1. INTRODUCTION
Collagen, a fibrous protein, plays a significant role in various physiological processes within the skin’s tendons, cartilage, bones, and connective tissue. Collagen protein constitutes around 30% of the total protein content in an animal’s body. Collagen has extensive applications across various industries, such as food, cosmetics, pharmaceuticals, medicine, and photography. Generally, bovine and porcine sources are the primary origins of commercial collagen. However, the availability of mammalian collagen has been diminishing due to concerns regarding the well-being of cattle, such as bovine spongiform encephalopathy, foot-and-mouth disease, and religious constraints [1]. Furthermore, the use of porcine collagen is restricted in many regions and by various religious factions, such as Muslims and Jews, due to religious and consumer concerns. Therefore, marine collagen is seen as a feasible alternative to mammalian collagen.
Marine collagen possesses properties that render it suitable for application as a biomaterial due to its solubility in water, compatibility with metabolic processes, and high accessibility [2]. The peanut worm (Siphonosoma australe) is a marine organism from the phylum Sipuncula that is shaped like a peanut. The main body wall of Sipuncula features a unique cuticle structure called the body papilla, composed of collagen fibers. Sipuncula consists of body fluids and the body wall, with the body wall making up to 80% of its total weight. Peanut worms are commonly used as a dietary source due to their substantial nutritional content and potential applications in traditional medicine [3].
Research findings indicate that marine collagen, such as that derived from peanut worms, possesses anti-inflammatory and wound-healing properties [4]. Inflammation is the body’s protective response to harmful stimuli, including pathogens, cellular damage, and irritants. The inflammatory process usually subsides at the stage of completion or healing, but sometimes, it can turn into severe inflammation, which poses a greater risk and can be fatal. Cyclooxygenase-2 (COX-2) is a significant mediator of inflammatory pathways, with its upregulation and overexpression primarily associated with inflammation [5].
Treatment of inflammation is generally divided into steroidal anti-inflammatory drugs (SAIDs) and non-steroidal anti-inflammatory drugs (NSAIDs). Both classes of anti-inflammatory drugs have side effects on the body. SAIDs can lead to peptic ulcers, decreased immunity, and fatty tissue atrophy, while NSAIDs can result in bleeding from stomach ulcers, kidney disorders, and anemia [6]. Due to the side effects of those drugs, alternative natural anti-inflammatory agents are increasingly being developed, including various types of plants, fish, algae, and peanut worms (Sipunculus nudus) [4]. Marine collagen peptides derived from Sipuncula peanut worms (S. nudus) have a high potential for accelerating wound healing [7]. Sangtanoo et al. discovered that two new peptides obtained from S. nudus exhibit anti-inflammatory properties [4]. In addition, Zhang et al. reported that extracts derived from S. nudus have characteristics that reduce inflammation and alleviate pain [8].
Until recently, there has been no information or research on using the peanut worm species S. australe as a raw material for collagen or its anti-inflammatory properties. The novel component of this study was the utilization of S. australe as a raw material for collagen, which can inhibit COX-2. The utilization of S. australe as a source of collagen and its ability to inhibit COX-2 present an opportunity for its development as a functional food, pharmaceutical drug, or supplement. Thus, this study aimed to determine the effect of acetic acid extraction time on the physicochemical characteristics of S. australe collagen and COX-2 inhibitory activity.
2. MATERIALS AND METHODS
2.1. Materials
Dried peanut worms (S. australe) averaging ±8 cm in length and ±5 g in weight were purchased from a local supplier in Rombo Village, Southeast Sulawesi Province, Indonesia, in February 2022. The samples were placed in polyethylene bags, brought to the laboratory under cold conditions, and stored at 4°C until further use. The chemicals utilized for extracting and analyzing the collagen were of analytical grade, including NaOH (Merck), acetic acid (Merck), 96% ethanol (Merck), and NaCl. In addition, the COX-2 inhibitor screening kit (Fluorometric) (MAK399) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA), while the regenerated cellulose membrane (Membra-Cel), size 14 kDa (MD44) was obtained from Viskase Co. (Lombard, Illinois, USA).
