1. INTRODUCTION
Proteases are a group of hydrolytic enzymes that are present in both prokaryotes and eukaryotes. The catalytic classification includes cysteine, serine, aspartic, glutamic, threonine, and metalloproteases. Their classification is based on the presence of unique amino acids or metal cofactors in their active sites [1]. In comparison to the eukaryotic proteases, bacterial proteases are considered to be more diverse [2]. Microbial proteases have been explored for their role in the healthcare and pharmaceutical industries. Proteases play a pivotal role in various metabolic pathways, immune responses, and pathogenicity of prokaryotes [3] as well as eukayotes. Microbial proteases are a major part of the normal microflora of humans, which thrive on the skin and on the mucosal membranes [4]. The gut microbiota of humans is reported to release proteases, as a part of homeostasis [5]. These proteases restore the host immune system by counteracting the virulence factors released by the pathogens and hydrolyzing them [6]. Researchers have reported the influence of a nutritious diet in the release of gut proteases. Most of the gut microbiota utilize host nutrients and protein to synthesize proteases. A balanced diet can be influenced by the consumption of probiotics, which can balance the gut microbiota [7]. Probiotic-based microorganisms such as Bacillus and Bifidobacterium are reported to release extracellular compounds like peptides (clausin, subtilisin, iturin) and proteins, which are reported to have antimicrobial properties [8]. One such probiotic of the genus Bacillus is Bacillus clausii, which is claimed to have immunomodulatory activity against diarrhea pathogens [9], [10].Proteases reported from B. clausii are shown to have anti-diarrheal effects on cell lines as well as on animal models.
Gastroenteritis or diarrhea is a major gut disorder that is caused by bacteria, viruses, and protozoa [11]. Among these pathogens, Bacillus cereus, a Gram-positive spore-producing bacteria is one of the major diarrhea-causing pathogens. The bacteria is considered a main cause of food poisoning and acute gastroenteritis [12]. Similarly, a broad range of Gram-negative bacteria, like Shigella, Salmonella, Escherichia coli, and Enterobacter spp., are also reported to be the causal agents of diarrhea. One such bacteria is Salmonella enterica, a causative agent of salmonellosis [13]. Salmonellosis is the onset of fever, abdominal pain, and diarrhea. The bacteria prevails in poultry and wild animals, which can enter the food chain through meat, eggs, or milk.
Our study aimed to explore the antimicrobial potential of the probiotic bacteria B. clausii UBBC07, one of the most common, commercially available over-the-counter medicines, which is effectively used to treat diarrhea [14]. The strain was first time isolated and reported by Unique Biotech Limited, India in 2005. Various in vitro and in vivo studies on B. clausii have already confirmed the presence of proteins, peptides, and proteases that counteract diarrhea pathogens like Clostridium difficile, B. cereus, Enterococcus faecalis, and Staphylococcus aureus [15]. In our primary study, the antimicrobial/bacteriostatic activity of B. clausii UBBC07 was further explored from the sporulating culture. The bacteriostatic nature of extracellular secretions was confirmed by agar well diffusion assay against a set of diarrhea-causing Gram-positive and Gram-negative pathogens (B. cereus and S. enterica). Further proteolytic activity in partially purified protein precipitates was confirmed with gel overlay assay against a set of diarrhea-causing pathogens. The ~ 23.4 kDa protein band in tricine SDS-PAGE coinciding with the zone of clearance was considered the target protein, which was further studied. MS/MS spectroscopy with MALDI-TOFF analysis confirmed the molecular weight and protein sequence. Our study aimed to explore the antimicrobial properties of the extracellularly secreted compound against diarrhea-causing pathogens. This was further attained by a set of in-vitro studies with the purified compound. The results were also supported by the MALDI-TOFF analysis which confirmed the purified protein to be a metalloenzyme with very strong protease activity.
