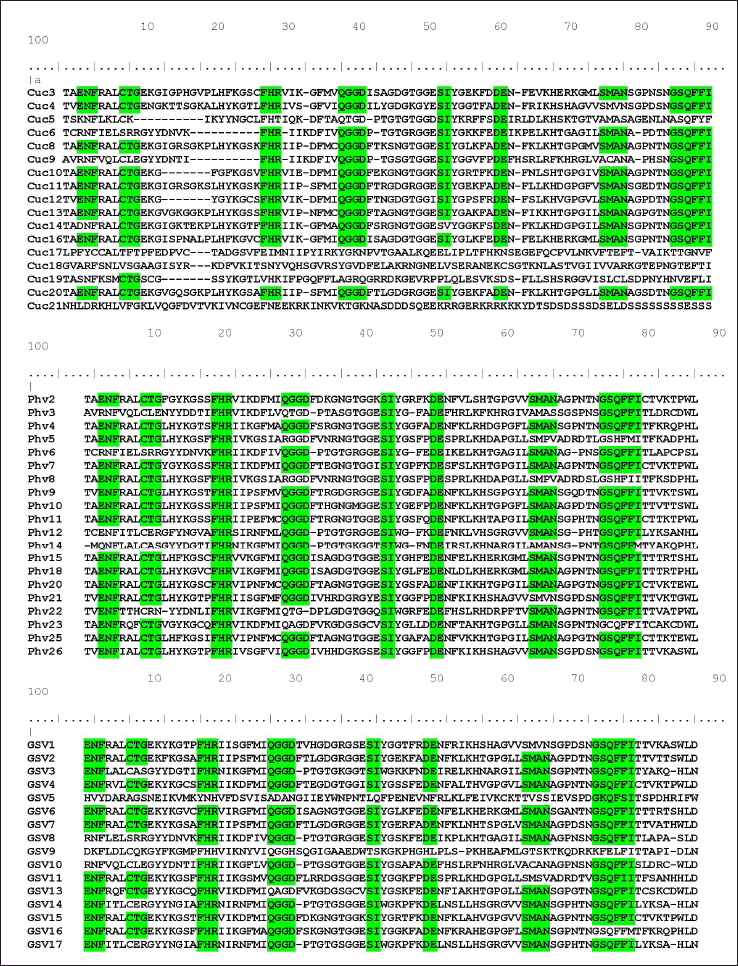 | Figure 3. Schematic representation of multiple sequence alignment for cyclophilins protein family showing conserved motifs. Protein sequences of cyclophilin family derived from (a) C. sativus, (b) P. vulgaris and (c) V. vinifera were aligned by using Clustal Omega tool (https://www.ebi.ac.uk/Tools/msa/clustalo) further visualization was done in Mview (https://www.ebi.ac.uk/Tools/msa/mview) and conserved regions are shown in different colour. The conservation of motifs was evident in the cyclophilins of all three plant species. [Click here to view] |
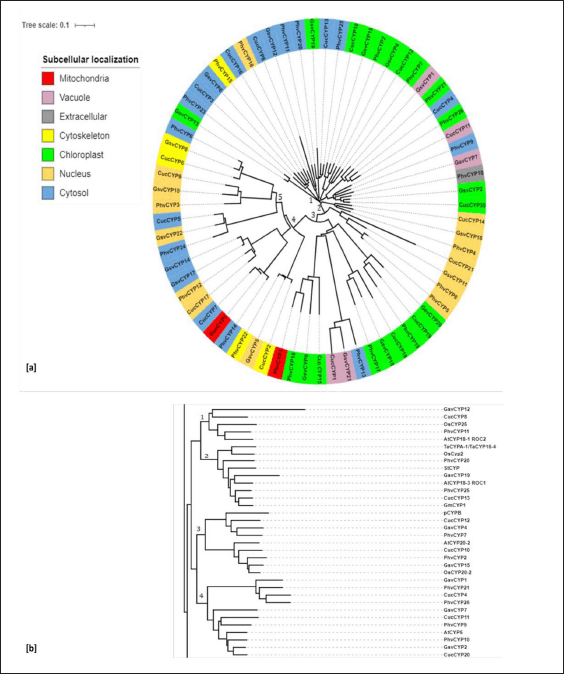 | Figure 4. Phylogenetic relationship of newly identified cyclophilin (a) Phylogenetic relationship among the identified cyclophilins of C. sativus, G. max, P. vulgaris and V. vinifera. Subcellular localization analysed by wolfpsort online tool where all cyclophilins were found to be localized in mitochondria, vacuole, extracelluar space, cytoskeleton, chloroplast, nucleus and cytosol in uneven manner. Color range represents predicted subcellular localization of cyclophilins. (b) Cladogram showing relationship among cyclophilins from C. sativus, G. max, P. vulgaris and V. vinifera with cyclophilins from various plants having role in abiotic stress. [Click here to view] |
In silico gene expression analyses were also carried out in P. vulgaris and data was retrieved from the phytozome database. The expression level of cyclophilins from P. vulgaris was studied in flower bud, flower, green mature pods, leaves, nodules, root on 10th day, root on 19th day, stem on 10th day, stem on 19th day, young pods and young trifoliate. The young pod showed high levels of mRNA transcript, which was the highest amongst all cyclophilin transcript data of P. vulgaris obtained from various tissues (Fig. 7b) (Supplementary Fig. S7). Results indicated that cytosol-localized Phvul.011G026900 (PhvCYP25) has higher levels (10–100x) of transcripts than the rest of cyclophilins and shared phylogenetic relationship with CucCYP13 and GmCYP1 (Fig. 4b).
