1. INTRODUCTION
Foods that are metastable are susceptible to spoilage during handling and storage. The spoilage may be due to microbes, enzymes, oxidation, and other environmental factors. Thus, packaging is a crucial step in food processing as it protects the food from undesirable contaminants. The paramount goal of packaging is to protect, contain, and communicate the contents of the package among three different environments: physical, human, and atmosphere [1]. In the food industry, packaging plays a vital role in maintaining the product’s quality, quantity, and hygiene. It protects from external elements such as light, moisture, and oxygen, thus preserving the food’s aroma and flavor [2]. Food packaging is important for preserving and delivering food safely. It can also help with marketing and traceability. Packaging materials come in rigid and flexible options, and the choice depends on various factors [3]. The characteristics of ideal packaging materials should be mass-produced, cost-effective, user-friendly, efficient, and suitable for their intended purpose and disposal. While rigid containers such as glass bottles and cans provide physical protection, flexible packaging options such as plastic sheets and foils can be easily shaped, heat-sealed, and printed.
Different packaging materials have varying levels of barriers against permeability. Paper and paperboards are biodegradable, printable, and have better mechanical properties, but they cannot be heat-sealed. Waxy and polymeric coatings can enhance their barrier properties. However, packaging materials can migrate into food, affecting its organoleptic properties. As the packaging industry revolves around plastic and its derivatives, about 26,000 tons of plastic are generated in India, of which 10,000 tons go for recycling [4]. Petrochemical polymers harm the environment as they are non-biodegradable and contaminate water bodies. This has raised global concern, with customers increasingly demanding eco-friendly packaging solutions. The food industry has responded with innovations such as biodegradable plastics and edible packaging to meet the demand for shelf-stable processed food [5].
Edible packaging is a type of “green packaging” that is made from sustainable sources. It is highly degradable and can be consumed by animals or humans without any risk. It includes coatings, films, and pouches, which are eco-friendly and safer for consumers. However, the sensory characteristics of the packaging must be compatible with those of the foods. Edible packaging is a potential alternative to conventional packaging due to its superior properties. It reduces source materials and environmental pollution [6]. They effectively reduce respiration and moisture loss in food products. The addition of certain additives can enhance these protective properties. Edible packaging serves as an efficient substitute for synthetic materials, further minimizing respiration and moisture loss in foods. This innovation extends shelf-life, maintains quality and sensory attributes, acts as a shield against contamination and mass transfer incidents, and incorporates active substances that enhance the food’s physical and sensory characteristics [7]. Edible packaging represents a significant advancement with far-reaching implications for the food industry. The thickness of edible packaging affects its sensory properties and the food inside. Thinner films are preferred for better taste and appearance.
Tensile strength and elongation at break are pivotal parameters related to a film’s mechanical properties and chemical structure. The mechanical property reflects that the edible packaging is effective in puncture resistance, bursting strength, stiffness, and tear resistance. Regulating the permeability of biomaterials is crucial for managing the environment around food. It is essential to reduce water vapor transmission in food packaging to prevent its impact on the food. The use of opaque packaging and materials that absorb UV–visible light is vital to preserve food quality by inhibiting oxidation and spoilage due to light exposure [8].
Advantages:
• Edibleness and biodegradability: Edible packaging can be consumed or discarded harmlessly due to its biodegradability.
• Health-enhancing nutrients: The composition is often incorporated with health-beneficial vitamins and plant extract, thus having a positive effect on the consumer’s health.
• Active substance carriers: These films have applications in many industries such as food, pharmaceuticals, nutraceuticals, and agrochemicals. They come in various forms such as capsules, microcapsules, soluble strips, flexible pouches, and coatings.
• Individual packaging: Unlike conventional packaging, edible packaging paves the way for small-sized food such as peas, beans, nuts, and similar items.
• Decreased usage of conventional packages reduces wasted generation and improves environmental impact [8].
Disadvantages:
• Thick Coating: Coating can affect flavor if it is too dense. Packaging efficiency is reduced with incorrect preparation.
• Nature of edible packaging: Edible packaging must be carefully selected to avoid hygroscopicity and microbial growth. Antimicrobial agents may be required.
• High Cost: The analysis of the properties of edible packaging on physio-mechanical properties could be complex in financing the development.
• Allergic reactions: Some ingredients in edible films and coatings, such as beeswax, may trigger individuals to become allergic to those ingredients.
• Insufficient intel: Insufficient data on edible film and coating machinery, compositions, and application techniques [9].
Despite many advantages in producing edible films and maintaining food quality during storage, their tensile and permeability characteristics are inferior to those of synthetic films. However, edible packaging cannot replace conventional packaging completely but can increase efficiency without compromising its quality. Hence, edible packaging is a promising eco-friendly alternative for packaging and coating food. This review covers the biopolymers, film-forming processes, and coating methods involved, as well as recent advances, applications, and future prospects.
2. EDIBLE FILMS
Edible packaging films use biopolymers such as hydrocolloids, lipids, and composites. Polysaccharide and protein-based hydrocolloid films have better oxygen, carbon dioxide, and lipid barriers than water vapor. Lipid films made of wax, triglycerides, acylglycerols, and fatty acids block moisture. These biopolymers exhibit low tensile strength and different material properties than usual plastics. Sometimes, the integration of additives such as plasticizers, antimicrobials, antioxidants, essential oils, nanoparticles, and others into packaging materials aids in the modification and advancement of its functionality and properties. Biopolymer packaging is a safe and reliable way to maintain food quality. The ingredients used are natural, renewable, and environmentally friendly, making them palatable, biodegradable, biocompatible, and antimicrobial, which poses no health concerns. Blending two distinct polymers can create superior films and coatings [10].
2.1. Biomaterials and Biopolymers Used for Edible Packaging
Biodegradable materials and biopolymers are different. Biodegradable material decomposes into simple compounds by microorganisms. Biopolymers are naturally derived from biodegradable polymer materials. Biopolymers are biodegradable, but biodegradable materials are not always biopolymers. The biopolymer production according to their renewable source:
1. Direct extraction from biomass (polysaccharides and proteins)
2. Chemical synthesis from bioderived monomers (PLA)
3. Production by microorganisms (pullulan, PHA, PHS, etc.)
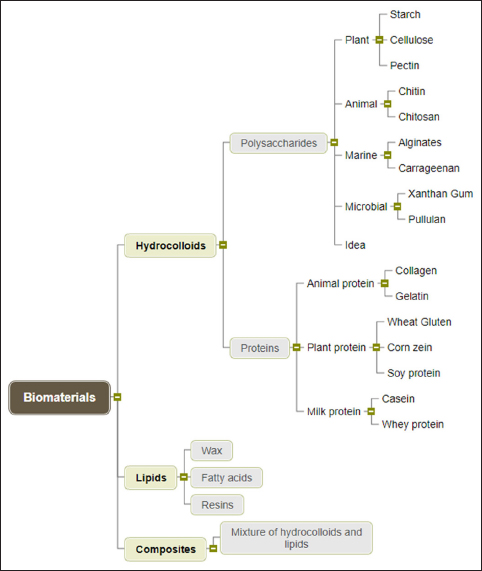
The biomaterials used in the preparation of edible films and coatings include polysaccharides (starch, cellulose, chitin, chitosan, pectin, alginates, gum arabic, xanthan gum, etc.), proteins (gelatin, casein, whey protein, wheat gluten, wheat, corn, and soy protein), lipids (waxes, acetylated triglycerides), and composites [Figure 1]. These materials are safe for human consumption, biodegradable, nontoxic, transport active compounds, and result in zero waste [11].
 | Figure 1: Schematic overview of biodegradable materials for polymer formation. [Click here to view] |
2.1.1. Polysaccharides
Edible films are made of naturally derived polysaccharides from plants, animals, marine, and microbial sources. They are tasteless, colorless, nontoxic, and selectively permeable to gases. Polysaccharides such as cellulose, pectin, and alginate offer a rigid structure and good barrier properties against gases [12]. Adding water-resistant particles and hydrophobic lipids enhances their low-barrier properties against water vapor. These materials have good mechanical properties such as tensile strength and percentage of elongation, making them ideal for formulating edible films and coatings. The properties and applications of polysaccharide-based films are presented in Table 1.
