1. INTRODUCTION
Insects are one of the most abundant organisms having remarkable evolutionary eminence. This can be attributed to their immune mechanisms that exhibit potent defenses against the multitude of disease-causing pests and pathogens. The increasing significance of research on insect immunity underscores its pivotal role in contemporary scientific investigations. Numerous insect model organisms have been studied over time in different research projects to unveil the intricacies of the same. Among them, studies on silkworm immunity are quite extensive due to its economic importance and its genetic homogeneity to humans to some extent [1]. As such, an in-depth study of silkworm immune system and a better understanding of the various diseases and their causative organisms are in all probability quite pertinent for future explorations of alternatives against potential pathogens.
Among the various diseases occurring in silkworms, bacterial flacherie is quite common. Just like other breeds of silkworms, the muga silkworm, Antheraea assama Helfer, also has quite a high occurrence of flacherie [2]. This causes about 40% loss of muga crops each year. One of the major causative agents of flacherie was reported to be Bacillus thuringiensis, having high toxicity levels, causing septicemia, and eventually death of the affected worms [3,4]. Though muga silkworms are susceptible to the infection of B. thuringiensis, they are not totally vulnerable to them and also to other such potentially pathogenic microbes and are often reported to develop resistance against such pathogens [5]. It is believed to be so because the muga worms are reported to have a well-developed immune system that includes both innate and adaptive elements [6]. The innate immune system and its components, especially antimicrobial peptides (AMPs), play a major role since it is the primary line of defense against microbes [7]. The AMPs are low-molecular-weight proteins synthesized by the fat body and then liberated into the hemolymph of the silkworm upon infection or injury [6]. For the synthesis of AMPs, the free amino acids in the hemolymph are utilized on immunization or in response to injury [8]. The AMPs have various modes of action; however, the most prominent mechanism is breakdown of the cell membrane or cell wall of microorganisms [9].
B. thuringiensis, known for its significant role as a causative agent of flacherie, is believed to trigger immune responses in silkworms, leading to the synthesis of immune proteins or AMPs. This production is expected to involve the utilization of free amino acids from the hemolymph repertoire following injury or infection by microbes. Therefore, the primary objective of this study was to assess the total protein content, total free amino acids, and antimicrobial activity in the hemolymph of B. thuringiensis-immunized muga silkworm larvae against both B. thuringiensis and Escherichia coli. While various silkworm breeds have been extensively studied, the muga silkworm, native to the northeastern region of Assam, has not received as much attention in this context. The successful exploration of other breeds has led to the discovery of numerous AMPs. Consequently, investigations into the Muga silkworm may prove crucial and highly significant, offering valuable insights into enhancing the quality of muga silkworm breeds and potentially uncovering novel avenues for antibiotic discovery.
2. MATERIALS AND METHODS
2.1. Insects
Disease-free eggs of Antheraea assamensis were procured from CSB sericulture farm, Gandhinagar, Tetelia, Assam, and reared on the farm itself. The fifth-instar healthy larvae (2–3 days post molt) developing from such eggs were considered for collection of hemolymph. Larvae were reared on Som plants (Machilus bombycina) in CSB sericulture farm, Gandhinagar, Tetelia, in prevailing climatic conditions with high humidity and temperature ranging from 32 to 35°C.
2.2. Bacterial Species Selection
Two bacterial strains were used in this study. B. thuringiensis (MTCC 1953) and E. coli (MTCC 40) were procured from the Microbial Type Culture Centre (MTCC), Chandigarh, and culture was carried out as per MTCC instructions using nutrient broth and nutrient agar media followed by reculture of bacteria after every 15–20 days. Immunization of the larvae was carried out by B. thuringiensis. Antimicrobial activity was assessed against both B. thuringiensis and E. coli.
2.3. Determination of Lethal Concentration of B. thuringiensis
Lethal concentration (LC50) value was found via the method of [3] with slight modifications. Different concentrations of B. thuringiensis (MTCC 1953) were prepared ranging from 101 to 109 CFU/ml by serial dilution. Ten groups of silkworms, each consisting of 10 individuals per concentration, were included in the experiment, along with a control group. Each group was injected with 20 µl of different bacterial concentrations while the control group was injected with sterilized phosphate-buffered saline (PBS). The number of silkworms dying at various concentrations was recorded for 24 h. Based on the observed data, LC50 value was calculated for 24 h using Probit analysis in the Biostat 2009 software package [10].