RNA seq data of V. vinifera was retrieved from the expression atlas database of EMBL-EBI for GsvCYP [72], where high-throughput sequencing was conducted using samples from three distinct stages of berry development, each infected with Botrytis cinerea rot. Stage 1 represents the initial infection phase, characterized by a color change from yellow to pink. In Stage 2, the infection progressed further, resulting in softer berry skin and dark pink coloration. Stage 3 samples were fully rotten but not yet dry. Expression data is presented in fold change against control. Results indicated a down regulation of GsvCYPs when infected with B. cinerea. VIT_211s0118g00810 (GsvCYP20) and VIT_201s0146g00110 (GsvCYP15) were found to be most down regulated viz. 160% on stage 3 and stage 2 of infection, respectively (Fig. 7c) (Supplementary Fig. S7). The least down regulated GsvCYPs are VIT_215s0048g01780 (GsvCYP3), VIT_204s0008g01370 (GsvCYP10), VIT_214s0081g00700 (GsvCYP12) and VIT_218s0001g14400 (GsvCYP19).
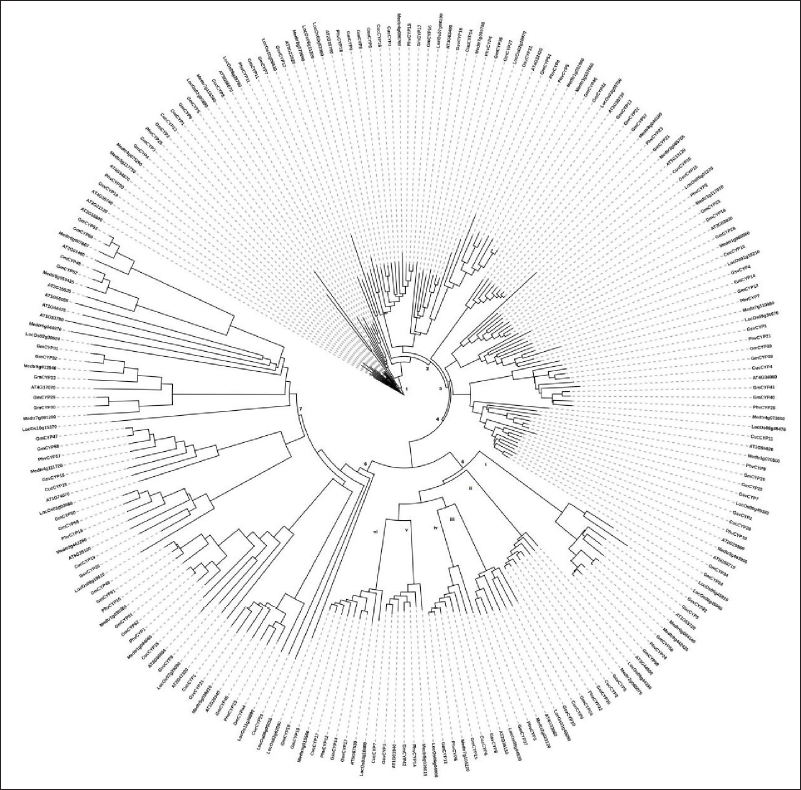 | Figure 5. Phylogenetic relationship of cyclophilin protein family for different plants. Phylogenetic correlation of cyclophilins from C. sativus, P. vulgaris and V. vinifera with previously reported G. max, A. thaliana, M. truncatula and O. sativa. Multiple sequence alignment of all amino acid sequences was carried out in MegaX using clustral W and tree was constructed using iTOL: Interactive tree of life. [Click here to view] |
4. RESULT
In summary, we identified 21, 26 and 22 cyclophilins from C. sativus, P. vulgaris and V. vinifera. C. sativus has 15 single domain cyclophilins while 6 cyclophilins are multi-domain proteins. Phaseolus vulgaris has 19 single domain cyclophilins while 7 are multi-domain cyclophilins. In case of V. vinifera 16 cyclophilins have single CLD domain while rest 6 cyclophilins are multi-domain. Multiple sequence analysis revealed conservation of eight motifs in all reported cyclophilins widespread in different regions. GSQFFI motif has been found to be longest with six amino acids one while SI and DE were smallest with only two amino acids. Phylogenetic analysis revealed grouping of cyclophilins into five major clades where cyclophilins having common domains and sub-cellular localization have close phylogenetic relationships. Evolutionary studies of cyclophilins with previously reported A. thaliana cyclophilins were also conducted where AT3G62030 (ATCYP20-3), AT5G13120 (ATCYP20-2), AT4G33060 (ATCYP57), AT2G16600 (ATCYP19-1), AT2G36130 (ATCYP18-2) and AT3G55920 (ATCYP21-2), AT1G01940 (ATCYP18-1) associated with light stress, P. syringae infection, drought and heat stress are orthologous to various cyclophilins in our study. Intron-exon analysis revealed number of introns ranges from 0 to 13 in C. sativus, P. vulgaris and V. Vinifera. All three plant species have maximum 14 exons however minimum number of exons varies in each. Synonymous and non-synonymous substitution analysis revealed the Ks values from cyclophilins from C. sativus ranged from 0.2 to 0.57. While the Ks values of P. vulgaris cyclophilins ranged from 0.2 to 0.56. In case of V. vinifera Ks values ranged from 0.03 to 0.6. Lastly, results from in-silico expression analysis in C. sativus showed higher expression of (CucCYP13) in roots in comparison to other cyclophilins. Cytosol-localized Phvul.011G026900 (PhvCYP25) also expressed multiple times in P. vulgaris than other cyclophilins. Whereas expression data of V. vinifera infected from B. cinerea revealed negative mRNA levels in all cyclophilins.
