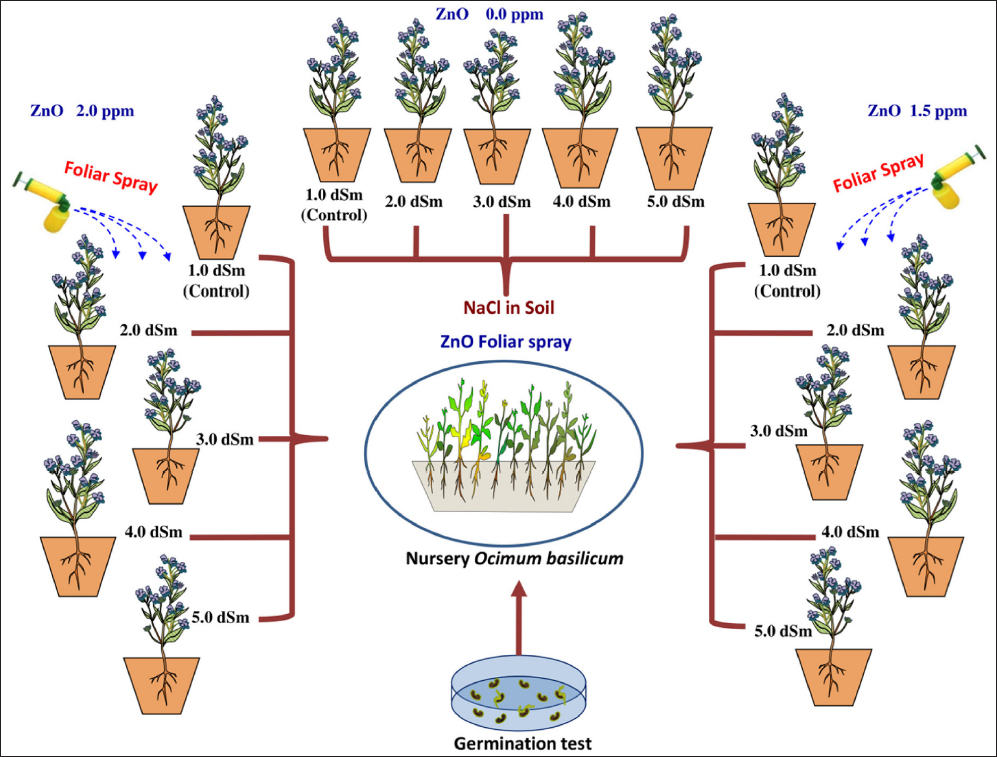
4. DISCUSSION
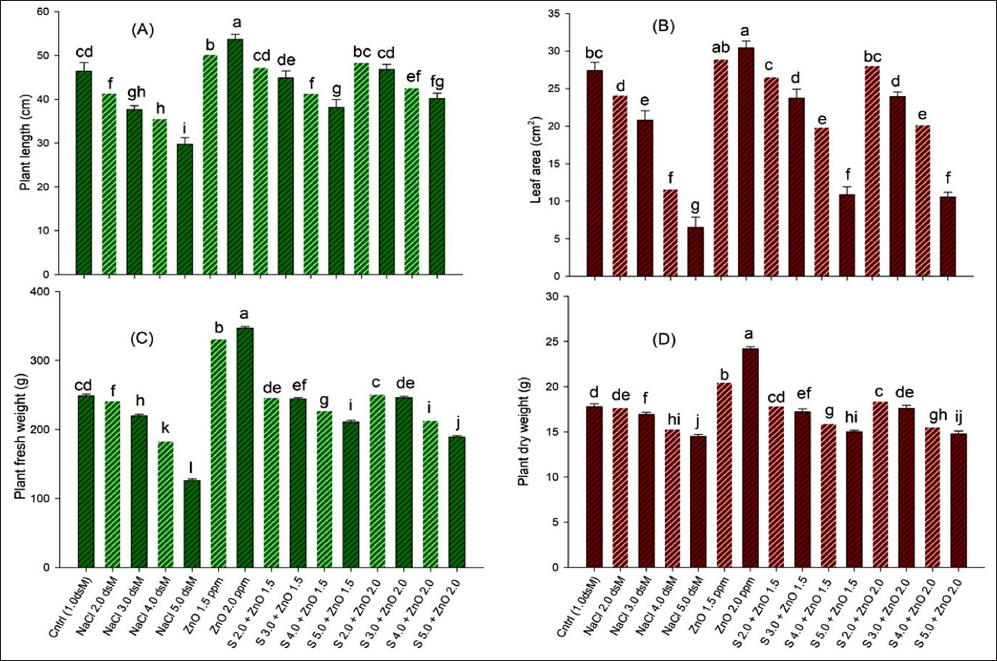
Salinity is considered a type of physiological stress that desiccates plant tissues to increase osmotic stress and check plant growth and yield [17,18,47]. However, different concentrations of soil salt have varying impacts on different crop species and varieties. Crops belonging to the glycophytic category of plants are sensitive to salt stress, and Ocimum genus plants belong to this category. Recently, various studies have been performed on plants in the Ocimum genus to evaluate the impact of salinity-induced toxicity on their growth, physiology, antioxidant system, and yield [48,49]. Metabolism perturbations and limited assimilative biochemical reactions due to salinity are reflected in the form of a loss in length, fresh and dry mass of the plant, and leaf area. The decrease in plant growth due to soil salinity is due to the inability of the plant roots to absorb water and nutrients from the root zone, mainly through Na+ accumulation in the root cells [50,51]. Salinity also causes nutrient imbalance in plants by disturbing the osmoticum [52], decreasing the rate of cell division and elongation, and ultimately reducing root and shoot length [53]. The length, fresh weight, and dry weight of plants are the outcomes of proper cell division and photosynthesis, leading to proper assimilation and accumulation of storage material ultimately resulting in proper plant growth [54]. Decreased water potential and oxidative stress by salinity could have adversely affected the enzymes of the carbon and nitrogen assimilation cycle, resulting in low root and shoot dry weight of affected plants [55-57]. A reduction in growth morphology by salt stress was also observed in different crop plants such as Solanum lycopersicum, Brassica juncea, Helianthus annuus, and O. basilicum plants [58-62].