2.4. Preparation of Control and Treated Groups
The fifth-instar larvae were inoculated with 20 µl of 105 CFU/m, that is, the one-tenth dose of the LC50 of B. thuringiensis, with the help of a sterile 1 ml disposable insulin syringe. The control group was injected with 20 µl of PBS and hemolymph was collected after 24 h. Samples of hemolymph were collected at various time intervals, namely, 6 h, 12 h, 18 h, and 24 h, following the induction of infection. Hemolymph were collected by incision of the last abdominal leg in previously chilled falcon tubes containing crystals of phenylthiourea and phenylmethane sulfonyl fluoride crystals to prevent oxidation as well as degradation of peptides. The hemolymph were mixed with the crystals and centrifuged at 3000×g (15 min at 4 °C) to remove any hemocytes, and supernatant with plasma was stored at −20 °C till further use.
2.5. Assessment of Antimicrobial Activity of Hemolymph Extracted at Different Hours
The cell-free hemolymph was assessed for probable antimicrobial activity by the well diffusion method by Haloi [11]. Two sterile Petri plates with nutrient agar medium were swabbed and spread with the test bacterial strains, that is, B. thuringiensis and E. coli. Five wells were formed in each plate with the help of a sterile 0.5 ml pipette tip, where 20 μl of sample hemolymph taken from four different time intervals (6 h, 12 h, 18 h, and 24 h) and PBS injected hemolymph collected after 24 h (control) were placed on the medium. In each plate, one well was kept as control by adding 20 μl of 1× PBS only. The plates were incubated overnight at 37 °C in an incubator. After 24 h, the diameter of the clear zone of inhibition was measured and documented by photography.
2.6. Comparison of Total Protein Content of Both Normal and Infected Hemolymph Samples of Larvae
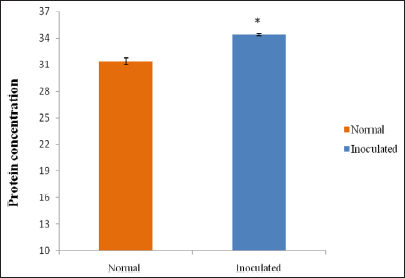
The sample showing the highest antimicrobial activity was then analyzed for total protein content following the method of Lowry et al. [12] and compared with a control sample. The intensity of the color was measured photometrically using a spectrophotometer at 670 nm.
2.7. Total Free Amino Acid Analysis
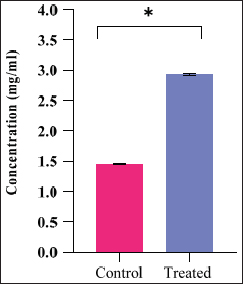
Free amino acid content was analyzed using the Ninhydrin method as per the protocol of Moore and Stein [13] using leucine as standard. Analysis was carried out with a spectrophotometer at 570 nm. The values were expressed in milligram of leucine per milliliter of hemolymph.
2.8. Protein Profile Study and Comparison of Hemolymph Samples
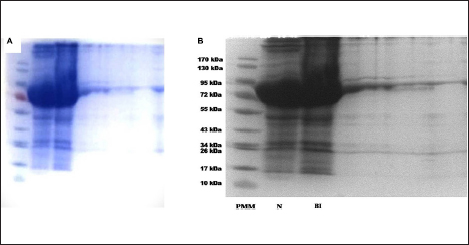
The protein profiles of normal and hemolymph sample having maximum antimicrobial activity were analyzed by 12% SDS-PAGE according to Laemmli [14] using electrophoresis apparatus from Bio-Rad and SDS-PAGE chemicals from GeNei. Control and immunized hemolymph samples were loaded into the wells of the gel along with a standard protein molecular marker (PMM), and the gel was run at a constant voltage of 100 V for 120 min. Once electrophoresis was complete, the gel was removed from the apparatus and stained overnight in a staining box with 0.2% Coomassie Brilliant Blue R-250 and destained in methanol and acetic acid in the ratio 2:1 [15]. The protein bands in the gel so obtained were then observed under a gel documentation system (UVITECH, TECHNE).
2.9. Statistical Analysis
Statistical analysis was performed using MS Office 2017 and SPSS 21. For protein content and free amino acid estimation, t-test was performed to determine the significance between the two groups using the same software (p<0.05). The results are expressed as mean ± standard deviation (X± SD). The data for inhibition zone measurement were subjected to ANOVA for the mean comparison using IBM SPSS 25.0 version. Differences between means were considered significant at p < 0.05.
3. RESULTS
3.1. Determination of Lethal Concentration of B. thuringiensis
In this study, the mean mortality values of muga silkworm were calculated for B. thuringiensis bacterial strains at 24 h. The lethal concentration was found to be 5.7 × 106 CFU/ml [Figure 1]. As such, for the later experiments, one-tenth of the concentration of LC50 was used, that is, about 105 CFU/ml. Regression plot was the same.