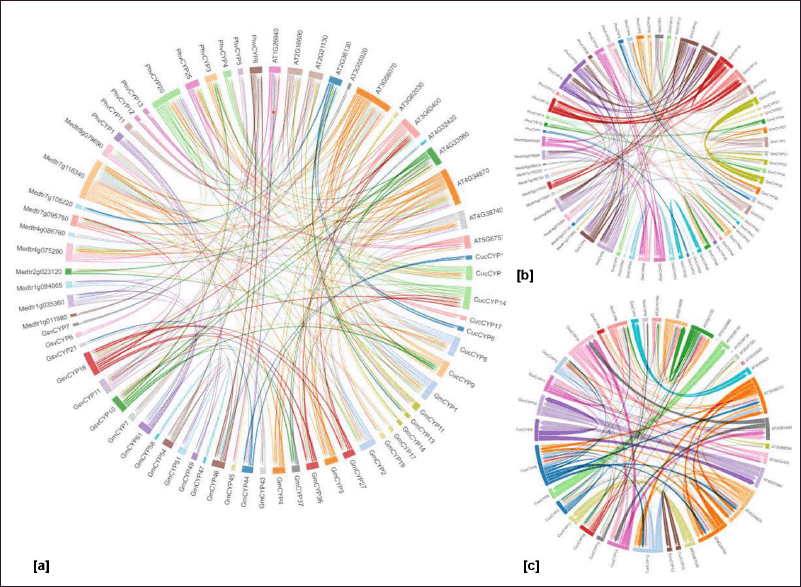 | Figure 6. Chord diagrams representing relation between cyclophilins based on sequence similarity regions (a) C. sativus, P. vulgaris and V. vinifera with previously reported G. max, ?. thaliana and M. truncatula (b) family wise orthology of leguminosae P. vulgaris, G. max and M. truncatula (c) A. thaliana and C. sativus. The count of colorful segments within each cyclophilin. indicates the presence of shared similarity regions among cyclophilins, which is determined by their amino acid sequences. The highest degrees of similarity were evident in PhvCYP20, GsvCYP16, and CucCYP14 when compared to other cyclophilins, represented by the presence of colored chords. [Click here to view] |
5. DISCUSSION
Cyclophilins are ubiquitous proteins found in all genera ranging from bacteria, fungi, and higher plants to humans [73]. Previously, a wide range of diversity was reported in cyclophilins from several crops such as 94 cyclophilins in Brassica napus, 83 in Triticum aestivum, 62 in Glycine max, 78 in G. hirsutum, 75 in G. barbadense, 40 in G. arboreum, 38 in G. raimondii, 33 in M. truncatula, and 29 in O. sativa [20–24]. Cyclophilins has been associated with different physiological processes such as transcription regulation, hormonal signaling, organogenesis, and plant-pathogen interactions [74, 75].
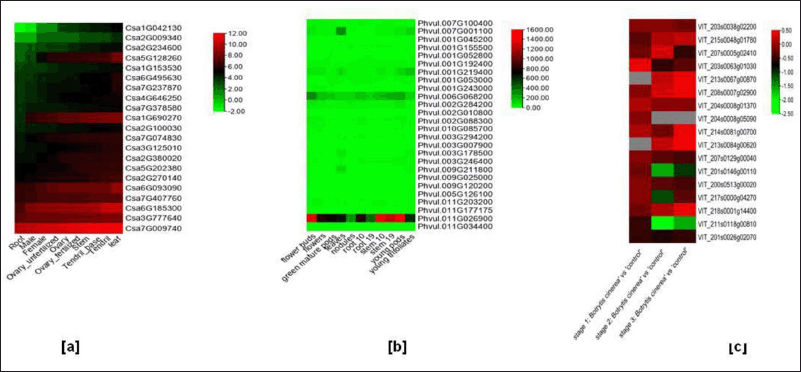 | Figure 7. Heatmaps of cyclophilin transcript expression variation in C. sativus and P. vulgaris. Expression data of P. vulgaris was obtained from phytozome (https://phytozome-next.jgi.doe.gov/) and C. sativus by using cucurbit genome database (CuGenDB) from bioprojectPRJNA80169 (http://cucurbitgenomics.org/rnaseq/cu/3). Heatmap for (a) C. sativus and (b) P. vulgaris (c) V. vinifera was constructed using TB tools software (https://bio.tools/tbtools). [Click here to view] |
In the present work, comparative and evolutionary studies were conducted in order to understand the role of cyclophilins family in three distinct species C. sativus, P. vulgaris and V. vinifera. We performed a comprehensive study that resulted in the identification of 21, 26 and 22 cyclophilins from C. sativus, P. vulgaris, and V. vinifera, respectively. Results from chromosomal distribution, sub-cellular localization and domain analysis revealed that cyclophilins are wide spread in cytosol and nucleus while few were detected in mitochondria and vacuole. In this study, we identified several cyclophilins from C. sativus, P. vulgaris and V. vinifera, which are orthologs to cyclophilins of other crops which play key roles in various biotic and abiotic stress tolerances. For example, orthologs of WD 40 domain containing cyclophilin were identified from all three plant species. The WD40 repeat (WDR) domain in cyclophilins from C. sativus, P. vulgaris, V. vinifera is conserved similar to previously reported G. max and A. thaliana (Supplementary Fig. S6). (WDR) domains are typically known for its β-propeller domains that were responsible for protein interaction scaffolds in multi-protein complexes [76]. Additionally, they could have a role in cell division, meristem organization, development of floral parts, regulation of secondary metabolites, and innate immunity [77,78]. The three cyclophilins viz. CucCYP2, PhvCYP22, and GsvCYP5 which are grouped with previously reported Arabidopsis CYP 71 (AT3g44600) which plays a key role in gene repression and organogenesis [79]. Disruption of CYP71 had resulted in ectopic activation of homeotic genes that regulate meristem development. The cyp71 mutant plants displayed dramatic defects, including reduced apical meristem activity, delayed and abnormal lateral organ formation, and arrested root growth. CYP71 was also associated with the chromatin of target gene loci and physically interacted with histone [79]. Similarly, other cyclophilins were also identified, which play a key role in other important traits. Cyclophilins GsvCYP6 in V. vinifera has 76.6% protein sequence similarity with previously reported OsCYP20 protein which regulated spliceosome assembly along with its interacting part OsSYF2 and thereby could contribute to long grain size and aid sugar metabolism in O. sativa [80]. Previous results have shown that OsCYP20–2 t1, a knock out mutant of OsCYP20–2 resulted in shorter grain and lacked cell elongation suggesting possibility for similar role of GsvCYP6.