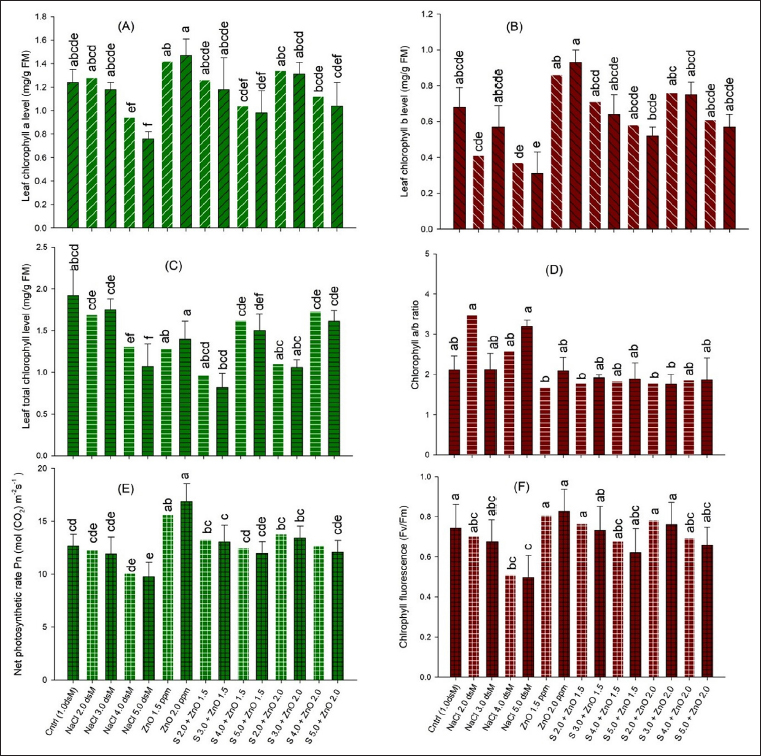
Salinity affects the cell division machinery and decreases plant photosynthesis and transpiration by closing stomata, inhibiting the genetic expression of genes involved in chlorophyll biosynthesis, and enhancing the biosynthesis of chlorophyllase enzymes by osmotic stress [63,64]. Due to osmotic stress and the ion toxicity of salinity, the decline in photosynthetic pigments in antenna molecules of thylakoids could ultimately limit the maximum quantum yield of PSII [65,66]. Further decreased leaf area due to limited turgor expansion of cells seems to result in decreased leaf photosynthetic area, causing the photosynthesis rate to be low [50,51]. Earlier studies by researchers [67-69] have shown a positive correlation between the net photosynthetic rate and chlorophyll level of leaves. Salt stress damages PSII electron transport [70], blocking electron transfer from the primary acceptor to the secondary acceptor plastoquinone (QA → QB) and leading to a decreased maximum quantum yield of PSII [66,71]. Furthermore, excess salt is taken up and accumulates Na+ ions in shoots (stem, leaves, and flowers), passing through roots and damaging roots, shoots, and leaf cells by ion toxicity, inducing lipid peroxidation, and causing electrolytic leakage in plant cells [72,73]. Furthermore, earlier studies revealed that salinity reduced K+ accumulation; however, Zn treatment enhanced K+ uptake in plants [13,27,33]. Similarly, salinity promoted Na buildup, whereas Zn treatment decreased Na concentration. The decreased biomass could be attributed to increased Na+ buildup and decreased K, Zn, Cu, and Mn concentrations [36,52,54].
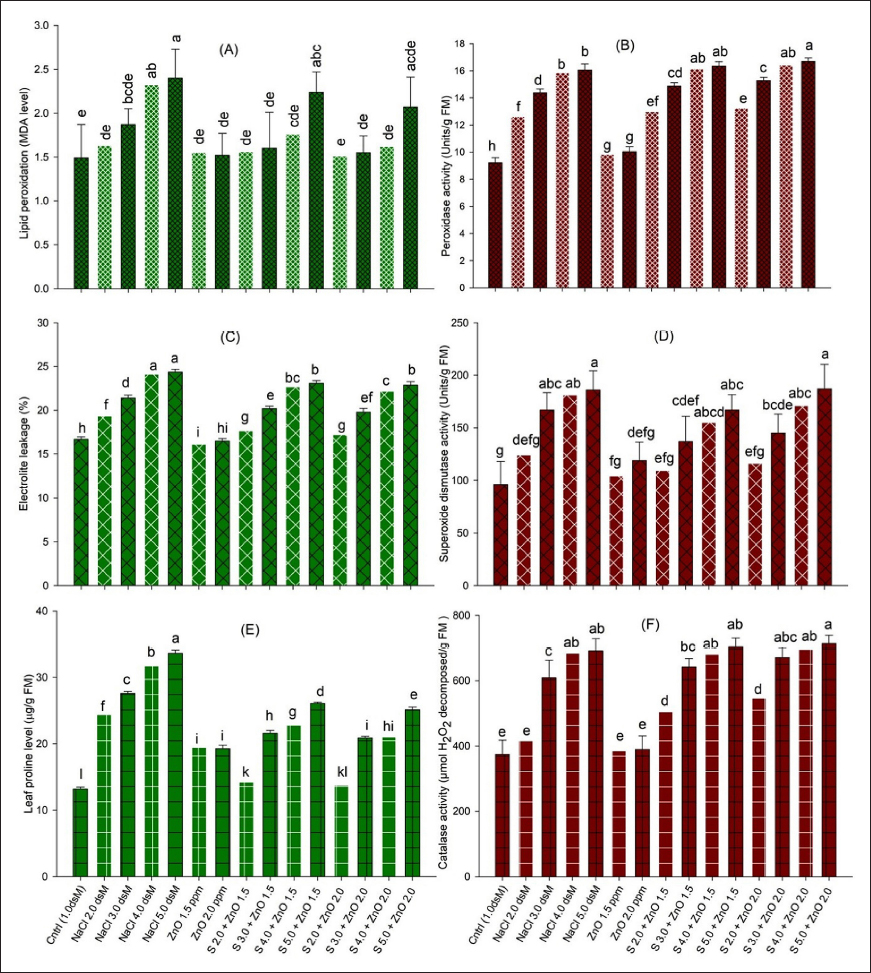