2.2. Pre-treatment for Sample
Pre-treatment procedures were conducted before the extraction of collagen. The purpose of the process was to remove any undesired elements and fully extract the collagens without any disruption. This method ensures that no additional particles interfere with the yield and properties of collagen production. Peanut worms were soaked in a 0.1 M NaOH solution at 4°C, using a ratio of 1:10 (w/v) for 6 h to eliminate non-collagen proteins, with the solution being changed every 3 h. This was followed by washing in cold distilled water until a neutral pH of 7 was achieved. Peanut worms were then demineralized using 0.5 M EDTA-2Na with a ratio of 1:10 (w/v) at 4°C, pH 7.4 for 48 h, the solution being changed every 24 h. Afterward, the sample is rinsed with cold water and then exposed to collagen extraction utilizing aqueous acetic acid [9].
2.3. Extraction of Acid-Soluble Collagen
The peanut worm S. australe, previously stripped of minerals, was submerged in a 0.5 M acetic acid solution in a 1:10 (w/v) ratio. The combination was then agitated for 0, 24, 48, and 72 h at 4°C. Finally, the mixture was passed through two layers of cheesecloth. To achieve a final concentration of 2.6 M, the collagen solution was precipitated by adding NaCl. The residue was obtained using the process of centrifugation at 15,000 g for 30 min at a temperature of 4°C. Subsequently, dialysis (MWCO of 14 kDa) using 30 volumes of a 0.1 M acetic acid solution and deionized water for 48 h. The dialysate obtained was subjected to freeze-drying and subsequently identified as acid-soluble collagen (ASC) from S. australe (ASC-SA) [9].
2.4. Collagen Yield
To determine the collagen yield, the dry weight of the freeze-dried collagen was compared to the dry weight of the peanut worm, which was calculated using the following formula [9].

2.5. Protein
The protein content was calculated using Kjeldahl. In a heat block (Kjeltec system 2020 digestor, Tecator Inc., Herndon, VA, USA) at 420°C for 2 h, 1 g of raw material was digested with 15 mL of concentrated sulfuric acid (H2SO4) and two copper catalyst tablets. Water (H2O) was added to hydrolysates after cooling for neutralization and titration.
2.6. pH
The pH values were determined by homogenizing 10 g of macerated sample with 90 mL of distilled water, followed by measurement using a calibrated pH meter (OHAUS STARTER 2100 Bench pH meter, OHAUS Instruments, USA).
2.7. Viscosity
A viscometer (Brookfield) with spindle No. 2 and a speed of 100 rpm was used to determine the viscosity of a 500 mL solution comprising collagen dissolved in 0.5 M acetic acid at a concentration of 0.3% (w/v) [10].
2.8. Amino Acid
The samples underwent hydrolysis using 6 N HCl at 110°C for 24 h, in the presence of 1% (w/v) phenol. The hydrolysate obtained was subjected to derivatization, followed by drying and dissolution in the solvent used for the sample. The samples were subsequently analyzed using LC-MS/MS (Waters Xevo TQD, Milford, MA) to determine their derivatives. The chromatogram was analyzed to determine the area beneath the peak of each amino acid. This area was then compared to standard amino acids; the results were given as the number of residues per thousand amino acids.
2.9. Functional Group of Extracted Collagens
Fourier transform infrared spectroscopy (FTIR) was employed to analyze the functional groups present in the collagen. Disks containing 1 mg of collagen were mixed with ± 100 mg of potassium bromide (KBr), compacted in a pellet mold, and vacuumed in a pellet molding machine. Measurements were conducted at wave numbers ranging from 4.000 to 400 cm−1 using an infrared spectrophotometer (Thermo Scientific Nicolet iS10, USA). Collagen functional groups were determined based on the absorption peaks of the wavenumbers detected with the absorption region for the protein functional groups [11].