Table 1: Properties of polysaccharide membranes and its food application.
| Polysaccharide | Source | Composition | Membrane Properties | Drawbacks | Remedies | Food Applications |
|---|---|---|---|---|---|---|
| Starch | Plants - cereals, grains, tubers, and nuts | Glucose | • Biodegradable • Brittle upon retrogradation • Transparent • Odorless and tasteless • High elongation • Tensile strength • Low oxygen permeability | • Highly hydrophilic • Poor mechanical strength • Less flexible | • Addition of plasticizers (glycerol, glycol, and sorbitol) • Polymer composites/blend • Chemical modification of starch | • Flexible and rigid packaging for fresh fruits and vegetables |
| Cellulose | Cell wall of plants—wood, cotton fibers, and sugarcane bagasse | Glucose | • Biodegradable • High mechanical strength • Transparent • Resistance to fats and oils • Low-density • Good film-forming capacity | • Highly sensitive to water • Rigid structure • Poor thermoplastic behavior | • Chemical modification • Use of plasticizers • Blending of polymers | • Cellophane membranes |
| Pectin | Plant components—apple pomace, citrus fruits, fruit waste, and by-products | β-D-galactopyranuronic acid | • Excellent mechanical property • Prevents ingression of oil, moisture, and gas • Prevents oxidation | • Poor barrier against water vapor • Low elongation and brittle | • Use of plasticizers | • Fresh and minimally processed fruit products |
| Chitin | Animal polysaccharide—crustaceans, insects, and fungi | N-acetylgluco-samine | • Biodegradable • Highly transparent films • Biocompatible • Nontoxic • Antimicrobial activity against bacteria and fungi | • Low oxygen permeability • Highly sensitive to moisture | • Blending with other natural or synthetic polymers | • Edible film packaging • Extruded bags • Capsules of coffee powder |
| Chitosan | Deacetylated derivative of chitin | D-glucosamine N-acetyl-D-glucosamine | • Biodegradable • Antifungal and antibacterial properties • Good mechanical properties • Barrier to gases • Biocompatible • Nontoxic | • Brittle • Highly permeable to water vapor • Low oxygen permeability | • Use of plasticizers • Incorporation of nanofillers, natural plant extracts, and essential oils (bioactive agents) | • Edible films and coatings for fruits and vegetables (strawberries, cherries, mango, guava, etc.) |
| Alginate | Marine brown algae (Phaeophyceae) | Mannuronic and glucuronic acid | • Biodegradable • Tasteless, odorless, and glossiness • Reduce shrinkage and moisture loss • Highly permeable to water vapor • Mechanical strength and flexibility • Cross-link with calcium • Enhance sensory characteristics • Retards microbial growth • Prevents off-flavor development due to oxidation | • Poor resistance to moisture • Fragile membranes | • Blending of polymers | • Biodegradable coatings • Fresh-cut fruits • Meat products • Coated foods can be made microwaveable |
| Carrageenan | Red seaweed (Rhodophyceae) | Galactose | • Biodegradable • Excellent mechanical properties • Encapsulation of flavor and aroma compounds • Reduce moisture loss • Prevent discoloration | • Fragile and ductile behavior | • Usually blended with other polymers | • Coating material for fruits, fish, meat, and poultry • Sausage casing • Dry solid foods |
| Xanthan gum | Xanthomonas campestris | Glucose Mannose Glucuronic acid Acetate pyruvate | • Biodegradable • Pseudo-plastic (shear-thinning) behavior • Reduce weight loss, moisture migration, and respiration process • Prevent discoloration • Increase shelf-life • Nontoxic | • High cost of production | • Used as an additive (suspending and thickening agent) | • Edible coating of fresh-cut fruits and meat • Act as a carrier of bioactive materials |
| Pullulan | Aureobasidium pullulans | Maltotriose | • Biodegradable and edible • Transparent • Resistant to oil and grease • Highly impermeable to oxygen • Good adhesion • Heat sealable • High water solubility and nontoxic • Odorless and tasteless • Promotes antimicrobial activity | • Hydrophilic • Brittle • Breakability • High cost | • Blending with other biopolymers, active agents, and additives | • Coating material Wrapping material • Inner package—Seasoning bag of instant noodles and instant coffee |
2.1.1.1. Plant polysaccharides
Starch
Starch is a storage polysaccharide found in plants, including cereals, grains, tubers, and nuts. It consists of amylose and amylopectin polysaccharides and can form films and coatings with low oxygen permeability. However, its high hydrophilic nature limits its suitability as a packaging material. Adding plasticizers such as sorbitol, glycol, and glycerol improves its flexibility, chain mobility, and water vapor barrier properties. But too much plasticizer can cause adverse effects. Glycerol is a common plasticizer used to impart flexibility and suppress retrogradation in starch-based edible films. The concentration of plasticizers plays an important role, and careful optimization is required to ensure desired polymer properties without triggering adverse effects [13].
Cellulose
Cellulose is a natural polymer found in plant cells and is abundant on Earth. It can be derived from various sources, including wood, cotton, and sugarcane bagasse. Bacterial cellulose, synthesized by aerobic bacteria, has gained attention due to its unique properties such as high mechanical strength, remarkable water-holding capacity, and heightened crystallinity [14]. Cellulose biosynthesis extends beyond higher plants, encompassing microorganisms such as bacteria, algae, and tunicates. Bacterial cellulose, particularly notable for its purity compared to plant cell walls, is a prime example of the promising alternatives within cellulose materials.
Pectin
Pectin is a heteropolysaccharide and is a significant source of dietary fiber. It is the main plant component that provides flexibility as an edible packaging material. Apple pomace and citrus fruits are commercial sources for pectin extraction. It also includes other fruits and their by-products, such as mango peel, banana peel, sunflower head, and soybean hull. Pectin and its derivatives are used in the preparation of edible packaging as it has an excellent mechanical property that prevents the ingression of moisture, oil, and gases, and oxidation of food. It can also be used along with plasticizers to enhance the barrier properties against water vapor in fresh and minimally processed fruit products [15].
Gum Arabic
Gum arabic (acacia gum) is a complex, branched heteropolysaccharide extracted from the sap of Acacia senegal and Acacia seyal trees. It has a wide range of properties and functionalities due to its intricate structure, which includes a mix of arabinogalactan oligosaccharides, polysaccharides, and glycoproteins. The unique structure of gum arabic limits its capacity to form micelles and hydrogen bonds in a solution, thereby restricting the effective immobilization of water despite its solubility and ability to form hydrogen bonds with water. It is also a versatile material with biocompatibility, renewability, nontoxicity, pH stability, cost-effectiveness, high solubility, and gelling properties. It is used in edible film production but faces challenges due to suboptimal mechanical properties, pronounced hydrophilicity, and limited barrier characteristics. Gum arabic is also used for wall formation in the production of active particles using spray drying techniques, particularly for encapsulating flavoring agents [16].
2.1.1.2. Animal polysaccharides
Chitin and Chitosan
Chitin is a natural polysaccharide synthesized by living organisms and found abundantly in the universe next to cellulose. It is identical to cellulose and obtained from crustaceans, insects, and fungi, whereas chitosan is the product of chitin after deacetylation. It is composed of N-acetyl-D-glucosamine produced commercially from prawn and crab wastes by the chemical extraction process. Both chitin and chitosan are highly basic polysaccharides having antimicrobial activity and are also consumable, thus used as a film or coating in edible packaging for food preservation. The chitosan polymer acts as a semipermeable membrane to gases with low and moderate permeability to oxygen and water vapor, respectively. Recently, nanofillers and bioactive agents such as natural plant extracts or essential oils were incorporated into chitosan polymer to strengthen its antimicrobial and antioxidant properties. Also, blending with other natural or synthetic polymers improves the mechanical and barrier properties of chitosan [17].
2.1.1.3. Marine polysaccharides
Alginates
Alginates are biopolymers extracted generally from marine brown algae (Phaeophyceae) or produced from certain bacteria (Pseudomonas and Azotobacter). It is isolated as salts of alginic acid (mannuronic and glucuronic acid) and is generally recognized as safe (GRAS). Alginates provide mechanical strength and flexibility during film formation, and they help to reduce shrinkage and enhance the sensory characteristics of the food product. Alginates used in the edible coating can retard microbial growth and off-flavor development due to oxidation. It is widely used in the edible coating of food products and possesses good mechanical properties such as tensile strength, flexibility, resistance to tear and oil, tastelessness, odorless, and glossiness. They do, however, have a high permeability to water and oxygen due to their porous nature [18].
Carrageenan
Carrageenan, a natural polysaccharide obtained from red seaweeds of the Rhodophyceae family, is hydrophilic in nature. Carrageenan is composed of sulfated galactose units in α-D-1,3 and β-D-1,4 forms and is classified into various fractions (λ, κ, ι, ε, and µ) based on its solubility in potassium chloride. Among these, the k-carrageenan form membranes with excellent mechanical properties. It acts as an emulsifier and stabilizers in food and is a food-grade additive. It also finds its application in edible films and coatings and is reported to encapsulate flavor compounds, reduce moisture loss, decrease gas exchange, and prevent discoloration in foods [19].
2.1.1.4. Microbial polysaccharides
Microbial gums are a group of polysaccharides derived from microbes such as bacteria and fungi. They are water-soluble and form viscous sugar solutions of glucose, fructose, and mannose at low concentrations. They are generally recognized as safe (GRAS) by FDA for their use in the food industry as gelling, stabilizing, viscous, and thickening agents. In addition, microbial gums such as xanthan, gellan, pullulan, bacterial cellulose, and bacterial alginates form films and coatings with adequate moisture and gas barrier properties by extrusion process [20].
Xanthan Gum
Xanthan gum is a heteropolysaccharide synthesized by the fermentation of Xanthomonas campestris culture. Xanthan gum readily solubilizes in aqueous media and imparts high viscosity at low concentrations that are stable at varying pH and temperature conditions. The pseudo-plastic (shear-thinning) rheological behavior of xanthan gum is responsible for the film formation [21].