Salinity also alters plant metabolism by generating oxidative stress through the generation of excess reactive oxygen species (ROS) that trigger the antioxidant response of cytoplasmic and membranous enzymes such as CAT, POX, and SOD and the generation of molecules such as glutathione, ascorbate, and proline to counter free radicals generated in response to biotic or abiotic stresses that attack plants [74,75]. ROS in excess damages various cell organelles and molecules [76,77]. Salt-induced increases in proline levels reported in different crops [69,78,79] maintain cell osmoticum and prevent cell protein enzymes from desiccation by improving water potential [80,81]. The increased activity of the antioxidant system was suggested to provide salt tolerance or a sensitive response in plant genotypes [61,82].
Earlier studies concluded that ion accumulation and selectivity declines have been well established in wheat, sorghum, maize, barley, and rice under salt stress conditions [54,83-86]. The productivity of any plant is dependent upon the photosynthesis rate which ultimately depends upon the gaseous exchange by the stomata of the plant. Na ion accumulation in saline-stressed plants disturbs the K ion concentration in the guard cells resulting in stomatal closure that decreases productivity and ultimately low growth, dry weight, and yield [14,51,54].
In recent studies, basil plant species showed great selectivity for K+ absorption, which increased the K+/Na+ ratio in salt stress conditions [83,84]. Zn influences the structural integrity and permeability of stem cell membranes, which decreases excessive Na uptake in saline environments. Zn supplementation reduces Na+ accumulation and improves the K+/Na+ ratio of plants exposed to salinity. As a result of Zn shortage, cell membranes exhibit significant permeability or leaking of certain chemicals from the roots [87]. Zn deficiency can result in harmful ion accumulation, such as Na+ and Cl– [86].