2.10. Molecular Weight
The molecular weight of ASC-SA was determined using SDS-PAGE with a 7.5% resolving gel and a 5% stacking gel. Collagen was dissolved in 10% (w/v) SDS, incubated at 85°C for 1 h, and then centrifuged at 10,000 g for 15 min. The supernatant was mixed with sample buffer at a ratio of 1:1 (v/v) and heated for 4 min. Samples were injected into the polyacrylamide gel, and electrophoresis was conducted at 180 V. The gel was stained with the staining solution for 30 min and immersed in the destaining solution until it became clear.
2.11. COX-2 Inhibition Assay
The in vitro inhibitory effect of collagen on COX-2 enzymes was evaluated using a COX-2 inhibitor screening kit (Fluorometric) manufactured by Merck. A 96-well plate was utilized to prepare a mixture consisting of arachidonic acid/NaOH solution (10 μL), recombinant COX-2 (1 μL), COX assay buffer (76 μL), COX-2 cofactor working solution (2 μL), test solution (10 μL), and COX probe solution (1 μL). The measurement of fluorescence kinetics was conducted for 10 min at a temperature of 25°C. A multimode microplate reader (Spark, Tecan, Switzerland) was utilized for this purpose, with excitation and emission wavelengths set at 535 nm and 587 nm, respectively. In the COX-2 experiments, celecoxib was employed as the positive control.
To determine the matching fluorescence values (RFU1 and RFU2), two points (T1 and T2) were chosen from the linear range of the plot. The slope (S) for all samples was determined by dividing the net change in fluorescence (ΔRFU = RFU2 − RFU1) by the appropriate period (ΔT = T2 − T1) using the following equation:

2.12. Statistical Analysis
The mean differences between the samples were determined using a one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test on the data. SPSS IBM 25 (IBM, Armonk, New York, USA) was utilized for the statistical analysis, with significance differences declared at a 5% level.
3. RESULTS AND DISCUSSION
3.1. Yield of Collagen
The yield indicates the efficiency of the collagen extraction procedure, with ASC-SA reaching a maximum yield of 3.55 ± 0.20% after 72 h of extraction (P < 0.05) [Table 1]. The longer acetic acid interacts with collagen telopeptides, the better the cross-linking solubility on the helical side of the telopeptide. Acetic acid is capable of dissolving cross-linked collagen [12]. The process of immersion in an acidic solution leads to skin expansion due to water penetrating the collagen fibers. The behavior under observation can be ascribed to the electrostatic forces between the polar groups on the collagen fiber and the hydrogen ions originating from the acid. The structural integrity of collagen fibers is compromised by the breakage of non-covalent linkages, leading to their dissolution in the acetic acid solution. According to Astiana et al., the optimal solubility of collagen was achieved through a three-day (72 h) extraction process [13]. Excessive immersion can lead to the depletion of collagen [14].
Table 1: Effect of acetic acid extraction time on yield, protein, and pH value of ASC-SA.
| Analysis | Extraction Time (h) |
|---|
| 0 | 24 | 48 | 72 |
|---|
| Yield (%) | 1.69. ± 0.11 | 2.68. ± 0.09 | 2.95bc ± 0.19 | 3.55. ± 0.20 |
| Protein (%) | 78.05. ± 0.28 | 83.28. ± 0.09 | 85.58. ± 0.10 | 85.52. ± 0.11 |
| pH | 3.61 ± 0.08 | 3.57 ± 0.12 | 3.49 ± 0.01 | 3.42 ± 0.01 |
The yield obtained in this study was higher than ASC from Haruan fish scale [15] and snapper fish scale collagen [9]. The observed variation is likely attributed to the selection of materials, which can be considered a factor influencing collagen yield. Discrepancies in collagen production are associated with differences in fish skin protein composition, variations in pre-treatment conditions, and diverse extraction methods [13].