Pullulan
Pullulan is a neutral exopolysaccharide made up of maltotriose units linked by α-1,6 glycosidic linkage. It is produced commercially from Aureobasidium pullulans, a yeast-like fungus. Pullulan is a water-soluble, nontoxic, tasteless, and odorless biopolymer with the potential to form thin, transparent, flexible, printable, and heat-sealable edible films and coatings. These films are highly impermeable to oil and oxygen, exhibit good adhesive properties, and inhibit fungal growth for antimicrobial activity. However, its hydrophilicity, brittleness, breakability, and high cost limit its application in food packaging. To overcome these limitations, pullulan can be blended with other biopolymers, active agents, and additives to produce multifunctional packaging materials with improved barrier and mechanical properties [22].
2.1.2. Proteins
Protein-based biopolymers are great for creating edible films due to their excellent gas barrier properties and mechanical strength. Proteins consist of amino acids and usually exist as fibrous and globular proteins. Multilevel protein structures offer potential raw materials for edible film formation. However, protein films are brittle and delicate due to the higher atomization energy per unit volume of the proteins. To improve the physical and functional properties of the film, reinforcing materials and active compounds can be added. Protein-based edible film coats individual food particles such as beans and nuts. It is also used at the interface between different layers of heterogeneous food to prevent the migration of food components [23]. Collagen and globular proteins such as gluten, zein, soy, and milk protein are excellent biomaterials for edible film production. The properties and applications of protein-based films are presented in Table 2.
Table 2: Properties of protein membranes and its food application.
| Protein | Source | Composition | Membrane Properties | Drawbacks | Remedies | Food Applications |
|---|---|---|---|---|---|---|
| Collagen Gelatin | Fibrous and structural protein part of the animal tissues—bones, skin, and connective tissue Thermal denaturalization of collagen | Amino acid with glycine, proline, and high hydroxyproline content | • Biodegradable • Biocompatible • Transparent • Thermo-reversible • Oxygen impermeable • Nontoxic • High tensile strength • High extensibility | • Low barrier against water vapor | • Modification of films | • Meat and meat products |
| Total milk protein | Milk | Casein, whey protein, & lactose | • Biodegradable • Sufficient mechanical strength • Control of mass migration | • Crystallization of lactose during drying results in the nonuniform film | • Removal of lactose | • Edible films |
| Casein | Milk | α, β, κ casein | • Biodegradable • Thermally stable • Nontoxic • Highly nutritive • Transparent and flexible • Low oxygen permeability • Resistance to thermal denaturation/coagulation | • Highly hydrophilic • Poor barrier against moisture • Films shrink and become brittle upon drying | • Addition of plasticizers, lipids, gelatin, and pectin • Increasing the cohesion between the protein polypeptide chain | • Milk products • Cheese |
| Whey protein | Milk | α-Lactalbumin, β-Lactoglobulin, bovine serum albumin (BSA), immunoglobulins (Ig), and proteose-peptones | • Biodegradable • Excellent barrier against oil and gases • Nutritive and nontoxic • Transparent and bland | • Rigid structure • Highly hydrophilic • Poor barrier against moisture • Poor mechanical properties | • Addition of plasticizers • Physical and chemical methods | • Cheese • Meat and meat cuts |
| Gluten | Wheat | Gliadin and glutenin | • Biodegradable • Strong and transparent • Glossy surface • Effective barrier against oxygen | • Limited resistance to water vapor • Lacks flexibility and mechanical properties | • Addition of plasticizers and cross-linking agents (glutaraldehyde) • Thermal treatment | • Perishable foods |
| Zein | Corn | Prolamins—proline, glutamine, and asparagines | • Biodegradable • Effective barrier against water vapor • Moderate oxygen barrier and mechanical properties | • Brittle • Low respiratory gas transmission rate | • Addition of plasticizers (glycerol, polyols, or fatty acids) and antimicrobial agents | • Cheese and milk products |
| Soy protein | Soybean | Conglycinin and glycinin | • Biodegradable • Increased firmness, rigidity, and tensile strength • Functional properties such as adhesion, cohesion, fiber formation, fat absorption, and texturizing capability • Good oxygen barrier | • Lack of water vapor barrier properties due to hydrophilic nature • Moderate mechanical properties | • Addition of plasticizers, hydrophobic compounds (lipids), etc. • Modification of protein chain through cross-linking | • Edible packaging film • Adhesives |
2.1.2.1. Animal proteins
Collagen and Gelatin
Collagen is found in animal tissues such as bones, skin, and connective tissue. Gelatin is a product obtained from collagen. It forms a clear, transparent, thermo-reversible film that is biocompatible, low-cost, and nontoxic. Gelatin is used in the production of biodegradable films and effectively encapsulates low-moisture food ingredients. The film formation process includes casting, extrusion, and blown-extrusion. Gelatin films produced are of two types: the cold cast film formed at lower temperatures (<35°C) has a spiral structure and the hot cast film prepared at higher temperatures of more than 35°C has a coil structure that makes it more brittle. Cast films have higher tensile strength while extruded films have extensibility. These films are modified to improve their barrier against water vapor and their functionality [24].
2.1.2.2. Milk proteins
Milk proteins include casein and whey protein. They are more nutritious and offer the potential components for edible coatings and films. The films formed from milk proteins impart excellent physical characteristics such as sufficient mechanical strength, control of mass migration, and sensory appeal. However, the film formation from total milk protein combined with lactose is quite hard as the sugar crystallizes during drying and results in a nonuniform film. Therefore, milk protein in the presence of lactose does not contribute to film formation. Individually, casein and whey proteins form a transparent, flexible, and flavorless film [25].
Casein
Casein is a water-soluble milk protein that makes up 80% of total milk protein. It has three principal components (a, β, and κ) that form casein micelles in milk, which are stabilized by a calcium–phosphate network. To extract casein, skim milk is acidified to its isoelectric point (pH 4.6), precipitating caseinates. By neutralizing the acid-coagulated caseins to pH 6.7 with an alkali (sodium, magnesium, calcium, phosphorous hydroxide), the process is completed. Caseinates are desirable biomaterials for making edible films due to their intermolecular interactions. Although casein films are transparent and flexible, they have poor water and oxygen barrier properties. Calcium caseinates have better barrier properties, while sodium caseinates have good optical and tensile properties. Plasticizers and other additives can improve the film characteristics, and increasing the cohesion between the protein polypeptide chain enhances the barrier properties [26]. Regardless of pH, temperature, or salt concentration, casein films remain stable.
Whey Protein
Whey proteins make up 20% of milk protein and have five fractions (α-lactalbumin (20%), β-lactoglobulin (52%), bovine serum albumin (BSA) (7%), immunoglobulins (Ig) (12%), and proteose-peptones (PP) (9%)). Whey protein isolate (WPI) and concentrate (WPC) having 90 and 25–80% protein are used to form edible films that are excellent barriers against oxygen, aroma, and oil solutions. The film formation depends on the behavior of whey proteins upon thermal denaturation. The physical and chemical methods such as UV, US, and alkalization significantly improve the mechanical properties of the film [27].
2.1.2.3. Plant proteins
Wheat Gluten
Gluten is a protein found in wheat and it acts as a binder and contributes to the stretching quality of food. The cohesive and elastic properties of gluten facilitate film formation, which is affected by protein structure, production method, and film-forming solutions. Glutenin has higher barrier properties than gliadins and whole gluten. Two methods of gelatin film formation include casting and thermos pressing, with cast films having higher tensile strength and thermos pressing offering strong resistance to rupture. Among the film-forming solutions, films from alkaline solutions reveal higher tensile strength than acidic solutions, and films from ethanol solutions demonstrate superior characteristics compared to acids and alkalis. Wheat gluten films are strong, transparent, and have a shiny surface. They provide an effective barrier against oxygen compared to water vapor due to their hydrophilic nature. The addition of plasticizers (glycerol) and cross-linking agents (glutaraldehyde) into the film-forming dispersion and thermal treatment results in higher flexibility and improved mechanical properties. However, an increased quantity of hydrophilic plasticizers may also tend to reduce the strength and elasticity of the film [28].
Corn Zein
Zein is a type of protein found in corn that is insoluble in water and makes up almost half of corn’s protein. It has a high content of nonpolar amino acids and lacks basic amino acids such as lysine and tryptophan, making it unsuitable for human consumption. Its hydrophobicity makes it useful for creating barriers against water vapor. Zein films are made by dissolving zein protein, plasticizers, and other raw materials in a solvent, casting the solution on a flat surface and peeling off the dried film. Different solvents affect the properties of the film, but adding plasticizers and antimicrobial agents improves flexibility and food preservation [29]. Zein has excellent film-forming abilities and can be used for coatings and biodegradable films.