2. MATERIALS AND METHODS
2.1. Selection of Bacteria and Antimicrobial Screening
The B. clausii strain UBBC07 an over-the-counter medicine under the name Bactolac was procured from a pharmacy in Jalandhar, India. The standard strains used in this study were E. faecalis (MTCC 3159), S. aureus (MTCC 1430), Escherichia coli (MTCC 293), Pseudomonas putida (MTCC 1194), Enterobacter cloacae (MTCC 7724), S. enterica (MTCC 1164), and B. cereus (MTCC 6629). All the strains were obtained from the CSIR-Institute of Microbial Technology, Chandigarh. The presence of antimicrobial compounds was examined as described by Maqbool et al. [16]. The standard strain of B. clausii UBBC07 was grown in Muller Hinton broth at different temperatures. The cell culture was removed at fixed time intervals and centrifuged at 10,000 rpm for 20 min and cell-free supernatant was tested for the presence of antimicrobial activity. Hundred microliters of centrifuged supernatant was added to the 6 mm deep well on overnight grown test strain plates. The effectiveness of bacterial supernatant was determined by the presence of a clearance zone. For each experiment, the guidelines of the Clinical and Laboratory Standards Institute (CLSI) were followed.
2.2. Partial Purification of Antimicrobial Compound from B. clausii Strain UBBC07
Protein was partially purified by acidic precipitation method [17]. Overnight grown bacterial culture was added to the sterile Muller Hinton broth in 1:100 proportion and incubated at 30 °C/180 rpm for 96 h to induce the synthesis of extracellular compounds. All experiments related to target protein extraction and purification were performed at 4ºC. The sporulated culture was then centrifuged at 10,000 rpm for 20 min to separate the cells from the clear supernatant. The supernatant was treated with 1N HCl in a 1:100 (HCl: supernatant) ratio for 1 h with constant stirring. The supernatant was again centrifuged at 10,000 rpm for 20 min to separate the protein precipitates from the supernatant. The precipitated protein was washed with absolute ethanol (molecular grade; Merck), centrifuged at 10,000 rpm, for 10 min, and decanted the supernatant. The protein pellet was dried and stored at −80°C till further use.
2.3. In-gel Activity Assay for Determination of Peptide Activity
The protein with proteolytic potential against diarrhea-causing pathogens was identified by zymography [18]. Tricine sodium-dodecyl-sulfate polyacrylamide gel electrophoresis (Tricine SDS-PAGE, 15% (w/v) was used in the process. The pellet of partially purified protein was dissolved in 1X phosphate buffer saline (pH 7.2), and the reducing dye was added for 1 h at room temperature. The sample was then loaded on 15% Tricine SDS-PAGE along with a protein ladder and run at 50 mA at 4°C. The samples were loaded in duplicates and after the gel run one of its sections was subjected to staining for band visualization and another part of the gel was washed with Tris-HCl buffer (pH 7.4) for 3 h and then placed on the plates of target pathogens. The plates were incubated at 37°C for up to 48 h to visualize the clearance zone.
2.4. Protein Purification
The target protein band was extracted from SDS-PAGE as described by Sakuma et al. [19]. The excised gel pieces were treated with the destaining solution for 2 h, with periodical vortexing. The protein sample was then subjected to centrifugation (4,000 rpm/5 min) and removed the supernatant. The sample was mixed with 500 µl of 50:50 acetonitrile and 20 mM ammonium bicarbonate to submerge the gel pieces thoroughly and incubated for 20 min. The sample solution was centrifuged and the supernatant was decanted. Elution buffer was added to the gel and incubated for 4 to 16 h to elute out the protein. Protein concentration was estimated by the Lowry method and Bradford assay.
2.5. Mass Spectrometry and Protein Identification
The protein identification was performed by mass spectroscopy [20]. 100 mM of dithiothreitol was added to the purified protein, and the solution was incubated at 56°C for an hour. After the first incubation was completed, 250 mM idoacetamide was added to the same solution, and the reaction mixture was kept at room temperature in the dark for 45 min. After reduction, chymotrypsin (20 ul) was added to the sample, and the mixture was incubated at 37°C, overnight. Digested peptides were extracted using 100 ul of 0.1% trifluoroacetic acid in water and 100% acetonitrile (1:1 ratio of 0.1% TFA in water and 100% ACN) and vacuum dried and dissolved in 5 μl of Tris buffer. External calibration of the instrument was done with standard peptide (PEPMIX Mixture) supplied by Bruker, with masses ranging from 1046 to 3147 Da. Further analysis was done with FLEX ANALYSIS SOFTWARE (Version 3.3) in reflectron ion mode with an average of 500 laser shots at a mass detection range between 500 and 5000 m/z for obtaining the MS /MS. The masses obtained in the MS/MS were submitted for Mascot search in the “Bacillus” database to identify the protein.