3.2. Protein of Collagen
The protein content of ASC-SA exhibited a range of 78.05 ± 0.28% to 85.58 ± 0.10% (P < 0.05) [Table 1]. The increased protein content observed in this study can be ascribed to the pre-treatment procedure involving NaOH and EDTA-2Na, which effectively removes impurities. The application of NaOH and EDTA-2Na in the pre-treatment process has been demonstrated to successfully remove 98% of inorganic components, including non-collagenous proteins and other impurities, during the demineralizing of collagen [16]. Applying this pre-treatment method will yield abundant, pure collagen from peanut worms, making the subsequent extraction process with acetic acid easier. The maximum protein content was seen at the end of a 48 h extraction period, followed by a slight reduction of 0.06% after 72 h. The hydrolysis of peanut worm body walls by acetic acid results in the production of collagen, which is a protein-rich substance. There is a positive correlation between the amount of extracted collagen protein and the duration of the extraction process. The acetic acid solution induces the formation of collagen fibers, which is attributed to a significant increase in hydrogen ions [17]. This process involves the cleavage of collagen telopeptides, disruption of ionic connections, and collagen synthesis. However, prolonged hydrolysis of peanut worm collagen protein by acetic acid eventually leads to degradation and decreasing protein quantity. Extended soaking can reduce collagen solubility, leading to potential collagen loss [14]. The ASC-SA in this study was higher than collagen derived from Spanish mackerel skin [18]. This difference is probably linked to geographic variations, affecting protein content and quality [19].
3.3. pH Value
The ASC-SA pH values varied between 3.42 and 3.61 [Table 1]. This value exceeds the collagen pH reported by Puspitasari et al., 0.17–0.85 [20]. There was no significant difference in collagen pH between treatments (P > 0.05), indicating that the pH value remained unaffected by the duration of collagen extraction. ASC exhibits high solubility at acidic pH (1–4), with maximum solubility at pH 3 and a significant decrease in solubility above pH 4 [15]. The limited solubility of collagen is attributed to hydrophobic interactions between its molecules, resulting in decreased solubility at the isoelectric point due to the integrity of cross-links [21]. Various factors can influence pH values, including the extraction method, acetic acid concentration, and the duration of the extraction process. The ultimate pH of collagen can be influenced by incorporating a neutralization procedure after extraction. Optimal neutralization can effectively reduce acid residues, leading to collagen samples with pH levels close to neutrality.
3.4. Viscosity of Collagen
The viscosity of ASC-SA decreased with increasing temperature, ultimately reaching a value of 52°C [Figure 1]. This occurs due to the denaturation of the collagen triple-helix structure as the temperature rises. Elevated temperatures cause the breakdown of hydrogen bonds and van der Waals bonds that hold the collagen structure. Collagen is a highly structured system with a high viscosity [10]. Thermal denaturation will permanently damage collagen, and at temperatures exceeding 40°C, collagen undergoes complete denaturation, resulting in a random mixture of single, double, and triple strands [22]. However, collagen is highly temperature-sensitive, and above ± 40°C, it transforms into gelatin.
Consistent with Chen et al., this study found that relative viscosity decreased rapidly from 83.37% to 54.27% at 37–45°C [23]. The highest viscosity value of peanut worm collagen was determined after 72 h (9 cP at 4°C), 24 h (8.88 cP at 4°C), 48 h (8.76 cP at 4°C), and 0 h (8.1 cP at 4°C). The high viscosity value of collagen can be attributed to its higher content of inter- and intramolecular cross-links, which stabilize the collagen structure [10].
3.5. Functional Group of Extracted Collagens
The ASC-SA was characterized using Fourier transform infrared (FTIR) spectroscopy. Collagen has five distinct amide absorption sites: amide A, amide B, amide I, II, and III [Figure 2]. The amide A of ASC-SA displayed a wavenumber in the absorption range of 3301–3304 cm−1. The amide A functional group signifies the presence of a detectable stretching NH group and serves as an index of hydrogen bonds in the amide group. The amide B region of ASC-SA can be found in the spectral range of 2927–2930 cm−1 and indicates a symmetrical stretching vibration of the CH2 group [24].