Soy Protein
Soybean consists of 38–44% protein with molecular weights ranging from 200 to 600 kg/mol. The protein from soybean meal is a complete protein that meets all the essential amino acid requirements in an individual’s diet. It often replaces animal protein with multiple health benefits, such as being low in saturated fat and absence of cholesterol. It also provides fiber, iron, calcium, zinc, and B vitamins and excellent functional properties to support human development. Approximately 90% of soy proteins are globular, insoluble in water, and composed mainly of polar and nonpolar side chains. These interactions enhance the hardness, stiffness, and tensile strength of soy protein-based films, making them ideal for producing edible and biodegradable films. Highly refined soy protein isolate (SPI), with a minimum protein content of 90%, serves as the primary source for soy protein films [30]. The various film formation methods include casting, extrusion, spinning, heating, and thermal compaction. Casting is the most common method of soy protein film formation that involves the following two-step process:
(i) Heating the film-forming solutions will result in the disruption of protein structure with split native disulfide bonds;
(ii) Forming new bonds through intermolecular interactions will lead to a network during the drying operation.
Soy protein films exhibit different characteristics in different film-forming solutions and soy protein fractions. They include adhesiveness, cohesiveness, emulsification, absorption of water and fat, formation of fiber, and texturizing capability. These films lack water vapor barrier properties due to their hydrophilic nature and require plasticizers to improve them. However, the enhancement of the soy protein film properties is achieved by incorporating hydrophobic compounds (lipids), sodium dodecyl sulfate (SDS), carboxymethyl cellulose (CMC), and cysteine, and modifying the protein chain through cross-linking [31].
2.1.3. Lipids
Lipids come from natural sources such as plants, animals, and insects. They include mono-, di- and triglycerides, phospholipids, fatty alcohol, phosphatides, terpenes, cerebrosides, and fatty acids. Lipids improve the hydrophobicity, compatibility, and barrier properties of edible films and coatings against water vapor. They include natural waxes, oils, neutral lipids, fatty acids, and resins. In addition, films prepared with lipid materials are usually opaque, brittle, and thicker but exhibit poor mechanical and barrier properties against gases such as oxygen and carbon dioxide [32]. To overcome the disadvantages of each polymer, they are combined to obtain composite films or coatings with improved barrier and mechanical properties.
Waxes
Wax is a lipid used to preserve fresh food. It is nonpolar and insoluble in water, which prevents it from spreading over the surface. It is derived from animals and plants and used to prevent moisture migration in edible films and coatings. Waxes are of two types: natural and synthetic [Figure 2]. Edible coating using wax is a potential alternative for preserving fruits and fresh-cut fruits. Waxes are the most effective lipid barrier against water vapor, and thus they have been used in a variety of products. Examples of wax-based coatings include a superhydrophobic coating created by dissolving candelilla and rice bran wax in hot ethanol, and another coating formulated by dispersing soy wax in hot ethanol, which displayed remarkable repellent properties against various non-Newtonian food liquids [33].
 | Figure 2: Classification of waxes. [Click here to view] |
The application of organic solvents in wax coatings can be toxic due to their high lipophilicity, which allows them to be dissolved in fats and distribute widely throughout the body upon exposure. Upon inhalation, dermal contact, or oral exposure, the lipophilic nature of these solvents promotes immediate absorption. Subsequent metabolism and deposition depend on exposure routes and the solvent’s chemical properties. Metabolism and elimination can occur promptly through organs such as the liver and lungs without entering the systemic circulation. Metabolites produced from these solvents vary in toxicity; some become less toxic, while others become severely toxic by-products. Unmetabolized solvents predominantly accumulate in fatty tissues, impacting the body for a long time. Some solvents used to dissolve natural compounds that can be harmful to cell cultures. All solvents showed dose-dependent cytotoxicity on various cell lines. Acetone and ethanol were compatible with cells, while dimethylformamide was highly cytotoxic [34].
Fats and Oils
Fats and oils are mixtures of animal and plant triglycerides. They have similar structures but differ physically at room temperature. They are water-insoluble but can form a stable monolayer, making them useful in edible films and coatings. Table 3 lists the significant fatty acids used for film formation with their physicochemical properties. From Table 3, it is clear that the melting point of fatty acids decreases with double bonds and increases with chain length. In addition, the water vapor permeability and hydrophobicity of these films incorporated with fatty acids depend on their structure, chain length, degree of saturation, and melting point. Short-chain triglycerides have good emulsifying properties and are used as emulsifiers to stabilize composite films. They enhance adhesion between layers of different hydrophobicity in bilayer films and between the film and the food. Vegetable oils such as lauric, stearic, and palmitic acids, and stearyl alcohol enhance the barrier properties of the edible film against moisture. In general, fatty acids derived from vegetable oils are considered GRAS substances in film formation. Edible film prepared from palm fruit oil exhibited water resistance, water vapor barrier, transparency, and elongation. An edible coat of sunflower oil and rice bran oil on meat and kiwi fruit prevented undesirable reactions and enhanced the quality by conserving color, taste, and firmness [35].
Table 3: Characteristics of fatty acids.
| Fatty Acid | No. of Carbon Atoms | No. of Double Bonds | Melting Point (°C) | Water Vapor Permeability (10−11 g/m s Pa) | Major Occurring Sources |
|---|---|---|---|---|---|
| Capric | 10 | 0 | 31.3 | Palmae seed fat, milk fat | |
| Lauric | 12 | 0 | 43.9 | Coconut oil | |
| Myristic | 14 | 0 | 54.4 | 3.47 | Butter, coconut oil, palm oil |
| Palmitic | 16 | 0 | 62.9 | 0.65 | Palm oil, butter, lard, tallow |
| Stearic | 18 | 0 | 69.6 | 0.11–0.22 | Tallow, cocoa butter, lard, butter |
| Oleic | 18 | 1 | 16.3 | Olive, peanut, lard, palm, tallow, corn, rapeseed, canola | |
| Linoleic | 18 | 2 | −5 | Soybean, safflower, sunflower, corn, cottonseed | |
| Linolenic | 18 | 3 | −11 | Soybean, canola | |
| Arachidonic | 20 | 4 | −49.5 | Lard, tallow | |
| Behenic | 22 | 0 | 80 | 13.8 | Peanut, rapeseed |
Resins
Resin is a substance produced by plants or insects. It is used to impart a glossy texture to products and includes shellac, wood rosin, and coumarone indene. Shellac resin is a widely used edible coating for fruits and vegetables and contains aleuritic and shelloic acids. It has improved moisture-barrier properties and increased glossiness in coated products [36]. Though it has a melting point between 115 and 120°C, it’s not considered a GRAS substance and is therefore permitted as an indirect food additive in food coatings and adhesives.
2.1.4. Composite Films
Biodegradable polymers are often preferred to ensure eco-friendliness. Composite films mix hydrocolloids and lipids to improve mechanical properties. However, compatibility between blend components is crucial to achieve optimal performance. The strategic combination of proteins (e.g., milk, soy, collagen, and gelatin) with polysaccharides (e.g., starches, alginates, cellulose, and chitosan) or other polymers offers an avenue to enhance the film’s barrier and physical characteristics. This amalgamation creates composite materials that exhibit improved properties [10]. Blend/composite films are listed in Table 4.
Table 4: List of possible blend/composite films.
| Type of Composite Film | Composite Film | Properties of the Composites | Inference |
|---|---|---|---|
| Protein–protein | Barely bran protein, gelatin | Mechanical properties: The thickness of the BBG film grew with higher amounts of BBP, gelatin, and sorbitol, despite using the same volume of film-forming solution. As the BBP content rose, both the tensile strength (TS) and elasticity (E) of the BBG film reduced. | Barley bran protein could be used as a suitable candidate because of excellent film-forming capability. Addition of grape seed extract to enhance as an antimicrobial agent. |
| Carbohydrate–carbohydrate | Rice starch, l-carrageenan | Thickness of rice starch-ι-car films varied between 0.084 and 0.114 mm. Film solubility ranged from 43.35% to 63.22%, which are good values for fruit applications. | The combination process improved TS and elongation at break properties. |
| Carbohydrate–protein | Chitosan, gelatin | Antimicrobial treated samples demonstrated 2–3 log CFU/g of L. monocytogenes reduced count compared to controls. Chitosan films containing Ziziphora clinopodioides. Essential oil (1%), pomegranate peel extract (1%), and cellulose nanoparticle (1%) demonstrated the best antimicrobial properties. | Chitosan and gelatin-based films were enriched with various active ingredients such as Ziziphora clinopodioides essential oil (ZEO), pomegranate peel extract (PPE), and cellulose nanoparticle, separately and in combinations. It was observed that composite films incorporated with ZEO and PPE had exhibited strong antimicrobial activity against L. monocytogenes. |
| Protein–carbohydrate | Whey protein isolate, chitosan | Mechanical properties: TS (MPa) 18.04 ± 1.30; elongation at break (%) 177.57 ± 9.54; barrier properties: water vapor permeability (10−9 g m−1 s−1 Pa−1) 1.82 ± 0.05; monolayer moisture content (g water/g dry solid) 0.207. | Films made by combining whey protein isolate and chitosan were mixed with sodium laurate and modified TiO2 nanoparticles, and their characteristics were studied. Introducing nanoparticles improved the films’ strength and promoted better interaction between the biopolymers while lowering water vapor permeability. |
| Carbohydrate–lipid | Tara gum, oleic acid | The contact angle increased from 76.75° to 107.28°, the TS decreased 18 from 57.4 to 26.8 MPa. | Oleic acid inclusion caused interruptions in the polymer structure, resulting in reduced tensile strength (TS) and decreased light transmittance in the tara gum film. Due to its hydrophobic nature, oleic acid could lower water vapor permeability (WVP) and moisture content in the TG film while enhancing the contact angle on its surface. Additionally, oleic acid marginally enhanced the thermal endurance of the TG film. |
3. METHODS OF FILM FORMATION
Edible packaging biopolymers need to form a cohesive structural matrix in the film. Two processes are used for film formation: wet process and dry process. After film formation, parameters such as film thickness, transparency, opacity, gas transmission rate, and thermal stability are determined. Polymers commonly used in solvent-casting film formation include chitosan, hemicellulose, agar, and alginate. In contrast, the dry process involves melting the film-forming materials. Polymers commonly used in extrusion film formation are polyhydroxyalkanoates and polylactic acid [37].