Additionally, cyclophilins have been reported to play key role in drought, light, salt, heat, chilling stress tolerance [81–85]. In this study, cyclophilins from C. sativus, P. vulgaris, and V. vinifera viz. CucCYP7, PhvCYP14 and GsvCYP3 cyclophilins shared conserved domains with AT1G01940 (ATCYP18-1) (Supplementary Fig. S7A), which was up regulated several folds and conferred heat tolerance [71]. Similarly, CucCYP6, PhvCYP6 and GsvCYP8, respectively, grouped together with AT2G36130 (ATCYP18-2) (Supplementary Fig. S7B), which interacted with AtSKIP and translocated to the nucleus from cytoplasm providing resistance against drought [69].
Genome duplication is base of evolution in plants and contributes to the expansion of gene families. Polyploidy has been the driving force in this evolution and expansion in plants. Angiosperms are reported to have many polyploidization events along with divergence that has resulted in varying gene families in different plant species. Here, we have identified 21 cyclophilins in C. sativus, 26 in P. vulgaris and 22 in V. vinifera. Species P. vulgaris and V. vinifera has only experienced core eudicot common hexa-ploidy (γ event) around 100 MYA and no recent whole genome duplication while entire Cucurbitaceae family had a tetra polyploidization event shortly after paleo-hexaploidy 90-102 MYA [86]. Both A. thaliana and G. max have experienced two additional polyploidization events, apart from the γ event. Specifically, in A. thaliana, these events occurred approximately 65–100 million years ago (α event) and 180 million years ago (β event), while in G. max, they occurred around 59 million years ago and 13 million years ago. These polyploidization events likely contributed to the expansion of the cyclophilin gene family in both species [87,88]. Some cyclophilins in C. sativus could have emerged from gene duplication or inter-chromosome rearrangement. All the three plant species showed Ka/Ks >1 denoting genes have gone through purifying selection. Varying number of cyclophilins in P. vulgaris and V. vinifera showed chromosomal level duplication or deletion [89,90]. The lowest divergence time between the gene pair GsvCYP17-GsvCYP14, compared to other gene pairs, indicates a probable recent divergence in V. vinifera.
Cyclophilin are also crucial in plant–microbe interactions. Recent studies have provided insights on how cyclophilins offer resistance against fungus like Magnaporthe oryzae, B. cinerea, Cryphonectria parasitica, and Puccinia triticina [91]. Cyclophilins hold high affinity for cyclosporin A, an immunosuppressive drug known for its antifungal properties [92]. Expression profile of cyclophilins from C. sativus was analyzed from publicly available data from cucurbit genome database (CuGenDB). CucCYP13 is highly expressed in roots and has highly similarity to GmCYP1 (97.7%) from G .max. Interestingly, GmCYP1conferres resistance against P. sojae, an oomycete that causes root and stem rot in soybean. GmCYP1 interactes with effector Avr3b which has PPIase-dependent enzymatic activity. Avr3b triggeres hypersensitive response and contributes in virulence against P. sojae strain P6497 [50,93]. Studies on CucCYP13 can be further conducted to study its role in biotic stress against P. sojae.
Cyclophilin play an important role in abiotic stress tolerance. The gene up-regulation of cyclophilins provides resistance against stress in M. domestica in response to abiotic stress [25]. Eight cyclophilins were responsive to salt stress and ten cyclophilins to drought stress by increasing expression. MdCYP16 showed upregulated response to both salt and drought indicating resistance to abiotic stresses [25]. Apart from this, cyclophilins from Cucumis family are believed to have an influence in biosynthesis of volatile compound resulting in fragrant aroma of melon. Cucumis melo shares consensus chloroplast simple sequence repeats in common with C. sativus and has recent divergence history [94,95]. Recent study indicated that overexpression of GmCYP2 from G. max confers salt tolerance by regulating photosynthetic and ionic homeostasis [96].
Cyclophilin have also been associated in improving biotic stress tolerance. Expression data of GsvCYs suggest a potential role of cyclophilins in the gray mold fungus B. cinerea as indicated by the down regulation of VIT_211s0118g00810 (GsvCYP20) and VIT_201s0146g00110 (GsvCYP15). Viaud et al. [97] demonstrated that mutants deficient in the CyP1 gene exhibited decreased formation of appressoria and was unable to penetrate the plant cuticle effectively. Restoring the wild-type CyP1 gene in the mutant strain reinstated virulence, confirming the crucial role of CyP1 as a virulence determinant in Magnaporthe grisea [97]. A similar pattern was also observed by Sun et al. [98] where deletion of the BcCyp2 gene in B. cinerea significantly impaired the fungus’s ability to infect its host plants, including tomato and Arabidopsis. Mutants lacking the BcCyp2 gene exhibited defects in infection-related development, such as reduced conidiation, decreased appressorium formation, and impaired penetration of plant tissues. Complementation of the mutant strain with the wild-type BcCyp2 gene restored virulence, conclusively confirming the role of BcCyp2 as a virulence factor in B. cinerea [98].
Similarly, isolation of 475 bp long cyclophilin from Gossypium arboretum has also proven to have links with epicuticular wax production in cotton. This wax loads provided tolerance against leaf curl virus in cotton and transmission via whitefly provides a new and important aspect of cyclophilin in plant defense mechanism [99]. Overexpression of cyclophilins from Panax ginseng reduces spore formation of Phytophthora cactorum, enhances proline synthesis in salt stress conditions and slows chlorophyll level to combat saline stress in A. thaliana [100].
Future studies on stress responsive expression, transcriptomics and proteomics can provide more significant role of reported cyclophilins in C. sativus, P. vulgaris and V. vinifera against various abiotic and biotic stress tolerance. Variations in cyclophilins can also be utilized as molecular marker to produce stress tolerant cultivars.