The increased concentration of Na+ ions generated by salt stress inhibits root potassium (K+) absorption. Potassium is the most abundant cation in living cells and is required for normal root cell turgidity as well as the action of numerous enzymes [62]. Due to a paucity of K+ ions, the root cell’s growth and development were halted [85]. Excessive Na+ absorption into the root cytoplasm can inhibit the action of important enzymes. When the Na+/K+ ratio is high, it might harm the plant roots [86]. Under salt stress, basil plants had the highest Na+ concentration and the lowest K+ concentration, according to recent findings [49,62]. In earlier studies, maximum K+ levels were evaluated in ZnO-NPs-treated basil plants compared with control and salinity stress plant roots [62,87]. Plants of maize and cotton also produced comparable results [54,56]. In earlier studies, a link was found between an increase in harmful ions (Na+ and Cl–) and a decrease in the absorption of critical components required for growth, as seen by the high Na+/K+ ratio for salt-treated basil plants [62,88]. Salinity also causes a significant reduction in the fruit and seed yield of commercially important crops [14,53,54,89,90].
Zn is an essential mineral nutrient for plants that is scarcely available for plant growth in the soil [72]. Enzymes, including dehydrogenases, aldolases, isomerases, transphosphorylases, and RNA and DNA polymerases, all require zinc to function [91,92]. Moreover, it contributes to tryptophan production, cell division, membrane structure maintenance, and photosynthesis and functions as a regulatory cofactor in protein synthesis [84,92,93]. Zn is important for plant growth, but its excess causes growth inhibition in plants. Reduced growth and plant biomass, restriction of cell elongation and division, wilting, curling, and rolling of young leaves, chlorotic and necrotic leaf tips, and suppression of root growth are all symptoms of Zn-induced toxicity in plants [94].
