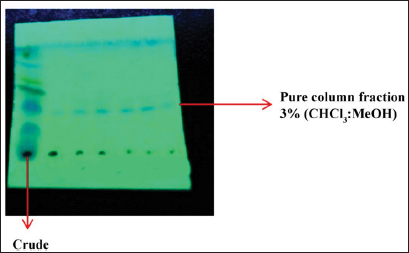
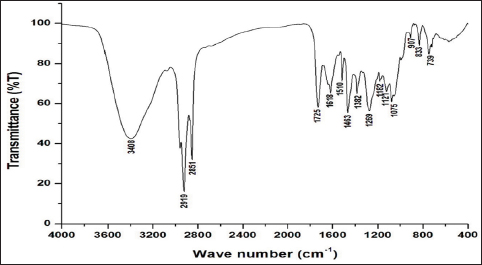
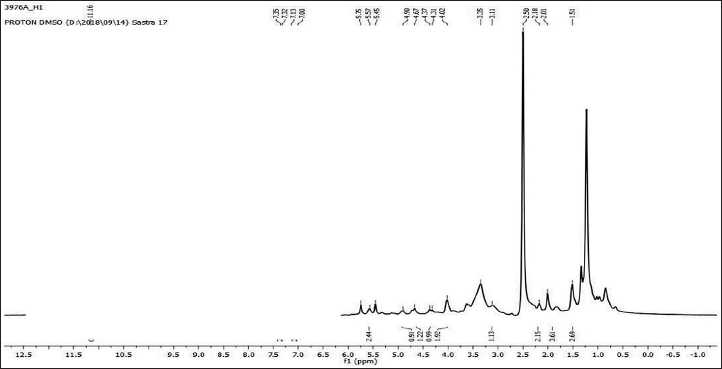
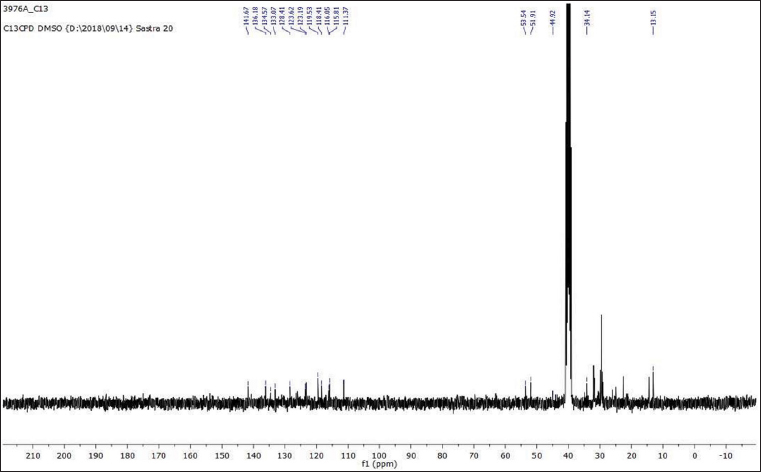
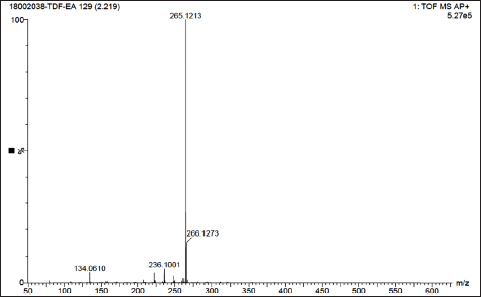
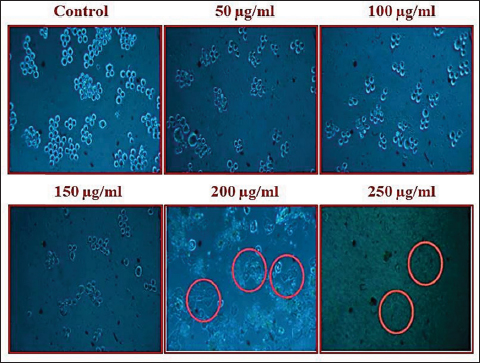
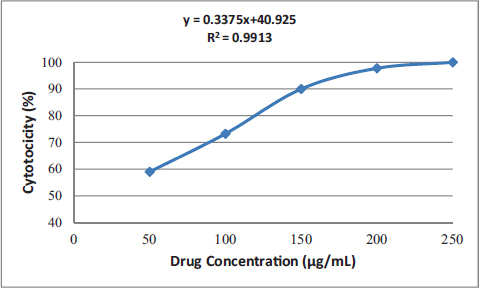
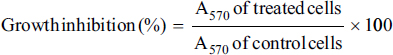Uncontrolled cell division and the spread of aberrant cells into surrounding tissues are hallmarks of the complicated disease known as cancer [1]. Retinoblastoma, which is the most common eye cancer in the world, primarily affects children under the age of 18 months, and 8000 new cases are reported each year. It is generated from the retinal tissue of the eyes [2-4]. Numerous alterations in both the environment and within organisms can lead to cancer. Most vertebrates, particularly mammals, have reduced amounts of CpG dinucleotide in their DNA. The remaining CpGs that group together in DNA areas are commonly called CpG islands (CGIs). The objective of this study was to assess gene expression analysis in cancer tissues because there has been an increasing interest in CGIs due to their enrichment in gene promoters, their ability to alter DNA methylation, and their crucial roles in controlling gene expression and silencing in biological processes such as X-chromosome inactivation, imprinting, and the silencing of intragenomic parasites. Additionally, CGIs may significantly aid in the discovery of epigenetic causes of cancer [5-8]. In 2020, as per the World Health Organization, cancer ranks as the second most common cause of death worldwide. Early detection and effective treatment must be accessible [9]. Treatments for retinoblastoma often involve radiation and chemotherapy, which can gradually harm good cells and increase their resistance to cancerous cells [3]. Current studies therefore concentrate on the possible therapeutic medication with the fewest possible adverse effects and several herbal medicines used in developed nations to address a wide range of health issues [3,10]. Plants produce a wide variety of phytochemicals and have rich sources of bioactive compounds such as alkaloids, phenolics, steroids, and terpenoids. Medicinal plants are reported as potent