Solvent Selection
The formation of a film depends on whether the polymer is dissolved or dispersed. The proper control of solvent evaporation rate is critical to avoid issues such as over wetting or premature drying. Traditionally, highly volatile organic solvents were preferred, but due to safety and environmental concerns, a shift toward aqueous-based systems has been necessary. The choice of solvent significantly impacts film properties, so it is important to consider its effect on the film’s inner structure and macromolecule binding. Water and ethanol are favored solvents for edible films, but their use can influence film properties significantly. For instance, in a study with zein films, ethanol initially resulted in higher tensile strength than acetone, but ethanol-based films were more susceptible to moisture. As humidity increased, the tensile strength of ethanol-based zein films decreased significantly compared to acetone-based films [38].
Chitosan films were prepared with different molecular weights and organic acid solvents. Higher molecular weight increased tensile strength, and different solvents influenced properties such as toughness and elongation. Acetic acid resulted in the most challenging films, while citric acid resulted in higher elongation values. Chitosan molecular weight did not affect water vapor permeability, but oxygen permeability varied with the type of acid used. In conclusion, solvent choice is critical for film characteristics. Water and ethanol are safe and eco-friendly options, but specific polymer needs and applications should be considered for optimal results [39].
3.1. Wet Process/Solution Casting
The solvent-casting method is widely used for film formation on a pilot scale in laboratories. The steps involved in this method include:
1. Choosing and solubilizing suitable biopolymer in the appropriate solvent. The solvent used in this solubilization process is a food-grade substance and is limited to water and ethanol.
2. Degassing the dissolved solution and casting onto the mold or Teflon-coated sheets.
3. Evaporating the solvent using driers such as hot-air ovens, tray dryers, vacuums, or microwave dryers to produce the polymeric film. This final step of casting determines the microstructure of the biopolymer film, and the film formed at this stage should be non-consistent and have a uniform surface [37].
This method of film production is cost-effective and results in a more uniform film with fewer defects. However, it has some drawbacks such as a lack of variability in edible films, longer drying time, and potential integration of toxic substances into the polymer solution. Further research is needed to scale up production from lab to commercial scale.
3.2. Dry Process/Extrusion
This method is a dry process for edible film formation that uses a minimal amount of water or solvent. The material is fed through a hopper and conveyed by a screw auger to the desired shape. The three zones involved in the process include feeding, kneading, and heating. The compressed granular biocomposite materials undergo increased pressure and temperature in the kneading zone [40]. At this stage, the compressed mixture enters the heating zone at high shear rates and other optimized conditions to obtain the film with the desired characteristics. This method is used widely in the manufacture of multilayer packaging films as it improves the functionality of developed films. The advantages of the extrusion process are the shorter processing time, low energy consumption at a wider range of temperatures and pressures, and easy handling practices. However, this method requires specialized equipment and higher maintenance during operation, and thus its use is limited to some extent [37].
4. METHODS OF EDIBLE COATING
Coating food products involves adhesion between the coating material and the product surface. Deposition methods include dipping, spraying, fluidized bed, and panning. The method and material chosen depend on the food’s physical properties, including size, shape, ripening patterns, and desired coating thickness [41]. The process of coating food products requires adhesion between the coating material and the product’s surface. The chosen method is dependent on the physical properties of the food and the characteristics of the coating materials. Methods include dipping, spraying, fluidized bed, and panning. Size, shape, ripening patterns, and coating thickness should be considered when choosing a method and material [37]. The application of coating is listed in Table 5.
Table 5: Application on edible coating.
| Method of Application | Coated Product | Coating Matrix | Result |
|---|---|---|---|
| Dip coating | Guava | Alginate, chitosan, nano-ZnO | Prevents rot in guavas with their antimicrobial solid properties but also delays fruit ripening, and prevents weight loss and lesions, resulting in maintaining their quality for up to 20 days in suitable storage conditions. |
| Panning (pharmaceutical, confectionery) | Rice crisp balls | Hydrolyzed collagen | Permeability was influenced by film thickness at identical cocoa butter concentrations. Films with more uniformity was produced. |
| Spraying | Fresh meat | Gelatin | Coating gelatin worked as an oxygen barrier reducing color deterioration, extending shelf-life, purging harbors microbial growth. No significant difference in flavor or aroma. |
| Electrospinning | Apple slices | Resveratrol–zein nanofiber | The electrospinning process successfully encapsulated resveratrol within zein nanofibers, demonstrating controlled release and retention of its antioxidant properties. The coated apple slices showed controlled moisture loss and better color retention than uncoated slices, indicating the potential for these nanofibers as a coating for preserving food quality. |
4.1. Dip Coating
It is the most popular coating method as it involves immersing the product in the coating solution. The steps involved in this method of coating include [42]:
1. Immersion of the food product in the coating solution for 5–30 s.
2. Dwelling (diffusion) of the coating material until the product’s surface is wet.
3. Deposition of a thin layer of coating material on the surface of the product.
4. Removal of the excess coating solution by draining.
5. Evaporation of the solvent from the surface by drying methods.
This method is used to enhance the taste and reduce moisture loss and oil absorption in fresh and frozen products. However, coating hydrophilic surfaces can be challenging. Multifaceted technology and nanometer-scale layer-by-layer electrodeposition can help [43]. Dipping fresh fruit in a coating solution with antimicrobials and antioxidants ensures microbial stability and anti-browning effects. Coating thickness and uniformity depend on specific gravity, viscosity, surface tension, and withdrawal rate.
The dipping method produces thicker and smoother films compared to spraying. Spraying highly viscous solutions is difficult, making dipping more feasible. The thickness and morphology of the films depend on various factors such as immersion time, withdrawal speed, and coating solution properties. Multiple dip coating processes can increase film thickness, but an intermediate consolidation step is required to prevent redissolution of wet films during subsequent immersions [44]. The dipping method has drawbacks such as dilution of coating, waste accumulation, microbe development, and degraded functionality of the external layer [37].
4.2. Spray Dry Method
Spraying is another method of applying an edible coating on food products in the industries [41]. This method uses a set of nozzles in which droplets with an increasing liquid surface are formed and then dispensed across the food surface. The spraying techniques include air spray atomization, air-assisted airless atomization, and pressure atomization.
4.2.1. Air spray atomization
This technique involves penetration of a high-speed air stream inside a fluid flowing at a low velocity. It disrupts the fluid flow and induces atomization. The air jet nozzle used in this method breaks the water jet into fine droplets for spraying the coating material on food products [45].
4.2.2. Air-assisted airless atomization
Spray guns employed in this method produce fine atomization of high viscous fluid materials containing high solids using higher fluid pressures. The atomization takes place in two stages. Initially, a special fluid nozzle tip partially atomizes the liquid, and subsequently, a small quantity of compressed air from the horn of the air nozzle completes the atomization.
4.2.3. Pressure atomization
The coating of the edible material on food products is by applying pressure. In this method, the high viscous coating solution is passed through a small-size nozzle to pressurize the fluid and provide surface tension. This technique is also known as airless atomization.
The spraying procedure involves cohesive and disruptive forces, which form a liquid surface between the product and the coating solution. Atomization of fine droplets depends on critical parameters such as atomization pressure (<30 bar), thickness of coating solution (30 µm), and size of droplet [46]. Spray coating technique forms a thin, uniform film with multilayer applications possible. The method is useful for low-viscosity coating solutions and does not contaminate the layer, but it is not suitable for highly viscous solutions [37]. The film formed is subsequently dried for adhesion to the product surface.
4.3. Fluidized Bed Method
Fluidized coating is a widely used method in research and food industries to coat dry particles of very low densities. It involves spraying the coating solution over the fluidized powder surface, which forms a shell-like structure [45]. The process is classified as a top spray, bottom spray, and rotary fluidized bed, with the former being the most effective. The fluidized bed of particles adheres, aggregates, and dries, providing complete adhesion of the coating material [47]. However, the process is expensive and requires a larger quantity of coating solution.