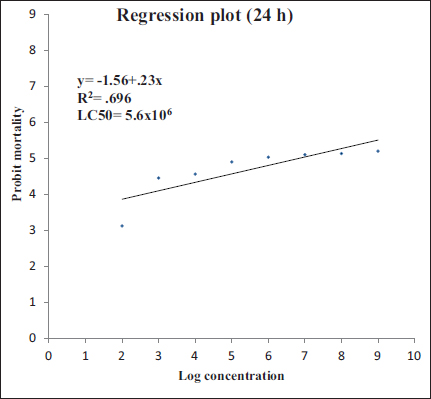 | Figure 1: Regression plot of probit mortality and log concentration for B. thuringiensis to A. assamensis fifth-instar larvae for 24 h.
[Click here to view] |
3.2. Assessment of Antimicrobial Activity of Hemolymph Extracted at Different Hours
Hemolymph samples were collected at different times after immunization with bacteria, that is, 6 h, 12 h, 18 h, and 24 h, and their antimicrobial activity was assessed against two test bacteria, B. thuringiensis and Escherichia coli, respectively. It was found that in both analyses the hemolymph extracted at 24 h showed clear inhibition zones against both bacteria while the hemolymph extracted at 6 h, 12 h, and 18 h failed to show any response [Figure 2]. It was also seen that the diameter of the inhibition zone developed against E. coli was 1.67 cm, which is larger than that against B. thuringiensis where the inhibition zone was 1.24 cm, suggesting greater antimicrobial activity against E. coli than B. thuringiensis. However, at p>0.05, these values were shown to be significantly different from each other [Figure 3].
 | Figure 2: Antimicrobial activity of fifth-instar muga larva hemolymph samples extracted at different time intervals after bacteria inoculation, that is, 6 h, 12 h, 18 h, and 24 h against (A) B. thuringiensis and (B) E. coli. PBS is taken as negative control.
[Click here to view] |
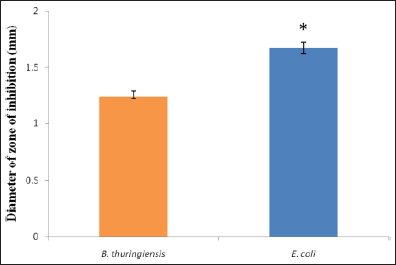 | Figure 3: Comparison of antimicrobial activity of induced hemolymph procured at 24 h after immunization against B. thuringiensis and E. coli. Hemolymph extracted at 6 h, 12 h, and 18 h were not considered as they did not show any antimicrobial activity. * indicates significant differences between groups (p>0.05); n = 3.
[Click here to view] |
4. DISCUSSION
Proteins associated with defense, in case of lower organisms like insects, serves as the first component and a key contributing factor in humoral immune response on invasion by microbes [16]. Each species is well equipped with their own set of specific AMPs, which are produced in response to different pathogens [17,18]. Our study basically compared and analyzed the protein profiles of noninduced hemolymph and induced hemolymph of muga silkworm larvae for the presence of proteins with potential antimicrobial activity against Gram-positive bacteria B. thuringiensis and Gram-negative bacteria E. coli.
In almost all the studies conducted on the AMPs of insects or immunity as a whole, it was seen that the immune system of the insect was activated or induced by immunizing with different doses of either bacteria, viruses, fungi, or other microorganisms. An explanation for this might be that the peptides produced as a result of induction are not absent but might be present below the detectable ranges under normal conditions. Only on exposure to a pathogen or on injury are the peptides transcribed rapidly, leading to their detection after a certain time post exposure [6].
Immunization in our study was carried out by B. thuringiensis into the abdominal cavity of muga silkworm larvae. It has been reported to cause flacherie in silkworms and is a very potent disease-causing bacteria having multiple toxic effects as it can cause sepsis in the midgut, leading to the penetration of the bacterial strains into the hemocoel that can cause death [3]. The lethal concentration of B. thuringiensis in this study was found to be 5.7 × 106 CFU/ml after 24 h, which is similar to that observed by Haloi [3] in muga silkworm with about 4.78 × 106 CFU/ml for 24 h as well. However, it was found to be much less in another study conducted against B. mori, with about 3.3 × 104 CFU/ml [19]. This might be due to the involvement of different strains of B. thuringiensis and host specificity.