Nanomaterials such as nanoparticles, nanobiochar, and nanofertilizers enhance the potential of plant resource use efficiency and reduce the environmental toxicity of different chemical salts [21,95]. In recent studies, various nanomaterials such as silicon (Si) nanoparticles and silicon fertilizers exhibited positive effects on the physiology and morphological traits of basil under salinity stress by increasing growth and development, chlorophyll level, and proline content in the leaves of basil under salt stress [96,97]. SiO2 NP application increased the fresh and dry weight of the leaf, chlorophyll level, and proline accumulation with increased antioxidant enzyme activity [96-98] and seedling growth under salt stress [99]. Si NPs have shown better physiological and biochemical responses under salt stress in various plants [100]. This resulted in improved photosynthesis, relative water content, photosynthetic pigments, and cell osmolites such as sugars and proline [101]. Proline content maintains cell osmoticum and excludes the toxic level of salts from the cell membrane, thus improving plant growth [102,103]. The application of Si NPs reduced MDA content (lipid peroxidation) and thus EL [101]. Exposure of onion seedlings to TiO2 NPs increased SOD activity. Seedling growth in onions was enhanced with the low-concentration application of TiO2 NPs [104]. However, the exact working mechanism of different element NPs is not yet understood in the case of desiccation stress (salt or drought).
Plants mainly uptake nutrients from the soil, and non-essential elements present in the soil hinder their uptake by overtaking the essential element channels. Therefore, the foliar spray of Zn as ZnO NPs helps provide the nutritional requirements of Zn in the plants [33]. In the agriculture sector, Zn doses proved potent to reduce salinity-induced toxicity on basil plants [62], but the foliar spray of NPs such as ZnO and other nutrients released in a controlled manner made the macromolecule delivery more selective and effective [21,88,105]. Foliar spray of ZnO NPs also enhances the expression level of genes encoding Rubisco- and chlorophyll-binding proteins, increases proline production and accumulation, and increases the antioxidant activity of plants by regulating the gene activation responsible for proline production and antioxidant activities [21,84,86,105].
Zn NPs (ZnO and ZnSO4) have been shown to promote seedling vigor, manifesting early flowering and higher leaf chlorophyll content [106-108]. ZnO NPs also effectively improved the stem and root growth and pod yield of the plants [106,109,110]. The role of different nanoparticles, including ZnO, in overcoming different abiotic stresses in plants, such as heavy metal toxicity and drought stress, has been reviewed [20,107]. In maize plants, it was suggested that ZnO and other nanoparticles mediated a reduction in salinity stress by reducing Na+ ion absorption by plant tissues, thus managing osmotic potential and Na+ toxicity [54,111,112]. Foliar spray of ZnO NPs efficiently absorbs the leaf surface inside, dilutes the toxic effects of salinity, and decreases Na+ ion accumulation by increasing the water potential and proline content, which minimizes electrolytic leakage and lipid peroxidation in plant cells [72,73]. ZnO NPs protect the photosynthetic machinery by hampering the activity of enzymes involved in the degradation of photosynthetic pigments such as chlorophyll and carotenoids [72,113]. ZnO NPs upregulate the genes that are involved in chlorophyll pigment biosynthesis, resulting in proper photosynthesis in plants [83,111]. ZnO NPs trigger the anti-oxidant activity of salt-stressed plants and help them mitigate the oxidative stress of salinity [85,86]. ZnO NPs induce the expression of genes that regulate carbon and nitrogen assimilation, resulting in improved growth and yield under saline conditions [83,105]. ZnO NP application also enhances the water uptake capability, leaf water potential, WUE, and transpiration rate in the plants grown under salt stress, maintaining the photosynthetic activity that results in greater biomass production [84].