6. CONCLUSION
Cyclophilins are widespread proteins present in various organisms, spanning a wide range from bacteria and animals to plants. Genome-wide analysis and identification of cyclophilins from few plants revealed their varying numbers in genome but their role remained ambiguous. We have conducted genome-wide search and identification of cyclophilins for the first time in three plant species C. sativus, P. vulgaris and V. vinifera which revealed presence of 21 cyclophilins in C. sativus, 26 in P. vulgaris and 22 in V. vinifera. Subsequently, a detailed analysis on chromosome location, domain, sub-cellular localization, gene structure, genome duplication, phylogeny, orthologous studies and expression analysis provided key insight on the evolution of cyclophilins in these three plant species. Results revealed that majority of cyclophilins in C. sativus, P. vulgaris and V. vinifera are single-domain containing cyclophilins with localization in cytosol. Additionally, evolutionary studies of these cyclophilins with previously reported cyclophilins from Arabidopsis suggested potential role of CucCYP2, PhvCYP22 and GsvCYP5 cyclophilins in organogenesis, meristem development and conferring immunity against oomycete. Leveraging the knowledge generated from present study combined with in-silico expression studies under stress conditions can be harnessed in advance crop breeding techniques and generation of superior varieties to combat global stress challenge and food security. This study provides comprehensive information for further research on cyclophilins from C. sativus, P. vulgaris and V. vinifera.
7. LIST OF ABBREVIATIONS
CucCYP, Cucumis sativus cyclophilin; CYP, Cyclophilin; GsvCYP, Vitis vinifera cyclophilin; MD, Multiple domain; PhvCYP, Phaseolus vulgaris cyclophilin; SD, Single domain.
8. ACKNOWLEDGMENTS
We thank Director, ICAR-Indian Institute of Soybean Research, Indore for providing various facilities required in our research work.
9. CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
10. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
11. FUNDING
There is no funding to report
12. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chou IT, Gasser CS. Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Plant Mol Biol 1997;35(6):873–92; http://doi.org/ 10.1023/a:1005930024796
2. Trandinh CC, Pao GM, Saier Jr MH. Structural and evolutionary relationships among the immunophilins: two ubiquitous families of peptidyl-prolyl cis-trans isomerases. FASEB J 1992;6(15):3410–20; http://doi.org/10.1096/fasebj.6.15.1464374
3. Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 1984;226(4674):544–7; http://doi.org/10.1126/science.6238408
4. Quesniaux VF, Schreier MH, Wenger RM, Hiestand PC, Harding MW, Van Regenmortel MH. Molecular characteristics of cyclophilin-cyclosporine interaction. Transplantation 1988;46(2 Suppl):23S–8S; http://doi.org/10.1097/00007890-198808001-00005
5. Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989;337(6206):476–8; http://doi.org/10.1038/337476a0
6. Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992;355(6355):33–45; http://doi.org/10.1038/355033a0
7. Schmid FX, Mayr LM, Mücke M, Schönbrunner ER. Prolyl isomerases: role in protein folding. Adv Protein Chem 1993;44:25–66; http://doi.org/10.1016/s0065-3233(08)60563-x
8. Horowitz DS, Lee EJ, Mabon SA, Misteli T. Acyclophilin functions in pre-mRNA splicing. EMBO J 2002;21(3):470–80; http://doi.org/10.1093/emboj/21.3.470
9. Shaw PE. Peptidyl-prolyl isomerases: a new twist to transcription. EMBO Rep 2002;3(6):521–6; http://doi.org/10.1093/embo-reports/kvf118
10. Faure JD, Gingerich D, Howell SH. An Arabidopsis immunophilin, AtFKBP12, binds to AtFIP37 (FKBP interacting protein) in an interaction that is disrupted by FK506. Plant J 1998;15(6):783–9; http://doi.org/v
11. Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci U S A 2002;99(4):1899–904; http://doi.org/10.1073/pnas.042529199
12. Deng W, Chen L, Wood DW, Metcalfe T, Liang X, Gordon MP, et al. Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proc Natl Acad Sci U S A 1998;95(12):7040–5; http://doi.org/10.1073/pnas.95.12.7040
13. Nigam N, Singh A, Sahi C, Chandramouli A, Grover A. SUMO-conjugating enzyme (Sce) and FK506-binding protein (FKBP) encoding rice (Oryza sativa L.) genes: genome-wide analysis, expression studies and evidence for their involvement in abiotic stress response. Mol Genet Genomics 2008;279(4):371–83; http://doi.org/10.1007/s00438-008-0318-5
14. Ahn JC, Kim DW, You YN, Seok MS, Park JM, Hwang H, et al. Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol 2010;10:253; doi: 10.1186/1471-2229-10-253
15. Andreeva L, Heads R, Green CJ. Cyclophilins and their possible role in the stress response. Int J Exp Pathol 1999 Dec;80(6):305–15; http://doi.org/10.1046/j.1365-2613.1999.00128.x
16. Singh H, Kaur K, Singh M, Kaur G, Singh P. Plant cyclophilins: multifaceted proteins with versatile roles. Front Plant Sci 2020;11:585212; http://doi.org/10.3389/fpls.2020.585212
17. Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR?. Trends Biochem Sci 1995;20(7):257–9; http://doi.org/10.1016/s0968-0004(00)89037-4
18. Van Nocker S, Ludwig P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics 2003;4(1):50; http://doi.org/10.1186/1471-2164-4-50
19. Hanhart P, Thieß M, Amari K, Bajdzienko K, Giavalisco P, Heinlein M, et al. Bioinformatic and expression analysis of the Brassica napus L. cyclophilins. Sci Rep 2017;7:1514; http://doi.org/10.1038/s41598-017-01596-5
20. Singh H, Kaur K, Singh S, Kaur P, Singh P. Genome-wide analysis of cyclophilin gene family in wheat and identification of heat stress responsive members. Plant Gene 2019;19:100197.