Once defense peptides are synthesized, they are rapidly released into the hemolymph [5]. Hemolymph samples were then collected at different time intervals, that is, 6 h, 12 h, 18 h, and 24 h after inducing infection since AMPs and other immune-related proteins have been reported to be first expressed at about 6 h after infection [20]. In our study, it was found that hemolymph extracted in 6 h, 12 h, and 18 h did not show any activity against both B. thuringiensis and E. coli. However, hemolymph extracted at 24 h showed very potent antimicrobial activity against both the test bacterial strains. This might be due to the intensity of transcription of AMPs peaking from 18 to 24 h, after which transcription decreases as such major production of AMPs might have occurred in the later hours after immunization. As in the hours just after immunization, the process of production of defense peptides might be slow and reaction against pathogens might not have fully developed as such. This theory was confirmed by Meister et al. [20].
In this study, the quantitative protein analysis of muga silkworm hemolymph showed an increase in protein concentration in the B. thuringiensis immunized larvae compared to the control group. Similar findings were recorded by Sharma et al. [21] in non-mulberry silkworm after inoculation of bacteria. The Eri silkworm Philosamia ricini also showed similar kinds of results when injected with E. coli and M. luteus bacteria [22]. Adamo [23] suggested that the bacteria-injected hemolymph might contain higher concentrations of proteins due to the presence of some inducible antibacterial protein synthesized by silkworm to defend themselves or as immune response against the injected bacteria.
Quantitative changes in free amino acids revealed changes in the hemolymph of control and bacterial-challenged muga silkworm larvae in the present studies. It was found to be higher than that of the normal larvae. Salama et al. [24,25] also reported changes in amino acid studies in Spodoptera littoralis hemolymph. Studies on total amino acid in B. thuringiensis-treated Plutella maculipennis by Narayanan [26] also reported similar observations like that of our study. Likewise, Reddy et al. [27] reported increases in free amino acids in thyroxine-treated larvae of Antheraea mylitta (tassar silkworm). It was suggested that amino acids contribute to the general pool for the production of new proteins among various other functions, and as such introduction of bacteria into the body of the insects might lead to the rapid production as well as utilization of the amino acid to produce defense proteins, which is reflected in our results. This theory is in accordance with Pant and Agrawal [28]. Similarly, Lazar and Mohamed [29] suggested that variations in the total amino acid pool change with corresponding changes in total protein content.
SDS analysis of the protein profiles of normal and bacteria-immunized muga silkworm showed that the bacteria-injected groups had new bands, which were completely absent in the normal groups. Similar results were derived from studies with Bombyx mori and Maduca sexta, where the hemolymph revealed the occurrence of a variety of new proteins in response to injury or microbe inoculation [30-32]. Ganjendra et al. [32] reported that such proteins are produced in the fat body and are released into the hemolymph of A. mylitta larvae too after bacterial introduction. It can be assumed that the newly observed proteins represented by the newer bands might be a response to bacterial stress as observed earlier in terms of an increase in free amino acids [33,34]. However, further proteomic analysis will be necessary to prove it conclusively.
REFERENCES
1. Meng X, Zhu F, Chen K. Silkworm: a promising model organism in life science. J insect Sci. 2017;17(5):97. [CrossRef]
2. Choudhury SN. Diseases, pests and parasites. In: Choudhury SN, editor. Muga Silk Industry, Directorate of Sericulture and Weaving. Govt. of Assam, Guwahati, India, 1981;74–81.
3. Haloi K, Kalita M, Nath R, Devi D. Characterization and pathogenicity assessment of gut-associated microbes of muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae). J Invertebr Pathol. 2016;138:73–85. [CrossRef]
4. Saad MSI, Elyamani EMY, Helaly WMM. Controlling of bacterial and fungal diseases that contaminating mulberry silkworm, Bombyx mori by using some plant extracts. Bull Natl Res Cent. 2019;43:172. [CrossRef]
5. Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect Immunity. Ann Rev Ento. 1997;42:611–43. [CrossRef]
6. Bulet P, Hetru C, Dimarcq JL, Hoffmann DL E. Antimicrobial peptides in insects; structure and function. Dev Comp Immun. 1999;23:329–44. [CrossRef]
7. Zhang W, Tettamanti G, Bassal T, Heryanto C, Eleftherianos I, Mohamed A. Regulators and signalling in insect antimicrobial innate immunity: functional molecules and cellular pathways. Cell Signal. 2021;83:110003. [CrossRef]
8. Mahmoud SM, Akila ME, Moustafa AA, El-Banna AA, Moustafa MN. Enzymatic activity of the silkworm, Bombyx mori L. hemolymph reared on different mulberry varieties. Egypt J Agricult Res. 2013;91:1407–13.