4.4. Panning Method
The Greek Arabian society first introduced the panning method for coating drugs in medical applications. In this coating method, the product placed in a rotating bowl known as a pan is dusted with the coating solution and then tumbled to evenly distribute the coating solution on the food product’s surface. The adhered coating layers are dried using forced air at room or higher temperatures, and this causes friction and generates heat during the process. Based on the characteristics of food products, the method is divided into hard, soft, and chocolate panning.
1. The hard panning method involves the continuous application of the coating material (sugar syrup) on the product’s surface, which eventually dries and crystallizes to form a hard shell.
2. The soft panning method uses a mixture of corn and sugar syrup on the surface, and by applying powdered sugar to it, the product dries to form a smaller number of soft and thick layers.
3. The chocolate panning method utilizes white and dark chocolate and cocoa-based confectionery as a coating solution to form a fatty layer on the product.
This method finds numerous applications in confectionery, food processing, and pharmaceutical sectors for applying either thin or thick layers of the coating solution around a core material [48].
4.5. Electrospinning
Electrospinning is a highly efficient method of producing high-quality polymeric fibers ranging from micro- to nanoscale diameters, typically between 10 and 1000 nm. This technology uses an electric field to draw and elongate a polymer solution, creating fine fibers. The process requires fundamental components:
• a high-voltage power supply
• a syringe with a needle for solution dispensing
• a pumping system controlling the flow rate
• a grounded collector screen for fiber collection
Electrospinning elongates a polymer solution droplet into a conical shape by applying a high-voltage electric field, creating fibers after solvent evaporation. The process is affected by factors such as polymer concentration, viscosity, surface tension, conductivity, as well as distance, flow rate, and voltage [49]. Electrospun fibers create a structural network that significantly reduces oxygen transmission and enhances adhesion between biopolymer layers, providing structural integrity to the composite structure [50].
4.6. Compression Molding
Compression molding is a thermal processing technique commonly used to transform various polymers into desired products. The method involves introducing the raw material into a heated mold, applying pressure to attain the desired shape. It is utilized as a preliminary step before extrusion to optimize conditions for subsequent processes [51]. Soy protein isolate–glycerol films were created through compression molding under specific conditions: an ideal temperature of 150°C, a pressure of 10 MPa, and a dwell time of 2 min [52].
4.7. Layer-by-Layer Assembly
Layer-by-layer technology involves depositing successive layers of oppositely charged polyelectrolytes onto a substrate to create nanolayered films. Computer-controlled slide strainers are used due to the extended time needed for multilayer formation. Robotic modifications in dipping systems involve spraying water on samples to prevent contamination. Films formed by dipping tend to be thicker, denser, and smoother than films with the same number of layers deposited by spraying [49].
5. APPLICATIONS OF EDIBLE FILMS AND COATING IN FOOD PRESERVATION
Food packaging materials depend on the characteristics of the packaged food and environmental factors during distribution and storage. Edible coatings with antimicrobial agents such as essential oils and polyphenols are an eco-friendly technology to preserve food products. The use of antimicrobial agents such as essential oils (clove, cinnamon, oregano, rosemary, and garlic extract) and polyphenols in the edible film formation was effective against major foodborne pathogens such as Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella species [53]. They provide barriers against moisture, oxygen, and other environmental factors and effectively inhibit pathogenic organisms and retard spoilage. The nature of coating material, its formation, and application of the edible coating on various food products are listed in Table 6.
Table 6: Application of edible packaging in food products.
| Biomaterials | Food Product | Type of Film Formation | Functions of Edible Film |
|---|---|---|---|
| Methylcellulose, carboxymethylcellulose (CMC), hydroxypropyl methylcellulose, and chitosan | Mandarin | Edible film | Retained firmness Reduced weight loss Glossy surface |
| Gum arabic incorporated with garlic extract, ginger extract, and aloe vera | Gola guava | Edible film | Reduced weight loss, skin browning, disease severity, and increased shelf life |
| Cassava starch, whey protein, beeswax, chitosan, glycerol, stearic acid, and glacial acetic acid | Blackberry | Edible coating | Improved physicochemical and sensorial properties |
| Rice starch-ι-carrageenan composite coating blended with sucrose fatty acid esters | Plum | Edible coating | Reduced weight loss and respiration rate Inhibited ethylene production |
| Cassava starch | Pineapple | Edible coating | Reduced weight loss and juice leakage Firmness retention |
| Mango kernel starch with plasticizers such as glycerol and sorbitol | Tomato | Edible coating | Delayed ripening Preserved sensory attributes |
| Cassava starch with carvacrol | Minimally processed pumpkin | Edible coating | Inhibited bacteria No change in pH, acidity, and TSS |
| 1% Chitosan | Sweet cherry | Edible film | Inhibited microbial growth Extended shelf-life |
| Chitosan/glycerol 30% films | Strawberry | Edible film | Retained sensory and textural attributes |
| Corn starch modified by extrusion | Mango | Edible film | Retained quality attributes |
| Blends of ellagitannins (ET), low methoxyl pectin (LMP), tara gum (TG) | Rubus chingii Hu | Edible film | Effective antioxidant, antimicrobial activity, and inhibitory activities against Escherichia coli and Staphylococcus aureus |
| Soybean protein isolate, chitosan | Apricot | Edible coating | Reduced weight loss Firmness retention Retention of TA, SSC, WSP, and CSP content |
| Carnauba wax, lemongrass oil | Grapes | Edible film | Retarded Salmonella and E. coli contamination Prevented degradation of phenolic compounds |
| Pectin–soy flour and transglutaminase | Apple | Edible film | Glossy film surface and uniformity Higher mechanical strength with reduced flexibility |
| Rhubarb extract alginate | Peach | Edible coating | Exhibited antifungal activity proactive in lessening post-harvest illnesses owing to Penicillium expansum Effectively prevented sensory deterioration |
| Gelatin, guar, chitosan | Barhi date | Edible coating | Extended shelf-life Preserved quality characteristics |
| Essential oils (thyme, oregano), alginate | Fresh-cut papaya | Edible coating | Decelerated pH changes Delayed organic acid consumption and senescence Reduced respiration rate Enhanced antimicrobial activity |
| Cassava starch, cinnamon bark | Apples | Edible film | Decreased respiration rate Effective antimicrobial activity against S. aureus and S. choleraesuis |
| Pectin, essential oil | Peach | Edible film | Retarded bacterial growth Increased antioxidant activity |
| Cassava starch | Pineapple | Edible coating | Barrier against gas and water vapor Extended storage life Preserves fresh-like quality attributes |
| Cassava starch based | Strawberry | Edible coating | Delays weight loss Retained firmness |
| Carboxymethylcellulose, aloe (Aloe vera) powder, and whey protein isolate | Fresh-cut mango | Edible coating | Reduced browning up to 10–11 days |
| Soy protein isolate with ferulic acid | Fresh-cut apple | Edible coating | Reduced weight loss Retained firmness |
| Sodium alginate, chitosan, and Aloe vera | Kiwi | Edible coating | Maintained firmness Prevented ascorbic acid loss and yellowing due to ripening, reduced microbial proliferation |
| Chitosan, ascorbic acid | Pomegranate | Edible coating | Preserved the visual quality of arils during storage Inhibited bacterial (mesophilic aerobic bacteria) and fungal growth |
| Gelatin, chitosan, calcium carbonate | Banana | Edible film | Good film structure with TS UV absorption property, antimicrobial Barrier against oxygen Nontoxic |
| Sodium alginate, citral nano-emulsion | Fresh-cut pineapple | Edible coating | Better color retention Lowered respiration rate Retarded microbial growth |
| Pectin, potassium sorbate, sodium benzoate, nisin, citric acid | Fresh-cut persimmon | Edible coating | Inhibited mesophilic aerobic bacterial growth No growth of molds, yeasts, and psychrophilic aerobic bacteria |
| Zinc oxide, xanthan | Hybrid tomatoes and apples | Edible coating | Reduced weight loss Improved storage quality |
| Chitosan, chlorogenic acid | Peach | Edible coating | Reduced weight loss Decay of index Delayed respiration rate |
| Beeswax, chitosan | Strawberries | Edible coating | Retarded fungal infection Reduced weight loss, respiration rate Firmness and color retention Preserved physicochemical properties (titratable acidity, pH, sugars, and TSS) |
| Soy protein isolate, honey | Fresh-cut kajari melon | Edible coating | Decreased microbial growth honey maintains the taste and color |
| Guar gum, Aloe vera, spirulina platensis | Mango | Edible coating | High firmness Decelerated TSS changes improved bioactive compounds such as phenol, ascorbic acid, and flavonoids |
| Multilayered coatings of chitosan, pectin, and trans-cinnamaldehyde | Fresh-cut cantaloupe | Edible coating | Extended shelf-life up to 9 days |
| Soy protein with cysteine | Eggplant | Edible coating | Controlled enzymatic browning Maintained the visual quality of fresh-cut eggplants for up to 8–9 days at 5°C |