21. Trivedi DK, Yadav S, Vaid N, Tuteja N. Genome wide analysis of cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signal Behav 2012;7(12):1653–66; http://doi.org/10.4161/psb.22306
22. Chen Q, Chen QJ, Sun GQ, Zheng K, Yao ZP, Han YH, et al. Genome-wide identification of cyclophilin gene family in cotton and expression analysis of the fibre development in Gossypium barbadense. Int J Mol Sci 2019;20(2):349; http://doi.org/10.3390/ijms20020349
23. Khatun K, Robin AHK, Islam MR, Jyoti SD, Lee D, Kim CK, et al. Genome-wide analysis of Solanum lycopersicum L. cyclophilins. J Plant Biotechnol 2022;49:15–29; http://doi.org/10.5010/JPB.2022.49.1.015
24. Ge L, Zhang K, Cao X, Weng Y, Liu B, Mao P, et al. Sequence characteristics of Medicago truncatula cyclophilin family members and function analysis of MsCYP20-3B involved in axillary shoot development. Mol Biol Rep 2020;47(2):907–19; http://doi.org/10.1007/s11033-019-05183-x
25. Qiao ZW, Wang DR, Wang X, You CX, Wang XF. Genome-wide identification and stress response analysis of cyclophilin gene family in apple (Malus × domestica). BMC Genomics 2022 Dec 6;23(1):806; http://doi.org/10.1186/s12864-022-08976-w
26. Mainali HR, Chapman P, Dhaubhadel S. Genome-wide analysis of cyclophilin gene family in soybean (Glycine max). BMC Plant Biol 2014;14:282; http://doi.org/10.1186/s12870-014-0282-7
27. Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int 2010;43:414–31; http://doi.org/10.1016/j.foodres.2009.09.003
28. Nwokolo E. Common bean (Phaseolus vulgaris L.). In: Nwokolo E, Smartt J (eds.). Food and feed from legumes and oilseeds, Springer, Boston, MA, 1996; http://doi.org/10.1007/978-1-4613-0433-3_16
29. Rao IM. Advances in improving adaptation of common bean and Brachiaria forage grasses to abiotic stresses in the tropics. In: Pessarakl M (ed.). Handbook of plant and crop physiology. 3rd edition, CRC Press, Taylor and Francis Group, Boca Raton, FL, pp 847–89, 2014.
30. W2150: Breeding Common Bean (Phaseolus vulgaris L.) for resistance to abiotic and biotic stresses, sustainable production, and enhanced nutritional – NIMSS [Internet]. Nimss.org 2015. Available from: https://nimss.org/projects/view/mrp/outline/11737
31. El-Sawy MA, Mohamed HAE, Elsharkawy MM. Serological and molecular characterisations of the Egyptian isolate of Bean common mosaic virus. Arch Phytopathol 2014;47(12):1431–43; http://doi.org/10.1080/03235408.2013.845470
32. Orellana RG, Fan FF. Nodule infection by bean yellow mosaic virus in Phaseolus vulgaris. Appl Environ Microbiol 1978 Dec;36(6):814–8; http://doi.org/10.1128/aem.36.6.814-818.1978
33. Anonymous. Ayurvedic pharmacopoeia of India. The Controller of Publication (NISCOM), New Delhi, India, 2001.
34. Patri G, Silano V, Anton R. Plants in cosmetics. Council of Europe Publishing, Strasbourg, France, 2002.
35. Xu X, Ji J, Ma X, Xu Q, Qi X, Chen X. Comparative proteomic analysis provides insight into the key proteins involved in cucumber (Cucumis sativus L.) adventitious root emergence under waterlogging stress. Front Plant Sci 2016;7:1515.
36. Liu D, Dong S, Bo K, Miao H, Li C, Zhang Y, et al. Identification of QTLs controlling salt tolerance in cucumber (Cucumis sativus L.) seedlings. Plants 2021;10:85.
37. Yu B, Yan S, Zhou H, Dong R, Lei J, Chen C, et al. Overexpression of CsCaM3 improves high temperature tolerance in cucumber. Front Plant Sci 2018;9:797.
38. Gupta S, Upadhyay RN, Kumar S, Razdan VK. Integrated management of downy mildew of cucumber. Indian Phytopathol 2014;67:203–12.
39. Kooistra E. Powdery mildew resistance in cucumber. Euphytica 1968;17:236–44; http://doi.org/10.1007/BF00021216
40. Ciofini A, Negrini F, Baroncelli R, Baraldi E. Management of post-harvest anthracnose: current approaches and future perspectives. Plants (Basel) 2022 Jul 15;11(14):1856; http://doi.org/10.3390/plants11141856
41. Rojas ES, Batzer JC, Beattie GA, Fleischer SJ, Shapiro LR, Williams MA, et al. Bacterial wilt of cucurbits: resurrecting a classic pathosystem. Plant Dis 2015 May;99(5):564–74; http://doi.org/10.1094/PDIS-10-14-1068-FE
42. Grape production [Internet]. Our World in Data. Available from: https://ourworldindata.org/grapher/grapes-production
43. Duchene E, Huard F, Dumas V, Schneider C, Merdinoglu D. The challenge of adapting grapevine varieties to climate change. Clim Res 2010;41:193–204; http://doi.org/10.3354/cr008
44. Van Leeuwen C, Destrac-Irvine A, Dubernet M, Duchêne E, Gowdy M, Marguerit E, et al. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019;9:514; http://doi.org/10.3390/agronomy9090514
45. Wong FP, Wilcox WF. Distribution of baseline sensitivities to azoxystrobin among isolates of Plasmopara viticola. Plant Dis 2000;84:275–81.
46. Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, et al. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant-Microbe Interact 2003;16:1118–28.
47. Belhadj A, Saigne C, Telef N, Cluzet S, Bouscaut J, Corio-Costet MF, et al. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J Agric Food Chem 2006;54:9119–25.