anthelmintic, schizonticidal, anticancer, anti-inflammatory, antioxidant, ascaricidal, antibacterial, insecticidal, anti-diarrheal, and larvicidal activities [11-13]. Tabernaemontana divaricata (Apocynaceae) is native to India and its evergreen shrub is now grown all across Southeast Asia. The phytochemical contents of the plant have been reported from the stem, root, flower, and leaves containing flavonoids, phenylpropanoids, terpenoids, enzymes, and steroids. The pharmacological properties of the plant are reported as analgesic, anti-diarrhea, antioxidant, anti-inflammatory, and reversible acetylcholinesterase inhibition effects [14-16]. The creation of novel, least-toxic drug moieties involves computational methods. Computational modeling of drugs is predicated on an understanding of the ligand and target receptor [17]. The molecular structures of the ligands can be linked to the biological activity by the application of either structure-based or ligand-based molecular design techniques. Both of these strategies rely on ligand and receptor data that are readily accessible to the general public [18]. In medicinal chemistry, computational studies are regarded as important tools that can expedite the drug-design process [19]. As far as we are aware, no scientific data exist to support the claim that T. divaricata flower extract inhibits Y79 human retinoblastoma cells. Hence, this study aimed to isolate and structurally identify the bioactive compound from ethyl acetate flower extract of T. divaricate by chromatography elucidation method, thin layer chromatography (TLC), column chromatography (CC), spectral approach of Fourier infrared (FTIR) spectroscopy, proton nuclear magnetic resonance (.H-NMR, 13C-NMR), and liquid chromatography-mass spectrometry (LC-MS). The cell viability test of the purified compound was evaluated against Y79 human retinoblastoma cells by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay.
REFERENCES
1. De Greef D, Barton EM, Sandberg EN, Croley CR, Pumarol J, Wong TL, et al. Anticancer potential of garlic and its bioactive constituents: a systematic and comprehensive review. In: Seminars in cancer biology. Academic Press; 2021. p. 219–64. (vol 73).
2. Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–31.