| Sweet orange essential oil and sodium alginate | Tomatoes | Edible coatings | Enhanced firmness up to 33% Decreased total mesophilic bacteria (Salmonella and Listeria) Reduced weight loss |
| Chitosan, gelatin, and essential oils | Shredded black radish | Edible film | Exhibited antimicrobial activity |
| Carvacrol and cinnamaldehyde incorporated in apple, carrot, and hibiscus-based films | Organic leafy greens | Edible film | Inhibited Salmonella |
| Chitosan, essential oil (clove, lemon), bioactive compound (bee pollen, ethanolic extract of propolis) | Broccoli | Edible coating | Inhibited the growth of mesophilic and psychotropic bacteria Controlled E. coli and L. monocytogenes survival |
| Alginate cross-linked with calcium chloride, oregano essential oil (OEO) | Tomato | Edible coating | Promoted surface adhesion Improved wetting capability Reduced the growth of the endogenous microbial flora Prolonged shelf-life |
| Mango kernel starch (MKS) | Red chili powder | Edible film | Retained color, pungency, and capsaicin content |
| Whey protein concentrate (WPC) and olive oil | Dried Peanuts | Edible film | Minimum moisture uptake Better crunchiness Delayed rancidity |
| Vegetable oil and egg proteins | Sweet baked goods | Edible film | High barrier against water No mold growth |
| Mung bean starch and guar gum containing sunflower seed oil/grape seed extract | Rice cakes | Edible coating | Suppressed the staling of rice cakes Antimicrobial effects |
| Soy protein, thyme/oregano essential oils | Beef | Edible coatings | Exhibited antibacterial activity against E. coli O157:H7, L. monocytogenes, and Staphylococcus aureus |
| Grape seed extract, chitosan, gelatin | Pork | Edible coatings | Enhanced antioxidant activity against meat oxidation |
| Rice protein concentrate/mineral oil | Egg | Edible coating | Maintained the interior quality such as weight loss, Haugh unit (HU), albumen pH, yolk index (YI), and shell strength |
| Alginate and pineapple peel | Beef | Edible film | Inhibited the microbial growth Maintained color Retarded the lipid oxidation |
| Gelatin, chitosan, and clove essential oil | Fish | Edible film | Antimicrobial activity against Pseudomonas fluorescens, Shewanella putrefaciens, Photobacterium phosphoreum, L. innocua, E. coli, and Lactobacillus acidophilus |
| Tea polyphenol, pectin, and chitosan | Beef | Edible coating | Remarkable slow-release Antioxidant and antiseptic activities |
| Corn starch, chitosan, eugenol | Fish | Edible film | Improved flexibility, barrier, hydrophobicity, antimicrobial, and antioxidant properties |
| Cassava starch, papain | Meat | Edible film | Improved the degree of tenderness and protein conformation in packaged beef |
| k-carrageenan, bee pollen, and honey extracts | Beef | Edible film | Increased antioxidant and antiradical activity of meat |
| Hydrocolloids: carrageenan and agar-agar, gel maltodextrin, sodium alginate, gelatin | Pork | Edible film | Good physicochemical and mechanical characteristics Provided sufficient stability to act as a vehicle to incorporate spices and condiments into entire pork Improved meat texture |
| Chitosan and gelatin | Nile tilapia | Edible coating | Increased adhesion |
| Chitosan, essential oils | Turkey | Edible coating | Effective antimicrobial coating Preserves the microbial quality of meat |
6. ADVANCEMENTS IN EDIBLE PACKAGING
Edible packaging has the potential to protect food but lacks mechanical and functional properties. Adding nanoparticles improves the film properties, enhancing characteristics such as strength, barrier properties, and shelf-life extension. Bio-nanocomposites, a hybrid form of nanostructured materials, are incorporated to overcome this shortcoming. They protect food, extend shelf-life, restrict conventional packaging use, and promote an environmentally friendly approach. Nanoparticles ranging from 10 to 100 nm can be added to packaging material to create a lightweight option [54]. Edible films can be reinforced with nanofillers such as nano starch, nanocellulose, nano chitosan, nano protein, and nano lipid to enhance mechanical, thermal, and barrier properties and impart active properties such as antimicrobial activity, oxygen scavenging, and biosensing [55]. Further research is necessary to adapt these films for commercial use.
7. SAFETY CONCERNS AND FUTURE OUTLOOK
Edible films and coatings for fruits and vegetables undergo strict regulations. FDA-approved materials that adhere to GRAS and GMP are eligible for use. Biopolymers without GRAS approval can be deemed safe through application. Assessing toxicity and allergenic potential is crucial, especially when incorporating essential oils for antimicrobial properties. Despite GRAS classification, these oils can cause allergies or toxicity in high doses. Balancing efficacy and potential toxicity is vital. Regulations on utilization and doses vary by country or export destination. Full ingredient disclosure on labels is essential as they become part of the produce. Another crucial regulatory aspect involves coatings with allergenic constituents such as milk-derived substances, soybeans, fish, peanuts, nuts, and wheat require declarations on labels and appropriate warnings for allergic consumers [56].
Food safety standards and regulations differ across countries. Edible film and coating must be classified as food ingredients, additives, contact materials, or packaging materials according to EU and US regulations. They must meet GRAS status under FDA regulations. The development process of films can cause potentially harmful changes. Cross-linking agents used to enhance film properties can create toxic compounds when interacting with gastrointestinal substances [57].
Edible film research is a constantly evolving field, exploring new materials, active packaging, and nanotechnology. The goal is to create biopolymers that match synthetic polymers while using agricultural by-products for cost-effective production. Key objectives include developing films with exceptional barrier and mechanical properties, and using cross-linking techniques to create composite biodegradable films. The focus is on preserving food safety, maintaining product integrity, and achieving complete biodegradability. The future of packaging looks promising, with nanotechnologies offering possibilities such as improving food contact materials, monitoring quality, and modifying sensory aspects. Food packaging, especially bio-nanocomposites, plays a significant role in shaping the future of edible films and food-related technologies [58].
8. CONCLUSION
Edible packaging is a green, innovative, and eco-friendly technology, which can act as potential alternative to synthetic packaging. Edible films provide better antimicrobial effects, retains color, flavor, and other natural properties. Edible films containing fruit purees exhibit antioxidant properties, which is suitable to prevent lipid oxidation but must be researched to understand its barrier properties. However, conventional packaging cannot be completely replaced because of cost, processing requirements, energy consumption, etc. Further research must be carried out to understand the scaling-up process, edibility, commercial use, layer-by-layer packaging, and so on.
9. ACKNOWLEDGMENTS
The authors would like to express thanks to Karunya Institute of Technology and Sciences (Deemed-to-be-University).
10. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design; drafted the article or revised it critically for important intellectual content; submitted to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
11. FINANCIAL SUPPORT AND SPONSORSHIP
There is no funding to report.
12. CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
13. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
14. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
15. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
16. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Otoni CG, Avena-Bustillos RJ, Azeredo HMC, Lorevice MV, de Moura MR, Mattoso LHC, et al. Recent advances on edible films based on fruits and vegetables—a review. Compr Rev Food Sci Food Safety. 2017;16(5):1151–69.
2. Avramescu SM, Butean C, Popa CV, Ortan A, Moraru I, Temocico G. Edible and functionalized films/coatings. Perform Perspect Coat. 2020;10(7):687.
3. Raheem D. Application of plastics and paper as food packaging materials-an overview. Emirates J Food Agricult. 2013; 25(3): 177–88.
4. Faraca G, Astrup T. Plastic waste from recycling centres: characterization and evaluation of plastic recyclability. Waste Manage. 2019;95:388–98.
5. Kabir E, Kaur R, Lee J, Kim KH, Kwon E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J Cleaner Prod. 2020;258:120536.
6. Janjarasskul T, Krochta, JM. Edible packaging materials. Annu Rev Food Sci Technol. 2010;1:415–48.
7. Pascall MA, Lin SJ. The application of edible polymeric films and coatings in the food industry. J Food Process Technol. 2013;4(2):116–7.
8. Candogan K, Barbosa-Cánovas GV, Çarkcioglu E. Edible films and coatings: sensory aspects. In: García MP, Gómez-Guillén MC, López-Caballero ME, Barbosa-Cánovas GV, editors. Edible Films and Coatings: Fundamentals and Applications. Boca Raton (FL): CRC Press; 2016. p. 515–36.
9. Meshram BD, Lule VK, Vyawahare S, Rani R. Application of Edible Packaging in Dairy and Food Industry. In: Tumuluru JS, editor. Food Processing and Packaging Technologies-Recent Advances. London: IntechOpen; 2022.
10. Dhumal CV, Sarkar P. Composite edible films and coatings from food-grade biopolymers. J Food Sci Technol. 2018;55:4369–83.
11. Popovic SZ, Lazic VL, Hromis NM, Suput DZ, Bulut SN. Biopolymer packaging materials for food shelf-life prolongation. In: Grumezescu AM, Holban AM, editors. Biopolymers for Food Design. Cambridge: Academic Press; 2018. p. 223–77.