Leaves of O. basilicum plants are the main economic product for farmers and are mainly cultivated for their leaves [87]. The essential oil constituents in basil leaves are responsible for the therapeutic properties of this plant species [114]. Under stress conditions such as salinity, the therapeutic values of these important phytochemicals decreased in the plants, making them less valuable. A study done by Ciriello et al. [87] revealed that Zn fortification helps enhance the antioxidant metabolite production in basil plants. Various studies revealed that foliar sprays of ZnO nanoparticles proved efficient in enhancing the photosynthetic activity and antioxidant and other phytochemical production-related activities in plants grown under saline soil [84]. ZnO NPs bind and activate the genes and proteins related to maintaining cell membrane leakage, lipid peroxidation, proline production, total soluble sugar content, stomatal conductance, transpiration activity, and chlorophyll production [21]. Essential oils are the key products of economically important plants such as mint, basil, mustard, soybean, sunflower, and flax [5,13,62,113,115,116]. Identification of the optimum dose or concentration of ZnO NPs foliar spray will be helpful to boost the large-scale cultivation of these plants and the oil content in their valuable parts under saline conditions [22].
Our results are in agreement with Tolay on basil, Ali et al. on barley, Rakgotho et al. on sorghum, and Singh et al. on rice exploring the impact of Zn and ZnO NPs on these plant species growing under saline conditions [62,84-86,88].
REFERENCES
1. Raven PH. Plants make our existence possible. Plants People Planet. 2021;3:2–6. [CrossRef]
2. Schaal B. Plants and people: our shared history and future. Plants People Planet. 2019;14–9. [CrossRef]
3. Shah A, Niaz A, Ullah N, Rehman A, Akhlaq M, Zakir M, et al. Comparative study of heavy metals in soil and selected medicinal plants. J Chem. 2013;621265. [CrossRef]
4. Tomar, O. Determination of some quality properties and antimicrobial activities of kombucha tea prepared with different berries. Turkish J Agri Forestry. 2023;47(2):252–62. [CrossRef]
5. Almoshari Y. Medicinal plants used for dermatological disorders among the people of the kingdom of Saudi Arabia: a narrative review. Saudi J Biol Sci. 2022;29(6):103303. [CrossRef]
6. Khair-ul-Bariyah S, Ahmed D, Ikram M. Ocimum basilicum: a review on phytochemical and pharmacological studies. Pak J Chem. 2012;2(2):78–85.
7. Celebi O, Fidan H, Iliev I, Petkova N, Dincheva I, Gandova V, et al. Chemical composition, biological activities, and surface tension properties of Melissa officinalis L. essential oil. Turkish J Agri Forestry. 2023;47(1):67–78. [CrossRef]
8. Ladwani AM, Salman M, Hameed AS. Chemical composition of Ocimum basilicum L. essential oil from different regions in the Kingdom of Saudi Arabia by using Gas chromatography mass spectrometer. J Med Plants Stud. 2018;6(1):14–9.
9. Dhama K, Sharun K, Gugjoo MB, Tiwari R, Alagawany M, Iqbal Yatoo M, et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Reviews Int. 2023;39:119–47. [CrossRef]
10. Aminian AR, Mohebbati R, Boskabady MH. The effect of Ocimum basilicum L. and its main ingredients on respiratory disorders: an experimental, preclinical, and clinical review. Front Pharmacol. 2022;12:805391. [CrossRef]
11. Teshome DT, Zharare GE, Naidoo S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front Plant Sci. 2020;11:601009. [CrossRef]
12. Torabian S, Zahedi M, Khoshgoftarmanesh A. Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J Agri Sci Tech. 2016;18(4):1013–25. http://dorl.net/dor/20.1001.1.16807073.2016.18.4.10.4
13. Torabian S, Zahedi M, Khoshgoftar AH. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J Plant Nutr. 2016;39(2):172–80. [CrossRef]