3. Kamarudin AA, Sayuti NH, Saad N, AbRazak NA, Esa NM. Induction of apoptosis by Eleutherinebulbosa (Mill.) Urb: bulb extracted under optimised extraction condition on human retinoblastoma cancer cells (WERI-Rb-1). J Ethnopharmacol. 2022;284:114–770.
4. Fernandes AG, Pollock BD, Rabito FA. Retinoblastoma in the United States: a 40-year incidence and survival analysis. J Pediatric Ophthalmol Strabismus. 2018;55(3):182–8.
5. Barazandeh A, Mohammadabadi MR, Ghaderi-Zefrehei M, Nezamabadipour H. Genome-wide analysis of CpG islands in some livestock genomes and their relationship with genomic features. Czech J Anim Sci. 2016;61:487.
6. Barazandeh A, Mohammadabadi MR, Ghaderi-Zefrehei M, Nezamabadipour H. Predicting CpG Islands and their relationship with genomic feature in cattle by hidden Markov model algorithm. Iran J Appl Anim Sci. 2016;6(3):571–9.
7. Mohammadabadi MR, Mozafari MR. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J Drug Deliv Sci Technol. 2018;47(1):445–53.
8. Zarrabi A, Alipoor Amro Abadi M, Khorasani S, Mohammadabadi MR, Jamshidi A, Torkaman S, et al. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules. 2020;25(3):638.
9. Imane B, Laila B, Fouzia H, Ismail G, Ahmed E, Kaoutar B, et al. Chemical characterization: antiproliferative activity and molecular docking of bioactive compounds from brown algae Fucusspiralis. Algal Res. 2022;68:102887.
10. Yadav M, Bhatia VJ, Doshi G, Shastri K. Novel techniques in herbal drug delivery systems. Int J Pharmaceut Sci Rev Res. 2014;28:83–9.
11. Mani JS, Johnson JB, Hosking H, Ashwath N, Walsh KB, Neilsen PM, et al. Antioxidative and therapeutic potential of selected Australian plants: a review. J Ethnopharmacol. 2021;268:113580.
12. Patra JK, Thatoi HN. Metabolic diversity and bioactivity screening of mangrove plants: a review. Acta Physiol Plant 2011;33:1051–61.
13. Alabi, MA, Muthusamy A, Kabekkodu SP, Adebawo OO, Satyamoorthy K. Anticancer properties of recipes derived from Nigeria and African medicinal plants on breast cancer cells in vitro. Scient Afr. 2020;8:e00446.
14. Pushpa B, Latha KP, Vaidya VP, Shruthi A, Shweatha A. Phytochemical analysis and antimicrobial evaluation of leaves extract of Tabernaemontana coronaria. J Chem Pharm Res. 2012;4:3731–3.
15. Gopinath SM, Suneetha TB, Mruganka VD, Ananda S. Evaluation of antibacterial activity of Tabernaemontana divaricata (L.) leaves against the causative organisms of bovine mastitis. Int J Res Phytochem Pharmacol. 2011;1(4):211–3.
16. Poornima K, Krishnan R, Aswathi KV, Gopalakrishnan VK. Toxicological evaluation of ethanolic extract of Tabernaemontana coronaria (L) R. Br. Asian Pac J Trop Dis. 2012;2:S679–84.
17. Correa-Basurto J, Bello M, Rosales-Hernandez MC, Hernández-Rodríguez M, Nicolás-Vázquez I, Rojo-Domínguez A, et al. QSAR, docking, dynamic simulation and quantum mechanics studies to explore the recognition properties of cholinesterase binding sites. Chem Biol Interact. 2014;209:1–13.
18. Wang Z, Cheng L, Kai Z, Wu F, Liu Z, Cai M. Molecular modeling studies of atorvastatin analogues as HMGR inhibitors using 3D-QSAR, molecular docking and molecular dynamics simulations. Bioorg Med Chem Lett. 2014;24:3869–76.
19. Singh A, Goyal S, Jamal S, Subramani B, Das M, Admane N, et al. Computational identification of novel piperidine derivatives as potential HDM2 inhibitors designed by fragment-based QSAR, molecular docking and molecular dynamics simulations. Struct Chem. 2016;27:993–1003.