2.6. Determination of Minimum Inhibitory Concentration (MIC)
Micro-dilution broth assay was performed using a 96-well microtiter plate [21]. 100 μl of sterile Luria Bertani broth was distributed in the first eight wells, and 100 μl of purified protein was added to the first well. The content of the first well was mixed well and 100 μl of this mixture was added to the second well, making it a two-fold dilution. The process was repeated till well 8. To each well 100 μl of test pathogen cells (105 CFU/ml) were distributed. The microtiter plate was incubated for 16 h at 37°C. The lowest concentration which hampered the bacterial growth was concluded to be the minimum inhibitory concentration.
2.7. Stability Analysis of Purified Protein
Protein’s sensitivity to various physicochemical factors like temperature, pH, reducing agents, and surfactants were analyzed [22]. The purified protein was subjected to a range of temperatures, i.e., 40°C, 50°C, 60°C, 70°C, and 100°C for 30 min and 121°C for 20 min. To study the pH sensitivity, the protein sample was added to different buffers, i.e., citrate phosphate buffer (pH 3 and 5), and Tris-HCl (pH 7.2, 7.4, 9, 10, and 11), followed by incubation at 37°C for 2 h. To study the effect of different detergents the purified protein was treated with 1% of Tween-20, Tween-80, Urea, Triton X-100, and sodium dodecyl sulfate for 2 h at 37°C. All incubated samples were later subjected to agar well diffusion assay against pathogens B. cereus and S. enterica. The untreated protein sample was taken as a control. All the experiments were performed in triplicates with two independent sets of experiments to obtain conclusive results.
2.8. Time Kill Kinetics
The antibacterial properties of purified protein were assessed by counting the colony number of pathogenic bacteria, collected at different time intervals [24]. The 96-well microtiter plate was inoculated with 100 μl of 105 CFU/ml of B. cereus and S. enterica separately with double the MIC concentration of purified protein (2 × 9.78 μM) in LB broth. The sample fractions from 96 well plates were collected at different time intervals, i.e., 0 min, 30 min, 1 h, 2 h, 3 h, and 4 h. Collected sample fractions were plated on LB agar plates, using the spread plate method. Plates were incubated at 37°C for up to 24 h and bacterial colonies were calculated. All the experiments were performed in triplicates with two independent sets of experiments to obtain conclusive results.
2.9. Action Mechanism Study
An action mechanism study was performed as described by Wilson et al. [24]. Extracellular and intracellular changes in B. cereus and S. enterica were studied using a compound microscope. Cell concentrations of 2 × 106 CFU/ml of both test pathogens were added to 2×MIC (9.78 μM) of purified protein, and incubated for 4 h at 37°C. Cells were twice washed with 0.1 M of 1X phosphate buffer saline (pH 7.4) and stored in 1X PBS only. Gram-stained samples were observed under 40× and 100 × magnification of a compound microscope. The test was performed in triplicates.
2.10. Statistical Analysis
The statistical analysis of data was done using Sigma Plot 12.0 and one-way analysis of variance (ANOVA). Values of probability, i.e., p > 0.05, were considered non-significant, while p < 0.001 was taken as significant.
3. RESULTS
3.1 Identification, Purification, and Structural Characterization of Antimicrobial Compound
Sporulated bacterial culture collected after 96 h of incubation [Figure 1A] showed a prominent antimicrobial activity against B. cereus and S. enterica. This was followed by the partial purification of extracellular proteins using acid precipitation, and a gel overlay assay (15% Tricine SDS PAGE) was performed to identify the protein with antimicrobial activity [Figure 1B]. After confirming the target protein band, the protein was excised out of the SDS PAGE gel, purified and observed for its antimicrobial properties [Figures 1C–E]. The antimicrobial activity of the purified protein was also tested against a series of pathogens using an agar well diffusion assay [Table 1].