The amide I region of ASC-SA is located at 1655–1656 cm−1. Amide I, characterized by a C=0 strain vibration, serves to identify the presence of serine in collagen protein [24]. C-O stretching vibrations in the polypeptide backbone are recorded at 1600–1700 cm−1 [25]. The ASC-SA exhibits an amide II region of 1543–1545 cm−1. The FTIR spectra revealed an intact collagen triple-helix structure with characteristic collagen amide II bands at 1538–1548 cm−1 [26]. The vibration of collagen amide II is related to C-N group stretching and N-H bending [25]. The amide III region of ASC-SA is found at 1239–1240 cm−1. The frequency of collagen amide III at 1229–1301 cm−1 is related to stretching C–N and bending N–H. C–N stretching and CH2 vibrations on the glycine backbone and proline side chains occur at 1238–1240 cm−1 [25].
The amide III analysis of ASC-SA demonstrated the preservation of a triple-helix structure in the collagen, signifying that denaturation did not occur during the extraction process, and the collagen remained unaffected, retaining its original state without transforming into gelatin. The absorption peak observed between 1236 and 1452 cm−1 provides evidence of this helical structure. Furthermore, the intensity ratio between the peak of the amide III absorption region and the peak at 1450 cm−1 indicates the collagen triple-helix structure [12].
3.6. Molecular Weight of Collagen
The SDS-PAGE analysis was employed to determine the molecular weight of ASC-SA, revealing that it consists of two chains: the α chain with molecular weights of 123 kDa and the β chain with 280 kDa [Figure 3]. This pattern suggests a general similarity between ASC-SA and fish collagen, indicating the duration of acetic acid hydrolysis influences the strength of collagen bands in peanut worms. Prolonged hydrolysis increases the contact time between acetic acid and collagen fibrils, leading to a more significant formation of collagen polypeptides. The cleavage of collagen fibrils is facilitated by acetic acid, which alters the ionic interactions within hydrogen bonds, ultimately increasing band intensity.
 | Figure 3: Effect of various extraction times on the molecular weight of ASC-SA.
[Click here to view] |
The SDS-PAGE analysis further reveals that ASC-SA comprises type I collagen, characterized by an α chain comprising two identical subunits, namely α1 and α2. The electrophoretic mobility of the α1 and α2 chains is not separated due to their relatively similar molecular weights. Type I collagen is made up of two identical subunits, α1 and α2 chain, which assemble as the primary component ([α1]2α2) [16]. Additionally, a high-molecular-weight constituent, identified as β chain dimers, indicates substantial collagen cross-linking [9]. This study is comparable to the profile of ASC from the Sipunculida coelomic wall, which shows two chains (α and β) [11] and follows a similar pattern as ASC of unicorn leatherjacket fish skin [10] and tilapia skin [26].
3.7. Amino Acid of Collagen
Analyses were conducted to determine the amino acid content of the collagen with the highest yield production (72 h). The amino acids with the highest content in the sample are glutamic acid, arginine, and aspartic acid, with values of 13.76%, 10.17%, and 7.77% of the total. The total contents of glycine, proline, and alanine, characteristic of ASC-SA collagen, were 2.65, 1.51, and 5.52%, respectively. These results were similar to ASC from salmon skin [27] but lower than ASC from Chirocentrus dorab fish skin [28]. Collagen amino acid content differences can be attributed to isolation methods, isolation materials, and amino acid determination methods.
The reduced collagen amino acid content might be attributed to the high concentration of acetic acid and the lengthy extraction time required to break down collagen telopeptides into minuscule free amino acids. During the dialysis procedure, these small amino acids can dissolve and exit through the pores of the 14 kDa dialysis bag. Peanut worms’ tropocollagen and telopeptide collagen structure are weaker than that of fish or animal skin, rendering it more susceptible to degradation when exposed to acetic acid. High amounts of acetic acid can affect the amino acid proportion of collagen through ion replacement, thereby influencing the protein’s structure [29]. Moreover, acids have been shown to effectively break down intermolecular bonds within amino acid chains, leading to permanent destruction through denaturation and dissolution processes.