20. Chethankumara GP, Nagaraj K, Krishna V, Krishnaswamy G. Isolation, characterization and in vitro cytotoxicity studies of bioactive compounds from Alseodaphne semecarpifolia Nees. Heliyon. 2021;7(6).
21. Kamal T, Muzammil A, Akintunde R, Rahma MS, Omar MN. Preliminary phytochemical screening test of Garcinia griffithii plant. Innova Ciencia. 2012;4(4):68–74.
22. Alfalluos KA, Alnade HS, Kollab WA, Alafid F, Edrah SM. Qualitative and quantitative phytochemical analysis and antimicrobial activity of “retama” extract grown in Zliten Libya. Int J Med Sci Clin Invent. 2017;4(4):2861–6.
23. Thilagavathi T, Arvindganth R, Vidhya D, Dhivya R. Preliminary phytochemical screening of different solvent mediated medicinal plant extracts evaluated. Int Res J Pharm. 2015;6(4):246–8.
24. Yunitasari N, Swasono RT, Pranowo HD, Raharjo TJ. Phytochemical screening and metabolomic approach based on Fourier transform infrared (FTIR): identification of α-amylase inhibitor metabolites in Vernonia amygdalina leaves. J Saudi Chemical Soc. 2022;26(6):101540.
25. Sadasivam S, Manickum A. Biochemical methods. New Age Intern (P) Ltd. Publication; 2005. p. 284–8.
26. Kumar T, Ray S, Brahmachary RL, Ghose M. Preliminary GC-MS analysis of compounds present in the root exudates of three mangrove species. Acta Chromatographica. 2009;21(1):117–25.
27. Pandey D, Gupta AK. Bioactive compound in Curcuma caesia (Roxb.) from Bastar and its spectral analysis by HPLC UV-visible FT-IR NMR and ESI-MS. Int J Pharma Sci Res. 2019;10:139–47.
28. Piruthiviraj P, Margret A, Krishnamurthy PP. Gold nanoparticles synthesized by Brassica oleracea (Broccoli) acting as antimicrobial agents against human pathogenic bacteria and fungi. Appl Nanosci. 2016;6:467–73.
29. Khan MSA, Ahmed N, Arifuddin M, Zakaria ZA, Al-Sanea MM, Khundmiri SUK, et al. Anti-nociceptive mechanisms of flavonoids-rich methanolic extract from Terminalia coriacea (Roxb.) Wight &Arn, leaves. Food Chemical Toxicol. 2018;115:523–31.
30. De Feo M, Paladini A, Ferri C, Carducci A, Del Pinto R, Varrassi G, et al. Anti-inflammatory and anti-nociceptive effects of cocoa: a review on future perspectives in treatment of pain. Pain Ther. 2020;9:231–40.
31. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63.
32. Ramakrishnan R, Thangaswamy S, Balasubramaian MG, Rajamanickam M, Srinivasan N, Ganesan DS. Bioactive Compounds of Schefflera stellata (Geartn) Baill. leaf methanolic extract and their cytotoxic effect on lung cancer cell line (A549). Indian J Pharmaceut Educ Res. 2022;56(3):S469–78.
33. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–9.
34. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open babel: an open chemical toolbox. J Cheminformatics. 2011;3(1):33.
35. Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics. 2012;4(1):17.
36. Halgren TA Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem. 1996;17(5 6):490–519.
37. Sanner MF, Olson AJ, Spehner JC. Reduced surface: an efficient way to compute molecular surfaces. Biopolymers. 1996;38(3):305–20.
38. Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61.
39. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.
40. Li X, Jiang H, Qu L, Yao J, Ma L, Wang X, et al. Phenolic compounds from Tabernaemontana divaricata and their anti-inflammatory activities. J Nat Prod. 2019;82(6):1671–81.
41. Ahmed M, Hossain MS, Mahal MJ, Islam MA, Hasan CM. Phytochemical investigation and bioactivities of Tabernaemontana divaricata (Linn.) leaves. J Pharm Negative Results. 2011;2(1):6–11.
42. Paulsamy S, Jeeshna MV. Preliminary phytochemistry and antimicrobial studies of an endangered medicinal herb Exacum bicolor Roxb. Res J Pharmaceut Biolog Chemic Sci. 2011;2(4):447–57.