48. Olejnik P, M?drzak CJ, Nuc K. Cyclophilins and their functions in abiotic stress and plant-microbe interactions. Biomolecules 2021 Sep 21;11(9):1390; http://doi.org/10.3390/biom11091390
49. Coaker G, Falick A, Staskawicz B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 2005;308:548–50; http://doi.org/10.1126/science.1108633
50. Mainali HR, Vadivel AK, Li X, Gijzen M, Dhaubhadel S. Soybean cyclophilin GmCYP1 interacts with an isoflavonoid regulator GmMYB176. Sci Rep 2017 Jan 11;7:39550; http://doi.org/10.1038/srep39550
51. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990 Oct 5;215(3):403–10; http://doi.org/10.1016/S0022-2836(05)80360-2
52. Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 2012 Jan;40(Database issue):D1178–86; http://doi.org/10.1093/nar/gkr944
53. Martin FJ, Amode MR, Aneja A, Austine-Orimoloye O, Azov AG, Barnes I, et al. Ensembl 2023. Nucleic Acids Res 2023 Jan 6;51(D1):D933–41; http://doi.org/10.1093/nar/gkac958
54. Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 2002;93(1):77–8.
55. Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res 2007 Jul;35(Web Server issue):W585–7; http://doi.org/10.1093/nar/gkm259
56. Tokmakov AA, Kurotani A, Sato KI. Protein pI and intracellular localization. Front Mol Biosci 2021 Nov 29;8:775736; http://doi.org/10.3389/fmolb.2021.775736
57. Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genom Proteom Bioinform 2010 Mar;8(1):77–80; http://doi.org/10.1016/S1672-0229(10)60008-3
58. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 1999;41:95–8.
59. Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 2021 Jul 2;49(W1):W293–6; http://doi.org/10.1093/nar/gkab301
60. Wang J, Chitsaz F, Derbyshire MK, Gonzales NR, Gwadz M, Lu S, et al. The conserved domain database in 2023. Nucleic Acids Res 2023 Jan 6;51(D1):D384–8; http://doi.org/10.1093/nar/gkac1096
61. Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 2015;31(8):1296–7.
62. Zheng Y, Wu S, Bai Y, Sun H, Jiao C, Guo S, et al. Cucurbit genomics database (CuGenDB): a central portal for comparative and functional genomics of cucurbit crops. Nucleic Acids Res 2018;47(2018):D1128–36.
63. Moreno P, Fexova S, George N, Manning JR, Miao Z, Mohammed S, et al. Expression atlas update: gene and protein expression in multiple species. Nucleic Acids Res 2022 Jan 7;50(D1):D129–40; http://doi.org/10.1093/nar/gkab1030
64. Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 2020 Aug 3;13(8):1194–202; http://doi.org/10.1016/j.molp.2020.06.009
65. Dominguez-Solis JR, He Z, Lima A, Ting J, Buchanan BB, Luan S. A cyclophilin links redox and light signals to cysteine biosynthesis and stress responses in chloroplasts. Proc Natl Acad Sci U S A 2008;105(42):16386–91; http://doi.org/10.1073/pnas.0808204105
66. Romano PG, Edvardsson A, Ruban AV, Andersson B, Vener AV, Gray JE, et al. Arabidopsis AtCYP20-2 is a light-regulated cyclophilin-type peptidyl-prolyl cis-trans isomerase associated with the photosynthetic membranes. Plant Physiol 2004 Apr;134(4):1244–7; http://doi.org/10.1104/pp.104.041186
67. Pogorelko GV, Mokryakova M, Fursova OV, Abdeeva I, Piruzian ES, Bruskin SA. Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene 2014;538:12–22.
68. Liu H, Shen J, Yuan C, Lu D, Acharya BR, Wang M, et al. The Cyclophilin ROC3 regulates ABA-induced stomatal closure and the drought stress response of Arabidopsis thaliana. Front Plant Sci 2021 May 25;12:668792; http://doi.org/10.3389/fpls.2021.668792
69. Lee SS, Park HJ, Yoon DH, Kim BG, Ahn JC, Luan S, et al. Rice cyclophilin OsCYP18-2 is translocated to the nucleus by an interaction with SKIP and enhances drought tolerance in rice and Arabidopsis. Plant Cell Environ 2015;38:2071–87.