3.8. COX-2 Inhibition Assay
The COX-2 inhibitor assay kit was used to test the anti-inflammatory properties of ASC-SA. The COX-2 enzyme plays a crucial role in the inflammatory process by converting arachidonic acid into prostaglandins, which mediate inflammation. The COX-2 inhibitor test demonstrated that the most effective collagen extraction results were achieved after 72 h, obtaining a 41.12 ± 0.58% (IC50 = 59.9 μg mL−1). The duration of the extraction process significantly impacts the COX-2 inhibitor properties of ASC-SA [Figure 4], as indicated by statistical significance (P < 0.05). This phenomenon occurs because acetic acid can cleave collagen fibers during the extraction process, forming less complex collagen molecules. These molecules feature peptide side chains containing hydrophobic and positively charged amino acids, which can attach to the active site of the COX-2 enzyme and inhibit its enzymatic activity. ASC-SA contains 21.87% hydrophobic amino acids, such as glycine, alanine, valine, proline, leucine, and isoleucine, as well as 16.23% positively charged amino acids, including histidine, lysine, and arginine [Table 2]. Most anti-inflammatory peptides contain hydrophobic amino acids, with one or more of these amino acids contributing to the peptide’s structure. Similarly, positively charged amino acids such as histidine, lysine, and arginine are essential for enhancing the anti-inflammatory activity of peptides [30].
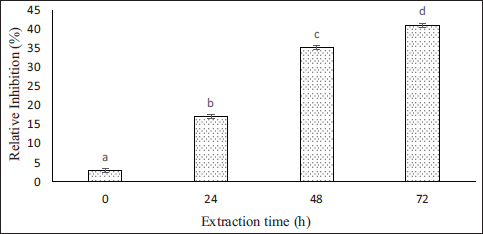 | Figure 4: Effect of various extraction times on the COX-2 inhibitions of ASC-SA.
[Click here to view] |
Table 2: Amino acids content of ASC-SA.
| Amino Acid | Peanut Worm (%) | ASC Salmon (%) [27] |
|---|
| Alanine | 5.52 ± 0.03 | 8.76 |
| Arginine | 10.17 ± 0.04 | 10.14 |
| Aspartic acid | 7.77 ± 0.03 | 5.69 |
| Glycine | 2.65 ± 0.01 | 27.98 |
| Glutamic acid | 13.76 ± 0.07 | 10.23 |
| Histidine | 1.49 ± 0.00 | 1.93 |
| Isoleucine | 2.84 ± 0.01 | 1.32 |
| Leucine | 6.77 ± 0.03 | 2.60 |
| Lysine | 4.57 ± 0.02 | 3.41 |
| Valin | 2.58 ± 0.01 | 1.92 |
| Phenylalanine | 1.71 ± 0.00 | 2.77 |
| Proline | 1.51 ± 0.00 | 12.07 |
| Serine | 3.51 ± 0.01 | 5.61 |
| Threonine | 4.50 ± 0.02 | 2.91 |
| Tyrosine | 2.70 ± 0.01 | 0.44 |
The inhibitory effect of ASC-SA on COX-2 enzyme activity is influenced by the interaction between the amino acid side chain of ASC-SA and the enzyme’s active site, and there is potential to further increase ASC-SA’s inhibitory value against the COX-2 enzyme. The process of hydrolyzing collagen into collagen peptides has the potential to enhance the presence or exposure of hydrophobic and positively charged amino acid side chains, as well as generate smaller peptide sizes. Consequently, this process could potentially improve the anti-inflammatory effect. Peptides with low molecular weights (less than 1 kDa) exhibit higher anti-inflammatory activity [30]. Peanut worm S. australe collagen demonstrates the capacity to mitigate the risk of inflammatory diseases and offers a novel therapeutic approach for anti-inflammatory intervention.
4. CONCLUSION
Findings from this study indicate that the optimal duration for extracting peanut worm collagen using acetic acid (ASC-SA) is 72 h. These ASC-SA have the properties of typical collagen and the activity of a COX-2 inhibitor, as high as 41.12 ± 0.58% (IC50 = 59.9 μg mL−1). Therefore, it can be considered a viable substitute for mammalian collagen. It can be used as an ingredient in food, cosmetics, and other industries. The application of ASC-SA as a COX-2 inhibitor can serve as a nutraceutical and medicinal option for preventing and treating inflammatory diseases.