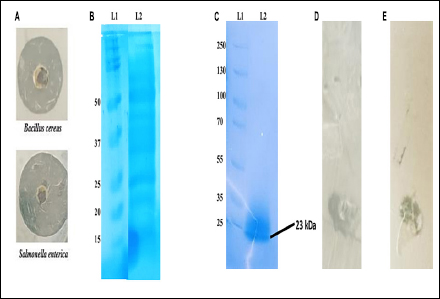 | Figure 1: Protein purification and in-gel activity using zymogram. (A) Agar well diffusion assay with 96 h incubated supernatant in Muller Hinton broth, a zone of clearance appeared against both pathogens, Bacillus cereus and Salmonella enterica. (B) Partially purified extracellular proteins using solvent-based extraction (L2) along with protein marker (L1). (C) Tricine SDS PAGE (15%) run of purified protein, extracted through gel excision method (L2) along with protein marker (L1). (D,E) Gel overlay assay on 1% agar showing clearance zone against both B. cereus and S. enterica confirming the antimicrobial nature of the purified protein.
[Click here to view] |
Table 1: Antimicrobial activity of purified protein against diarrhea-causing pathogens.
| S. no. | MTCC number of strain | Name of the test strain | Zone of clearance (mm) |
|---|
| 1 | MTCC 6629 | Bacillus cereus | 7 |
| 2 | MTCC 1164 | Salmonella enterica | 6 |
| 3 | MTCC 3159 | Enterococcus faecalis | 7 |
| 4 | MTCC 1430 | Staphylococcus aureus | 8 |
| 5 | MTCC 293 | Escherichia coli | 8 |
| 6 | MTCC 1194 | Pseudomonas putida | 6 |
| 7 | MTCC 7724 | Enterobacter cloacae | 5 |
Mass spectrometry analysis of the target protein [Figure 2A] showed molecular mass coinciding with the protein band from SDS-PAGE analysis [Figure 1C]. Peptide mass fingerprinting revealed the mass detection range between 500 and 5,000 m/z. The obtained peptide sequences were analyzed using FLEX ANALYSIS SOFTWARE (Version 3.3) using a Mascot search in the “Bacillus” database to identify the protein. The identified peptide fragment sequences of the target protein showed 36% sequence similarity to the DinB family protein (Accession number AQA268P3I3) of Souchella clausii (KSM-16) [Figure 2B].
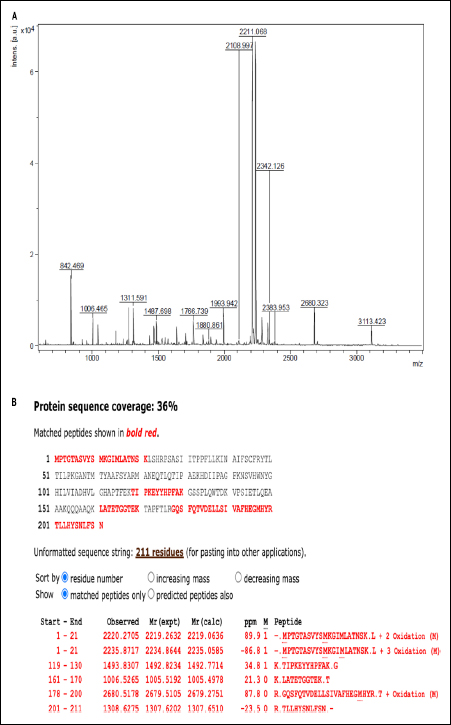 | Figure 2: (A) MALDI-TOF mass spectra of purified protein. (B) The partial amino acid sequence of protein obtained from peptide mass fingerprinting shows maximum similarity to the sequence of Dinb protein of Souchella clausii.
[Click here to view] |
3.2. Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration of purified protein against both target pathogens remained the same, i.e., 9.78 μM [Table 2].