70. Park SW, Li W, Viehhauser A, He B, Kim S, Nilsson AK, et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc Natl Acad Sci U S A 2013 Jun 4;110(23):9559–64; http://doi.org/10.1073/pnas.1218872110
71. Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci U S A 2006 Dec 5;103(49):18822–7; http://doi.org/10.1073/pnas.0605639103
72. Blanco-Ulate B, Amrine KC, Collins TS, Rivero RM, Vicente AR, Morales-Cruz A, et al. Developmental and metabolic plasticity of white-skinned grape berries in response to Botrytis cinerea during Noble Rot. Plant Physiol 2015 Dec;169(4):2422–43; http://doi.org/10.1104/pp.15.00852
73. Galat A. Variations of sequences and amino acid compositions of proteins that sustain their biological functions: an analysis of the cyclophilin family of proteins. Arch Biochem Biophys 1999 Nov 15;371(2):149–62; http://doi.org/10.1006/abbi.1999.1434
74. Romano PG, Horton P, Gray JE. The Arabidopsis cyclophilin gene family. Plant Physiol 2004;134(4):1268–82; http://doi.org/10.1104/pp.103.022160
75. Hansen J, Jørgensen JE, Stougaard J, Marcker KA. Hairy roots—a short cut to transgenic root nodules. Plant Cell Rep 1989;8(1):12–5; http://doi.org/10.1007/BF00735768
76. Schapira M, Tyers M, Torrent M, Arrowsmith CH. WD40 repeat domain proteins: a novel target class. Nat Rev Drug Discov 2017;16(11):773–86; http://doi.org/10.1038/nrd.2017.179
77. Miller JC, Chezem WR, Clay NK. Ternary WD40 repeat-containing protein complexes: evolution, composition and roles in plant immunity. Front Plant Sci 2016;6:1108; http://doi.org/10.3389/fpls.2015.01108
78. Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 1999;24(5):181–5; http://doi.org/10.1016/s0968-0004(99)01384-5
79. Li H, He Z, Lu G, Lee SC, Alonso J, Ecker JR, et al. A WD40 domain cyclophilin interacts with histone H3 and functions in gene repression and organogenesis in Arabidopsis. Plant Cell 2007;19(8):2403–16; http://doi.org/10.1105/tpc.107.053579
80. Ge Q, Tang Y, Luo W, Zhang J, Chong K, Xu Y. A cyclophilin OsCYP20-2 interacts with OsSYF2 to regulate grain length by pre-mRNA splicing. Rice (N Y) 2020;13(1):64; http://doi.org/10.1186/s12284-020-00425-0
81. Kim JH, Nguyen NH, Nguyen NT, Hong SW, Lee H. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis. Plant Cell Rep 2013 Dec;32(12):1843–53; http://doi.org/10.1007/s00299-013-1497-z
82. Ge Q, Zhang Y, Xu Y, Bai M, Luo W, Wang B, et al. Cyclophilin OsCYP20-2 with a novel variant integrates defense and cell elongation for chilling response in rice. New Phytol 2020;225(6):2453–67; doi:10.1111/nph.16324
83. Gou H, Gan J, Liu J, Deng S, Gan L, Wang X, et al. The GmCYP2-GmHAL3 module regulates salt tolerance in soybean seedlings. Environ Exp Bot 2023;218:105604; http://doi.org/10.1016/j.envexpbot.2023.105604
84. Kai H, Iba K. Temperature stress in plants. In: eLS, John Wiley & Sons Ltd., 2014; http://doi.org/10.1002/9780470015902.a0001320.pub2
85. Jo SH, Park HJ, Lee A, Jung H, Park JM, Kwon SY, et al. The Arabidopsis cyclophilin CYP18-1 facilitates PRP18 dephosphorylation and the splicing of introns retained under heat stress. Plant Cell 2022;34(6):2383–403; http://doi.org/10.1093/plcell/koac084
86. Wang J, Sun P, Li Y, Liu Y, Yang N, Yu J, et al. An overlooked paleotetraploidization in Cucurbitaceae. Mol Biol Evol 2018;35(1):16–26; http://doi.org/10.1093/molbev/msx242
87. Simillion C, Vandepoele K, Van Montagu MC, Zabeau M, Van de Peer Y. The hidden duplication past of Arabidopsis thaliana. Proc Natl Acad Sci U S A 2002;99(21):13627–32; http://doi.org/10.1073/pnas.212522399
88. Patil G, Valliyodan B, Deshmukh R, Prince S, Nicander B, Zhao M, et al. Soybean (Glycine max) SWEET gene family: insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genomics 2015;16(1):520; http://doi.org/10.1186/s12864-015-1730-y
89. Zhou Y, Massonnet M, Sanjak JS, Cantu D, Gaut BS. Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc Natl Acad Sci U S A 2017;114(44):11715–20; http://doi.org/10.1073/pnas.1709257114
90. Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet 2014;46(7):707–13; http://doi.org/10.1038/ng.3008
91. Singh K, Winter M, Zouhar M, Ryšánek P. Cyclophilins, less studied proteins with critical roles in pathogenesis. Phytopathology 2018;108(1):6–14; http://doi.org/10.1094/PHYTO-05-17-0167-RVW
92. Wang P, Heitman J. The cyclophilins. Genome Biol 2005;6:226; http://doi.org/10.186/gb-2005-6-7-226
93. Kong G, Zhao Y, Jing M, Huang J, Yang J, Xia Y, et al. The activation of Phytophthora effector Avr3b by plant cyclophilin is required for the Nudix hydrolase activity of Avr3b. PLoS Pathog 2015;11(8):e1005139; http://doi.org/10.1371/journal.ppat.1005139
94. Maryanto SD, Wibowo WA, Daryono BS. Phenotypic characters and identification CYPs (Cyclophilin) gene in Cucumis melo L. cv. Gama Melon Parfum. Biodiversitas 2021;22:3007–14.
95. Chung SM, Staub JE, Chen JF. Molecular phylogeny of Cucumis species as revealed by consensus chloroplast SSR marker length and sequence variation. Genome 2006 Mar;49(3):219–29; http://doi.org/10.1139/g05-101
96. Gou H, Gan J, Liu J, Deng S, Gan L, Wang X, et al. The GmCYP2-GmHAL3 module regulates salt tolerance in soybean seedlings. Environ Exp Bot 2024 Feb 1;218:105604.
97. Viaud MC, Balhadère PV, Talbot NJ. A Magnaporthe grisea cyclophilin acts as a virulence determinant during plant infection. Plant Cell 2002 Apr;14(4):917–30; http://doi.org/10.1105/tpc.010389
98. Sun J, Sun CH, Chang HW, Yang S, Liu Y, Zhang MZ, et al. Cyclophilin BcCyp2 regulates infection-related development to facilitate virulence of the gray mold fungus Botrytis cinerea. Int J Mol Sci 2021 Feb 8;22(4):1694; http://doi.org/10.3390/ijms22041694
99. Sher Z, Majid MU, Hassan S, Batool F, Aftab B, Rashid B. Identification, isolation and characterization of GaCyPI gene in Gossypium arboreum under cotton leaf curl virus disease stress. Phyton 2021;90(6):1613.
100. Sun T, Zhang M, Geng H, Wang Y, Liu Z, Xue D, et al. Overexpression of the Panax ginseng CyP gene enhances abiotic and biotic stress tolerance in transgenic Arabidopsis. Physiol Mol Plant Pathol 2024 May 1;131:102294.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: Link here [https://jabonline.in/admin/php/uploadss/1268_pdf.pdf].