9. Haloi K., Kalita M.K., Nath R., Devi D. Characterization and pathogenicity assessment of gut-associated microbes of muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae), J Invertebrate Pathol. 2016;138:73–85. [CrossRef]
10. Finney DJ. Profit analysis. Cambridge: Cambridge University Press; 1980. p. 333.
11. Haloi K. Immune response of muga silkworm Antheraea assamensis Helfer with special reference to flacherie disease. 2017. Available from: http://hdl.handle.net/10603/223378
12. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
13. Moore S, Stein WH. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1968;211:907–13.
14. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
15. Delkash-Roudsari S, Zibaee A, Bigham Z. Purification and characterization of a phenoloxidase in the hemocytes of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae): effects of insect growth regulators and endogenous inhibitors. J Enzyme Inhib Med Chem. 2015;30(4):569–74. [CrossRef]
16. Brey PT, Hultmark D. Molecular mechanisms of immune responses in insects. London: Chapman & Hall; 1998.
17. Mak P, Zdybicka-Barabas A, Cytrynska M. A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev Comp Immunol. 2010;34:1129–36. [CrossRef]
18. Kaito C, Usui, K, Kyuma T, Sekimizu K. Isolation of mammalian pathogenic bacteria using silkworms. Drug Discov Ther. 2011;5:66–70. [CrossRef]
19. Hoffman JA. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. [CrossRef]
20. Meister M, Hetru C, Hoffmann JA. The antimicrobial host defense of Drosophila. In: Du Pasquier L, Litman GW, editors. Origin and Evolution of the Vertebrate Immune System. Curr. Top. Microbiol. Immunol., vol. 248. Berlin: Springer; 2000. [CrossRef]
21. Sharma J, Archana Y, Unni BG, Kalita MC. Antibacterial proteins from non-mulberry silkworms against flacherie causing Pseudomonas aeruginosa AC-3. Curr Sci. 2005;89(9):1613–8.
22. Ravindar G, Ragamalika G, Nagaraja Rao P. Analysis of proteins profile and antibacterial activity in haemolymph of Eri silkworm, Samia cynthia ricini after bacterial inoculation. Int J Adv Res. 2015;3(1):186–92.
23. Adamo SA. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J Insect Phys. 2004;50:209–16. [CrossRef]
24. Salama HS, El-Moursy A, Aboul-Ela R, Razek AA. Potency of different varieties of Bacillus thuringiensis (Berliner) against some lepidopterous stored product pests. J Appl Ento. 1991;112:19–26.
25. Salama HS, Ragaei M, Sabbour M. Biochemistry of the haemolymph of Phthorimaea operculella larvae treated with Bacillus thuringiensis. J Islamic Acad of Sci. 1994;7:163–6.
26. Narayanan KS, Jayaraj S, Subramanian T. Sensitivity of Bacillus thuringiensis Berliner to two antibiotics and sulphanilamide. Hind Anti biot Bull. 1973;15:14–5.
27. Reddy KD, Chaudhuri A, Thangavelu K. L-thyroxine (T4) elevates the free amino acid pool of haemolymph plasma of tasar silkworm, Antheraea mylitta drury (Lepidoptera: Saturniidae). Horm Metab Res. 1994;26(12):570–3. [CrossRef]
28. Pant R, Agrawal HC. Free amino acids of the hemolymph of some insects. J Insect Physiol. 1964;10:443–6.
29. Lazar KV, Mohamed UVK. The high titre of free amino acids in the larval haemolymph of the moth, Spodoptera mauritia boisduval. Ins Biochem. 1988;18:331–5.
30. Abraham EG, Nagaraju J, Salunke D, Gupta HM, Datta, R.K. Purification and partial characterization of an induced antibacterial protein in the silkworm, Bombyx mori. J Invert Pathol. 1995;65:17–24. [CrossRef]
31. Dickinson L, Russell V, Dunn PE. A family of bacteria-regulated, cecropin D-like peptides from Manduca sexta. J Biol Chem. 1988;263:19424–9. [CrossRef]
32. Ganjendra, PS, Kumar SA, Roy DK, Alok S, Kallahally N, Madhusudhan P, Kiran K. Cellular and biochemical changes of Antheraea mylitta D, on immuniztion with attenuated Antheraea mylitta cytoplasmic polyhedrosis virus. Int J Zoo Res. 2011;7(3):263–71. [CrossRef]
33. Hughes JA, Hurlbert RE, Rupp, RA, Spence KD, Bacteria-induced haemolymph proteins of Manduca sexta pupae and larvae. J Insect Physiol. 1983;29:625–32.
34. Hulbert LJ, Gruenewald GVT. Textural and compositional features of chromite in the lower and critical zones of the Bushveld complex south of Potgietersrus. Econ Geol. 1985;80(4):872–95. [CrossRef]