Table 2: MIC values of purified protein against Bacillus cereus and Salmonella enterica.
| S. no. | MTCC number of strain | Name of the test strain | MIC (μM) of protein |
|---|
| 1 | MTCC 6629 | Bacillus cereus | 9.78 |
| 2 | MTCC 1164 | Salmonella enterica | 9.78 |
3.3. Effects of Temperature, pH, and Detergents on Purified Protein Activity
Various physicochemical parameters like temperature, pH, and detergents were studied on the efficacy of purified protein against pathogens B. cereus and S. enterica. Untreated purified protein was taken as a control for all experiments with 100% stability. The average mean and standard deviation were used to describe protein stability. The protein remained stable at 37ºC showing 100% activity. At 40ºC 96.8 % activity was retained against B. cereus. At 50 and 60ºC 73% activity was retained [Figure 3A]. At 70ºC 50% activity, at 100ºC 28 % activity was retained. Protein, when subjected to autoclave at 121ºC for 20 min, completely lost its activity. Similar results were obtained for protein treatment when tested with S. enterica. The protein retained 100% activity from 37ºC to 50ºC. At 60ºC, 80% activity was retained, at 70ºC and 100ºC the activity was reduced to 65% and 50.7%, respectively. Also, protein treatment at 121ºC for 20 min retained no activity at all. ANOVA analysis showed p < 0.0007 and p < 0.0006 for B. cereus and S. enterica, respectively.
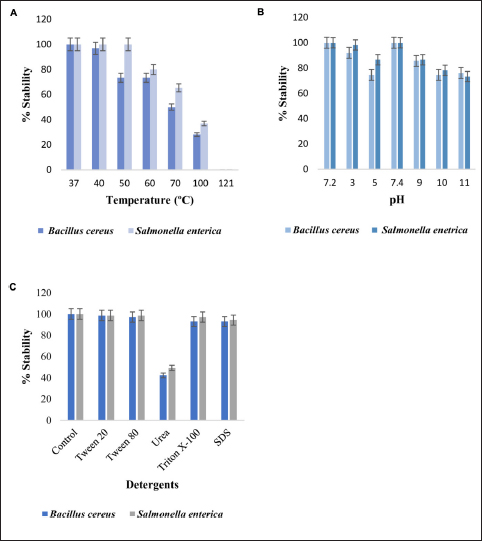 | Figure 3: (A) Protein stability comparison after the treatment at a range of temperature for 30 min and at 121ºC for 20 min. (B) Protein stability at different pH, the purified protein was treated with various buffers for 2 h at 37ºC, and stability was observed as the zone of clearance against the pathogens. (C) Purified protein was treated with different detergents and reducing agents for 2 h at 37ºC. An untreated pure protein sample was used as a control for all experiments.
[Click here to view] |
Protein had 100% stability at pH 7.2 and 7.4. For pH 3, 5, and 9, the protein stability varied between 98% and 86% activity against B. cereus and S. enterica [Figure 3B]. At pH 10 and 11, 74 to 76% stability was observed against B. cereus. For S. enterica at pH 10 and 11, activity above 73% was retained. Treatment with different detergents and surfactants had a variable effect on the stability of the protein [Figure 3C]. The p values for B. cereus and S. enterica were p < 0.001 and p < 0.004.
Protein treatment with Tween 20 and Tween 80, retained 98% activity against Bacillus cereus, with urea 42% activity retained. With Triton X-100 and sodium dodecyl sulfate, 93% activity was recorded. Similarly for Salmonella enterica 98% activity was retained with Tween 20 and Tween 80.With urea, Triton X-100, and sodium dodecyl sulfate 49%, 97%, and 94.3% of activities were retained. p values for Bacillus cereus and Salmonella enterica were < 0.00009 and < 0.00005.
3.4. Time Kill Kinetics Assay
Time kill kinetics assay was performed to analyze the killing of pathogenic strain cells (B. cereus and S. enterica) based on a variable time profile. A sharp decline in log10 (CFU/ml) of both bacterial strains was obtained within 4 h of treatment [Figure 4] confirming the antibacterial potential of purified protein.