5. ABBREVIATIONS
ANOVA: Analysis of variances. ASC: Acid-soluble collagen. ASC-SA: Acid-soluble collagen of Siphonosoma australe. COX: Cyclooxygenase. COX-2: Cyclooxygenase-2. FTIR: Fourier transform infrared spectroscopy. IC50: Inhibition concentration 50%. LC-MS/MS: Liquid Chromatography–tandem mass spectrometry. MWCO: Molecular weight cut-off. NSAIDs: Non-steroidal anti-inflammatory drugs. pH: Potential of hydrogen. PSC: Pepsin-soluble collagen. SAIDs: Steroidal anti-inflammatory drugs. SDS: Sodium dodecyl sulfate. SDS-PAGE: Sodium dodecyl sulfate polyacrylamide gel electrophoresis. SPSS IBM: Statistical Product and Service Solutions International Business Machines Corporation.
6. ACKNOWLEDGMENTS
The authors express gratitude to the Center for Higher Education Funding and Indonesia Endowment Funds for Education under grant number 202101121326. The Ministry of Finance, The Republic of Indonesia, for providing a scholarship through the Beasiswa Pendidikan Indonesia (BPI) in the Food Science Doctoral Study Program, Universitas Gadjah Mada.
7. AUTHOR CONTRIBUTIONS
Concept and design: S, CH, TDW, RI; data acquisition and data analysis: S; drafting manuscript: S; critical revision of manuscript: CH, TDW, RI; statistical analysis: S; obtained funding: S; supervision: CH, TDW, RI; final approval of the article: all authors.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Rodríguez MIA, Barroso LGR, Sánchez ML. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17:20–6. [CrossRef]
2. Geahchan S, Baharlouei P, Rahman MA. Marine collagen: a promising biomaterial for wound healing, skin anti-aging, and bone regeneration. Mar Drugs. 2022;20:61. [CrossRef]
3. Li J, Xie X, Zhu C, Guo Y, Chen S. Edible peanut worm (Sipunculus nudus) in the Beibu Gulf: resource, aquaculture, ecological impact and counterplan. J Ocean Univ China. 2017;16:823–30. [CrossRef]
4. Sangtanoo P, Srimongkol P, Saisavoey T, Reamtong O, Karnchanatat A. Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculus nudus) in lipopolysaccharide-induced RAW264.7 macrophages. Food Funct. 2020;11:552–60. [CrossRef]
5. Gandhi J, Khera L, Gaur N, Paul C, Kaul R. Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front Microbiol. 2017;8:538. [CrossRef]
6. Rinayanti A, Dewanti E, Adelina M. Test of the anti-inflammatory effect of water fraction of mahkota dewa leaves (Phaleria macrocarpa (Shecff.) Boerl.) on white rats (Rattus norvegicus L.). Pharm Sci Res. 2016;1:78–85. [CrossRef]
7. Lin H, Zheng Z, Yuan J, Zhang C, Cao W, Qin X. Collagen peptides derived from Sipunculus nudus accelerate wound healing. Molecules. 2021;26:1385. [CrossRef]
8. Zhang CX, Dai ZR, Cai QX. Anti-inflammatory and anti-nociceptive activities of Sipunculus nudus L. extract. J Ethnopharmacol. 2011;3:1177–82. [CrossRef]
9. Chuaychan S, Benjakul S, Kishimura H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). LWT - Food Sci Technol. 2015;63:71–6. [CrossRef]
10. Ahmad M, Benjakul S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010;120:817–24. [CrossRef]
11. Su XR, Sun B, Li YY, Hu QH. Characterization of acid-soluble collagen from the coelomic wall of Sipunculida. Food Hydrocoll. 2009;23:2190–4. [CrossRef]
12. Liu D, Zhang X, Li T, Yang H, Zhang H, Regenstein JM, et al. Extraction and characterization of acid- and pepsin-soluble collagens from the scales, skins and swim-bladders of grass carp (Ctenopharyngodon idella). Food Biosci. 2015;9:68–74. [CrossRef]