43. Thaniarasu R, Anitha A. Phytochemical screening antioxidant activity and identification of active phytochemical compounds of ScutellariawightianaBenth.an endemic plant to peninsular India. Res J Biotechnol. 2023;18(5).
44. Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatograp A. 2005;1065(2):145–68.
45. Sultana N, Ata A, Malik A. A new flavonol glycoside from Tabernaemontana divaricata (L.) R Br J Asian Nat Prod Res. 2008;10(6):579–84.
46. Patel JK, Tripathi P, Sharma V, Chauhan NS, Dixit VK. Phospholipase C-γ1 signaling involvement in apoptosis and cell cycle arrest of retinoblastoma cells induced by alcoholic extract of Tabernaemontana divaricata. Tumor Biol. 2017;39(7):1010428317719786.
47. Prashant K, Selvakumar S, Singh SK, Singh MM. Antioxidant activity and HPTLC analysis of Tabernaemontana divaricata (L.) R Br. J Adv Pharm Technol Res. 2010;1(1):68–74.
48. Deshpande SR, Sathe SS, Salunkhe VR, Vaikos NP, Govindwar SP, Pande VV. Characterization of the major bioactive indole alkaloids from Tabernaemontana heyneana wall. Drug Test Anal. 2018;10(9):1441–7.
49. Gobinath P, Packialakshmi P, Vijayakumar K, Abdellattif MH, Shahbaaz M, Idhayadhulla A, et al. Synthesis and cytotoxic activity of novel indole derivatives and their in silico screening on spike glycoprotein of SARS-CoV-2. Front Mol Biosci. 2021;8:637989.
50. Parveen N, Khan TH, Ahamad J. Revisiting secondary metabolites from Tabernaemontana divaricata: chemical characterization and biological applications. Arabian J Chem. 2019;12(8):4189–99.
51. Rekha S, Vijayan P, Jeya A, Guruvayoorappan C. Bioactive compound apparicine isolated from Tabernaemontana divaricata induces G2/M cell cycle arrest and apoptosis in A549 cells through p53-dependent pathway. Environ Toxicol. 2018;33(1):99–113.
52. Yallappa S, Manjappa S, Dhananjaya BL, Vishwanath BS. Synthesis characterization and in vitro anticancer evaluation of novel benzofuran-isatin derivatives. Bioorg Med Chem Lett. 2016;26(4):1272–6.
53. Vijayakumar R, Arunkumar A, Muthusamy V, Senthilkumar K, Gopinath M, Ramesh Kumar A, et al. Phytochemical analysis and in vitro antimicrobial activity of Tabernaemontana divaricata (L.) R.Br. ex Roem: Schult, Flowers against human pathogens. J Acute Med. 2013;3(1):35–42.
54. Muthukrishnana J, Seifert K, Homannc KH, Lorenz MW. Inhibition of juvenile hormone biosynthesis in Gryllusbimaculatus by Glycosmis pentaphylla leaf compounds. Phytochemistry. 1999;50(2):249–54.
55. Chakravarti D, Chakravarti RN, Cohen LA, Dasgupta B, Datta S, Miller HK. Alkaloids of Glycosmis arborea—II: structure of arborine. Tetrahedron. 1961;16(1-4):224–50.
56. Paiva SR, Figueiredo MR, Aragão TV, Kaplan MA. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Memorias do Instituto Oswaldo Cruz. 2003;98:959–61.
57. Ranjithkumar R, Gokulakrishnan R, Viswanathan V, Sampathkumar P, Yuvarajan R, Rajesh R. Anticancer activity of Tabernaemontana divaricata and its bioactive compounds: in vitro and in vivo studies. Biomed Pharmaco Ther. 2019;109:346–58.
58. Hu Y, Yang W, Han H, Wang P, Zhou Y, Zhang H, et al. The indole alkaloid apparicine induces apoptosis of colon cancer cells via suppression of Akt/NF-κB signaling pathway. Front Pharmacol. 2020;11:241.
59. Kitchen DB, Decornez, H, Furr, JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3(11):935–49.
60. Lot M, Hamblin MR, Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Int J Clin Chem Diagnostic Lab Med. 2020;508:254–66.