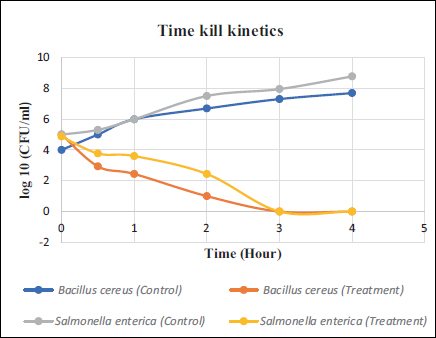 | Figure 4: Time kill kinetics of purified protein against B. cereus and S. enterica. 2 × MIC (9.78 μM) of protein was used against 2 × 106 CFU/ml of bacterial strains. Results were represented as a mean of two independent sets.
[Click here to view] |
3.5. Action Mechanism Studies
Protein-treated bacterial cells were observed using compound microscopy. The cell membrane integrity was taken as a major of cell viability. Control and treatment cells were washed, Gram-stained, and observed for cell surface changes. The set of treatment cells from both bacteria appeared damaged and showed debris formation around healthy cells when compared to the control set. This confirmed the proteolytic property of purified protein [Figure 5].
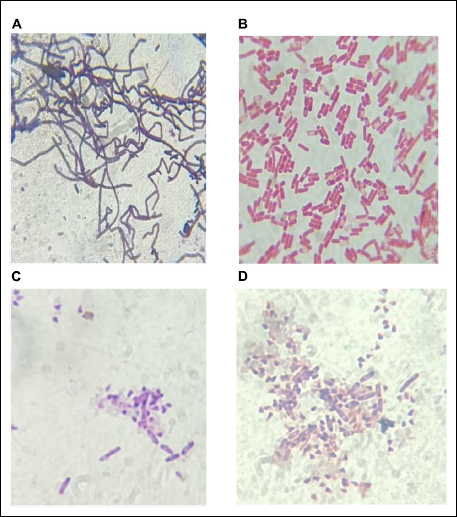 | Figure 5: Gram staining of B. cereus and S. enterica. (A,B) Untreated control group cells of Bacillus cereus and Salmonella enterica, respectively (C,D) 2 × 106 CFU/ml B. cereus and S. enterica cells treated with purified protein, showed disrupted cells and debris.
[Click here to view] |
4. DISCUSSION
Bacillus clausii is a GRAS-classified bacteria, which is utilized as a probiotic drink worldwide [17]. Extracellularly secreted proteases from B. clausii hold great significance as an alternative medicine, as they can reduce the risk of development of antibiotic resistance [12]. A study reported by Ripert et al. [12] describes the antimicrobial action of purified serine protease from B. clausii O/C strain, which counteracted the toxins of C. difficile and B. cereus in cell lines and reduced toxicity [12]. Subtilisin is another protease with strong antimicrobial activity, isolated from the genus Bacillus, which is explored in many disease models like colitis as it can degrade gluten [25]. Studies on viral infections have shown proteases targeting the viral glycoproteins and restraining their entry into host cells [26]. Researchers have reported that B. clausii, not only can tolerate bile salts and a pH 2 environment [27], but also successfully germinate in the intestinal environment [26].
In our study, we identified and isolated a protein of the Din b protein family that showed inhibitory action against B. cereus and S. enterica. The protein’s molecular mass and sequence determination were done by MALDI-TOFF spectroscopy. The molecular mass of protein was 23.4 kDa, which is similar to the weight observed on SDS-PAGE during zymography. The results denoted that the sporulation of bacteria is efficient in the production of antimicrobial protein. Cell-free supernatant from 96-hour incubation, as well as the purified protein, hindered the growth of target Gram-positive B. cereus and Gram-negative S. enterica. Peptide mass fingerprinting has confirmed the protein sequence similarity with 36% homology to the Din B protein family. The MS spectroscopy analysis has confirmed the protein to be a metalloenzyme with strong protease activity, which was further supported by the in vitro experiments. Enzymatic degradation was not compatible with the remaining protein sequence; hence, only partial protein sequence was determined. This outcome also concluded that the isolated target protein must have some unique amino acid sequence, which did not find any protein similarity in the Bacillus database using MASCOT software. Physicochemical analysis of the protein concluded its stability at a variable range of temperature (up to 70°C), pH (3 to 11), and detergents.