13. Astiana I, Nurjanah N, Nurhayati T. Characterization of acid soluble collagen from redbelly yellowtail fusilier fish skin (Caesio cuning). J Pengolah Has Perikan Indones. 2016;19:79–93. [CrossRef]
14. Mulyani S, Hintono A, Adefatma NR, Pahlawan IF. Collagen extraction from buffalo skin using acetic acid. J Ris Ind. 2021;37:51–8. [CrossRef]
15. Pamungkas BF, Murdiati AS, Indrati R. Characterization of the acid- and pepsin-soluble collagens from haruan (Channa striatus) scales. Pak J Nutr. 2019;18:324–32. [CrossRef]
16. Wang B, Wang YM, Chi CF, Luo HY, Deng SG, Ma JY. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar Drugs. 2013;11:4641–61. [CrossRef]
17. Hardiyanti R. Optimization of extraction and characterization of skin collagen from sea bass (Lates calcarifer) [Internet]. Master’s thesis, IPB University; 2017. [cited 2023 Oct 10]. Available from: http://repository.ipb.ac.id/handle/123456789/87727
18. Li ZR, Wang B, Chi C, Zhang QH, Gong Y, Tang J, et al. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013;31:103–13. [CrossRef]
19. Puspitojati E, Cahyanto MN, Marsono Y, Indrati R. Production of angiotensin converting enzyme (ACE) inhibitory peptides during the fermentation of jack bean (Canavalia ensiformis) tempe. Pak J of Nutr. 2019;18:464–70. [CrossRef]
20. Puspitasari DAP, Bintoro VP, Setiani BE. The soaking effect on different hydrocloride acid level and soaking time on pH, swelling percentage and collagen yield of chicken shank bone. J Indones Trop Anim Agric. 2013;38:98–102. [CrossRef]
21. Carpio KCR, Bezerra RS, Cahú TB, Do Monte FTD, Neri RCA, da Silva JF, et al. Extraction and characterization of collagen from the skin of Amazonian freshwater fish Pirarucu. Braz J Med Biol Res. 2023;56:e12564. [CrossRef]
22. Wong DWS. Mechanism and theory in food chemistry. 2nd ed. New York (NY): Springer Cham; 2018. [CrossRef]
23. Chen X, Guo Z, Zhang J, Li Y, Duan R. A new method for determining the denaturation temperature of collagen. Food Chem. 2021;343:128393. [CrossRef]
24. Mberato SP, Rumengan IFM, Warouw V, Wullur S, Rumampuk NDT, Undap SL, et al. Determination of the molecular structure of the collagen scales of parrotfish (Scarus sp.) based on the molecular absorption of FTIR waves (Fourier-transform infrared spectroscopy) analysis. J Pesisir Laut Tropis. 2020;8:7–14. [CrossRef]
25. Riaz T, Zeeshan R, Zarif F, Ilyas K, Muhammad N, Safi SZ, et al. FTIR analysis of natural and synthetic collagen. Appl Spectrosc Rev. 2018;53:703–46. [CrossRef]
26. Zhang Q, Wang Q, Lv S, Lu J, Jiang S, Regenstein JM, et al. Comparison of collagen and gelatin extracted from the skins of Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus). Food Biosci. 2016;13:41–8. [CrossRef]
27. Afifah A, Suparno O, Haditjaroko L, Tarman K, Setiyono A, Nugraha AW. Isolation and characterization of collagen from salmon skin (Salmo salar) using acid method. Squalen. 2023;18:139–47. [CrossRef]
28. Safithri M, Tarman K, Suptijah P, Widowati N. Physicochemical characteristics of acid soluble collagen from dorab wolf-herring fish skin (Chirocentrus dorab). J Pengolah Has Perikan Indones. 2019;22:441–52. [CrossRef]
29. Nurhayati N, Tazwir T, Murniyati M. Extraction and characterization of acid-soluble collagen from tilapia skin (Oreochromis niloticus). J Pascapanen Bioteknol Kelaut Perikan. 2013;8:84–92. [CrossRef]
30. Liu W, Chen X, Li H, Zhang J, An J, Liu X, et al. Anti-inflammatory function of plant-derived bioactive peptides: a review. Foods. 2022;11:2361. [CrossRef]