12. Galgano F, Condelli N, Favati F, Di bianco V, Perretti G, Caruso MS. Biodegradable packaging and edible coating for fresh-cut fruits and vegetables. Italian J Food Sci. 2015;27(1):1–20.
13. Mali S, Karam LB, Ramos LP, Grossmann MV. Relationships among the composition and physicochemical properties of starches with the characteristics of their films. J Agricult Food Chem. 2004;52(25):7720–5.
14. Duan J, Reddy K, Ashok B, Cai J, Zhang L, Rajulu A. Effects of spent tea leaf powder on the properties and functions of cellulose green composite films. J Environ Chem Eng. 2016;4(1):440–8.
15. Kumar M, Tomar M, Saurabh V, Mahajan T, Punia S, Contreras M, et al. Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends Food Sci Technol. 2020;105:223–37.
16. Nieto MB. Structure and function of polysaccharide gum-based edible films and coatings. In: Huber K, Embuscado M, editors. Edible Films and Coatings for Food Applications. New York (NY): Springer; 2009. p. 57–112.
17. Priyadarshi R, Rhim J. Chitosan-based biodegradable functional films for food packaging applications. Innov Food Sci Emerg Technol. 2020;62:102346.
18. Xiao Q, Gu X, Tan S. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem. 2014;164:179–84.
19. Prajapati VD, Maheriya PM, Jani GK, Solanki HK. Retracted: carrageenan: a natural seaweed polysaccharide and its applications. Carbohydr Polymers. 2014;105:97–112.
20. Freitas F, Alves V, Coelhoso I, Reis M. Production and food applications of microbial biopolymers. Contemporary Food Engineering. 2013. p. 61–88.
21. Nur Hazirah M, Isa, Sarbon N. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag Shelf Life. 2016;9:55–63.
22. Kanmani P, Lim S. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013;141(2):1041–9.
23. Guerrero P, de Caba K. Protein-based films and coatings. In: Cerqueira MAPR, Pereira RNC, Ramos OLDS, Teixeira JAC, Vicente AA, editors. Edible Food Packaging. Boca Raton (FL): CRC Press; 2016. p. 81–120.
24. Andreuccetti C, Carvalho R, Galicia García T, Martinez Bustos F, González Nunez R, Grosso C. Functional properties of gelatin-based films containing Yucca Schidigera extract produced via casting, extrusion and blown extrusion processes: a preliminary study. J Food Eng. 2012;113(1):33–40.
25. Wagh Y, Pushpadass H, Emerald F, Nath B. Preparation and characterization of milk protein films and their application for packaging of cheddar cheese. J Food Sci Technol. 2014;51(12):3767–75.
26. Bonnaillie L, Zhang H, Akkurt S, Yam K, Tomasula P. Casein films: the effects of formulation, environmental conditions and the addition of citric pectin on the structure and mechanical properties. Polymers. 2014;6(7):2018–36.
27. Ramos O, Fernandes J, Silva S, Pintado M, Malcata F. Edible films and coatings from whey proteins: a review on formulation, and on mechanical and bioactive properties. Crit Rev Food Sci Nutr. 2012;52(6):533–52.
28. Gennadios A, Weller CL, Testin RF. Modification of physical and barrier properties of edible wheat gluten-based films. Trans Am Soc Agricult Biol Eng. 1993;36(2):465–70.
29. Soliman E, Mohy Eldin M, Furuta M. Biodegradable zein based films: influence of γ-irradiation on structural and functional properties. J Agricult Food Chem. 2009;57(6):2529–35.
30. Tian H, Guo G, Fu X, Yao Y, Yuan L, Xiang A. Fabrication, properties and applications of soy-protein-based materials: a review. Int J Biol Macromol. 2018;120:475–90.
31. Sabato S, Ouattara B, Yu H, D’Aprano G, Le Tien C, Mateescu M, et al. Mechanical and barrier properties of cross-linked soy and whey protein-based films. J Agricult Food Chem. 2001;49(3):1397–403.
32. Embuscado ME, Huber KC. Edible films and coatings for food applications. New York (NY): Springer; 2009.
33. Shen T, Fan S, Li Y, Xu G, Fan W. Preparation of edible non-wettable coating with soybean wax for repelling liquid foods with little residue. Materials. 2020;13(15):3308.
34. Jamalzadeh L, Ghafoori H, Sariri R, Rabuti H, Nasirzade J, Hasani H, et al. Cytotoxic effects of some common organic solvents on MCF-7, RAW-264.7 and human umbilical vein endothelial cells. Avicenna J Med Biochem. 2016;4(1):e33453.
35. Rodrigues D, Cunha A, Brito E, Azeredo H, Gallao M. Mesquite seed gum and palm fruit oil emulsion edible films: influence of oil content and sonication. Food Hydrocolloids. 2016;56:227–35.
36. Baldwin EA, Hagenmaier R, Bai J. Edible coatings and films to improve food quality. 2nd ed. Boca Raton (FL): CRC Press; 2012.
37. Suhag R, Kumar N, Petkoska AT, Upadhyay A. Film formation and deposition methods of edible coating on food products: a review. Food Res Int. 2020;136:109582.
38. Felton LA. Mechanisms of polymeric film formation. Int J Pharm. 2013;457(2):423–7.
39. Park SY, Marsh KS, Rhim JW. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J Food Sci. 2002;67(1):194–7.
40. Calderon Castro A, Vega García M, de Jesús Zazueta Morales J, Vargas F, Carrillo López A, Gutiérrez Dorado R. Effect of extrusion process on the functional properties of high amylose corn starch edible films and its application in mango (Mangifera indica L.) cv. Tommy Atkins. J Food Sci Technol. 2018;55(3):905–14.
41. Senturk Parreidt T, Müller K, Schmid M. Alginate-based edible films and coatings for food packaging applications. Foods. 2018;7(10):170.
42. Costa C, Conte A, Del Nobile MA. Effective preservation techniques to prolong the shelf life of ready-to-eat oysters. J Sci Food Agricult. 2014;94:2661–7.
43. Vargas M, Pastor C, Chiralt A, McClements DJ, Gonzalez Martinez C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit Rev Food Sci Nutr. 2008;48(6):496–511.
44. Puetz J, Aegerter MA. Dip coating technique. In: Aegerter MA, Mennig M, editors. Sol-gel Technologies for Glass Producers and Users. New York (NY): Springer; 2004. p. 37–48.
45. Valdes GA, Ramos M, Sanahuja AB, Jimenez A. State of the art of antimicrobial edible coatings for food packaging applications. Coatings. 2017;7(4):56.
46. Andrade RD, Skurtys O, Osorio FA. Atomizing spray systems for application of edible coatings. Compr Rev Food Sci Food Safety. 2012;11(3):323–37.
47. Chen Y, Yang J, Mujumdar A, Dave R. Fluidized bed film coating of cohesive Geldart group C powders. Powder Technol. 2009;189(3):466–80.
48. Pandey P, Turton R, Joshi N, Hammerman E, Ergun J. Scale-up of a pan coating process. Am Assoc Pharm Sci. 2007;7(4):125–32.
49. Bertuzzi MA, Slavutsky AM. Standard and new processing techniques used in the preparation of films and coatings at the lab level and scale-up. In: García MP, Gómez-Guillén MC, López-Caballero ME, Barbosa-Cánovas GV, editors. Edible Films and Coatings: Fundamentals and Applications. Boca Raton (FL): CRC Press; 2016. p. 21–42.
50. Fabra MJ, Busolo MA, Lopez-Rubio A, Lagaron JM. Nanostructured biolayers in food packaging. Trends Food Sci Technol. 2013;31(1):79–87.
51. Sothornvit R, Olsen CW, McHugh TH, Krochta JM. Tensile properties of compression-molded whey protein sheets: determination of molding condition and glycerol-content effects and comparison with solution-cast films. J Food Eng. 2007;78(3):855–60.
52. Cunningham P, Ogale AA, Dawson PL, Acton JC. Tensile properties of soy protein isolate films produced by a thermal compaction technique. J Food Sci. 2000;65(4):668–71.
53. Du WX, Olsen CW, Avena Bustillos RJ, Friedman M, McHugh TH. Physical and antibacterial properties of edible films formulated with apple skin polyphenols. J Food Sci. 2011;76(2):149–55.
54. Jeevahan J, Chandrasekaran M. Nanoedible films for food packaging: a review. J Mater Sci. 2019;54:12290–318.
55. De Azeredo MCH. Nanocomposites for food packaging applications. Food Res Int. 2009;2:1240–53.
56. Galus S, Arik Kibar EA, Gniewosz M, Krasniewska K. Novel materials in the preparation of edible films and coatings—A review. Coatings. 2020;10(7):674.
57. Giteru SG, Cridge B, Oey I, Ali A, Altermann E. In-vitro degradation and toxicological assessment of pulsed electric fields crosslinked zein-chitosan-poly (vinyl alcohol) biopolymeric films. Food Chem Toxicol. 2020;135:111048.
58. Kumar A, Hasan M, Mangaraj S, Pravitha M, Verma DK, Srivastav PP. Trends in edible packaging films and its prospective future in food: a review. Appl Food Res. 2022;2(1):100118.