Gram-positive B. cereus is a known cause of food poisoning and a causative agent of diarrhea [28]. Likewise, all serotypes of S. enterica cause diarrhea, which may appear fetal in many cases [29]. In this study, both target pathogens’ growth was constrained with a minimum inhibitory concentration of 9.78 μM of purified protein. The protein’s ability to inhibit a broad range of bacteria and resistance to variable temperature, pH, and detergent treatment makes it suitable for drug design. The proteolytic action of protease was observed after time-kill kinetics against pathogenic bacteria. Under 100× magnification of a compound microscope treated cells showed debris formation, while the control bacterial cells remained intact [12] have also mentioned the proteolytic activity of purified serine protease against C. difficile and B. cereus. A similar observation was reported by Aquaro et al [30], where protease-based drugs were interacting with the cell membranes of viruses and disrupting them to cause cell lysis [31].
5. CONCLUSION
The extracellularly released protein from B. clausii UBBC07 inhibited the growth of B. cereus and S. enterica. Such secreted proteins/ proteases of B. clausii can be a strong tool in the treatment of diarrhea infection. The use of protein engineering can result in providing better alternatives to antibiotics and probiotics, reducing the multiple drug resistance, and a cure with cost-effectiveness for the masses. In conclusion, to reduce the multidrug resistance formulation of protease-based proteins isolated from B. clausii, modified with genetic engineering can be utilized as antimicrobial agents.
6. ACKNOWLEDGMENTS
We are thankful to our HOD and technical staff for providing all laboratory facilities. The authors acknowledge the generous support from the research supporting project (RSPD2024R713) by King Saud University, Riyadh, Kingdom of Saudi Arabia.
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
The data supporting this study's findings are available on request from the corresponding author.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Caminero A, Guzman M, Libertucci J, Lomax AE. The emerging roles of bacterial proteases in intestinal diseases. Gut Microbes. 2023;15:1, 2181922, [CrossRef].
2. Kriaa A, Jablaoui A, Rhimi S, Soussou S, Mkaouar H, Mariaule V, et al. SP-1, a Serine protease from the gut microbiota, influences colitis and drives intestinal dysbiosis in mice. Cells. 2021; 10(10):2658. [CrossRef]
3. Souza D, Bonnart C, Tapias NS, Marcellin M, Gilmore B, Alric C, et al. Functional proteomic profiling of secreted serine proteases in health and inflammatory bowel disease. Sci Rep. 2018; 8:7834. [CrossRef].
4. Singh T, Kaur G, Kaur A. Dysbiosis—an etiological factor for cardiovascular diseases and the therapeutic benefits of gut microflora. Adv Gut Microb Res. 2023; Article ID 7451554, 8 pages, [CrossRef
5. Burgers K, Lindberg B and Bevis JZ. Chronic diarrhea in adults: evaluation and differential diagnosis. Am Fam Phys. 2020; 101(8). https://www.aafp.org/afp/2020/0415/p472-s1.html.
6. Edogawa S, Edwinson AL, Peters SA, Chikkamenahalli LL, Sundt W, Graves S, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. 2020;69:62–73. [CrossRef].
7. Moayyedi P, Simren M, Bercik P. Evidence-based and mechanistic insights into exclusion diets for IBS. Nat Rev Gastroenterol Hepatol. 2020;17(7):406-13. [CrossRef].
8. Stoica RM, Moscovici M, Tomulescu C, Casarica A, Babeanu N, Popa O, et al. Antimicrobial compounds of the genus Bacillus: a review. Roman Biotechnol Lett. 2019;24(6):1111-9. [CrossRef]
9. Jovanovic J, Ornelis VFM., Madder A, Rajkovic A. Bacillus cereus food intoxication and toxic infection. Comp Rev Food Sci Food Saf. 2021. [CrossRef].
10. Khokhlova E, Colom J, Simon A, Mazhar S, García-Lainez G, Llopis S, et al. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms. 2024;11(2):240. [CrossRef].
11. Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15(2):182–93.e3.
12. Ripert G, Racedo SM, Elie AM, Jacquot C, Bressollieret P, Urdaci MC, et al. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob Agents Chemother. 2016;60:3445-54. [CrossRef].
13. Guillier L, Thébault A, Fravalo P, Gras L, Silva N, David J, et al. Risk factors for sporadic salmonellosis: a systematic review and meta-analysis. Microbial Risk Anal. 2021;17:100138. ISSN 2352-3522, [CrossRef].
14. Ahire JJ, Kashikar MS, Madempudi RS. Comparative accounts of probiotic properties of spore and vegetative cells of Bacillus clausii UBBC07 and in silico analysis of probiotic function. 3 Biotech. 2021;11:116.
15. De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33(3):e00181-19. [CrossRef].
16. Maqbool H, Visnuvinayagam S, Zynudheen A, Safeena MP, Kumar S. Antibacterial activity of beetroot peel and whole radish extract by modified well diffusion assay. Int J Curr Microbiol Appl Sci. 2020. January, [CrossRef].
17. Liu Z, Guan X, Zhong X, Zhou X, Yang F. Bacillus velezensis DP-2 isolated from Douchi and its application in soybean meal fermentation. J Sci Food Agricult. 2020. [CrossRef].
18. Nargotra P, Sharma V, Sharma S, Bangotra R, Bajaj BK, Purification of an ionic liquid stable cellulase from Aspergillus aculeatus PN14 with potential for biomass refining. Environ Sustain. 2022;5:313-23. [CrossRef].
19. Sakuma C, Tomioka Y, Li C, Shibata T, Nakagawa M, Kurosawa Y, et al. Analysis of protein denaturation, aggregation, and post-translational modification by agarose native gel electrophoresis. Int J Biol Macromol. 2021; 172: 589–596. ISSN 0141-8130, [CrossRef].
20. Noor Z, Ahn SB, Baker MS, Ranganathan S, Mohamedali A. Mass spectrometry-based protein identification in proteomics – a review. Brief Bioinform. 2021; 22(2):1620–38. [CrossRef].
21. Yi L, Luo L, Lu X. Efficient exploitation of multiple novel bacteriocins by a combination of complete genome and peptidome. Front Microbiol. 2018;9:1567. [CrossRef].
22. Taggar R, Jangra M, Dwivedi A, Bansal K, Patil P. Bacteriocin isolated from the natural inhabitant of Allium cepa against Staphylococcus aureus. World J Microbiol Biotechnol. 2021; 37:20. [CrossRef].
23. Jangra M, Kaur M, Tambat R, Rana R, Mauryaet SK, Khatri N, et al. Tridecaptin M, a new variant discovered in mud bacterium, shows activity against colistin and extremely drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63:6. [CrossRef].
24. Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, et al. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Res Rev J Eng Technol. 2017;6(4).
25. Wei G, Darwish G, Oppenheim FG, Schuppan D, Helmerhorst EJ. Commensal bacterium Rothia aeria degrades and detoxifies gluten via a highly effective subtilisin enzyme. Nutrients. 2020; 12:3724. [CrossRef]
26. Gioia M, Ciaccio C, Calligari P, De Simone G, Sbardella D, Tundo G, et al. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem Pharmacol. 2020;182:114225. ISSN 0006-2952, [CrossRef].
27. Bueno A, Abreu A, Guarner, F, et al. Bacillus clausii for gastrointestinal disorders: a narrative literature review. Adv Therapeut. 2022;39:4854–74. [CrossRef].
28. Dubey S, Sharma N, Thakur S, Patel R, Reddy BM, Bacillus cereus food poisoning in Indian perspective: a review. Pharma Innov J. 2021;10(9):970-5.
29. Zhang J, Peng Z, Chen K, Zhan Z, Shen H, Feng S, et al. Genomic characterization of Salmonella enterica serovar Weltevreden associated with human diarrhea. Microbiol Spectr. 2023;11:e03542-22. [CrossRef]
30. Aquaro S, Borrajo A, Pellegrino M, Svicher V. Mechanisms underlying antiretroviral drugs in different cellular reservoirs with a focus on macrophages. Virulence. 2020; 11(1):400–13. [CrossRef].
31. Caminero A, Guzman M, Libertucci J Lomax AE. The emerging roles of bacterial proteases in intestinal diseases. Gut Microb. 2023; 15:1. 2181922. [CrossRef]