14. Stavridou E, Hastings A, Webster RJ, Robson PRH. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB-Bioenergy. 2017;9(1):92–104. [CrossRef]
15. Yadav S, Irfan M, Ahmad A, Hayat S. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 2011;32(5):667–85. PMID: 22319886
16. Adhanom OG. Salinity and sodicity hazard characterization in major irrigated areas and irrigation water sources, Northern Ethiopia. Cogent Food Agric. 2019;5(1):1673110. [CrossRef]
17. Egamberdieva D, Wirth S, Bellingrath-Kimura SD, Mishra J, Arora NK. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front Microbiol. 2019;10:2791. [CrossRef]
18. El-Sabagh A, Islam MS, Skalicky M, Ali Raza M, Singh K, Anwar Hossain M, et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: adaptation and management strategies. Front Agron. 2021;3:661932. [CrossRef]
19. Ioannou A, Gohari G, Papaphilippou P, Panahirad S, Akbari A, Dadpour MR, et al. Advanced nanomaterials in agriculture under a changing climate: the way to the future? Env Exp Bot. 2020;176:104048. [CrossRef]
20. Mittal D, Kaur G, Singh P, Yadav K, Ali SA. Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. Front Nanotechnol. 2020;2:579954. [CrossRef]
21. El-Saadony MT, Saad AM, Soliman SM, Salem HM, Desoky EM, Babalghith AO, et al. Role of nanoparticles in enhancing crop tolerance to abiotic stress: a comprehensive review. Front Plant Sci. 2022;13:946717. [CrossRef]
22. Alamdari S, Mirzaee O, Nasiri Jahroodi F, Tafreshi MJ, Ghamsari MS, Shik SS, et al. Green synthesis of multifunctional ZnO/chitosan nanocomposite film using wild Mentha pulegium extract for packaging applications. Surf Interfaces. 2022;34:102349. [CrossRef]
23. Gadewar M, Prashanth GK, Babu MR, Dileep MS, Prashanth PA, Rao S, et al. Unlocking nature’s potential: green synthesis of ZnO nanoparticles and their multifaceted applications -a concise overview. J Saudi Chem Soc. 2024;28(1):101774. [CrossRef]
24. Alamdari S, Sasani Ghamsari M, Lee C, Han W, Park HH, Tafreshi MJ, et al. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl Sci. 2020;10(10):3620. [CrossRef]
25. Elsamra RM, Masoud MS, Zidan AA, Zokm GM, Okbah MA. Green synthesis of nanostructured zinc oxide by Ocimum tenuiflorum extract: characterization, adsorption modeling, cytotoxic screening, and metal ions adsorption applications. Biomass Conversion Biorefinery. 2023;1–14. [CrossRef]
26. Mazumder JA, Khan E, Perwez M, Gupta M, Kumar S, Raza K, et al. Exposure of biosynthesized nanoscale ZnO to Brassica juncea crop plant: morphological, biochemical and molecular aspects. Sci Rep. 2020;10(1):8531. [CrossRef]
27. Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica. 2018;56:678–86. [CrossRef]
28. Pooja, Munjal R, Bhaumik J, Kaur R. Role of zinc oxide nanoparticles in mitigation of drought and salinity. Int J Curr Microbiol App Sci. 2020;9(11):467–81.
29. Al-Qurainy F, Khan S, Alansi S, Nadeem M, Alshameri A, Gaafar AR, et al. Impact of phytomediated zinc oxide nanoparticles on growth and oxidative stress response of in vitro raised shoots of Ochradenus arabicus. Biomed Res Int. 2021;2021:6829806. [CrossRef]
30. Sedghi M, Hadi M, Toluie SG. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann West Univ Timisoara Ser Biol. 2013;16(2):73–8.
31. de la Rosa G, López-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X-ray absorption spectroscopy studies. Pure Appl Chem. 2013;85:2161–74. [CrossRef]
32. Semida WM, Abdelkhalik A, Mohamed GF, Abd El-Mageed TA, Abd El-Mageed SA, Rady MM, et al. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in Eggplant (Solanum melongena L.). Plants (Basel). 2021;10(2):421. [CrossRef]
33. Faizan M, Hayat S. Effect of foliar spray of ZnO-NPs on the physiological parameters and antioxidant systems of Lycopersicon esculentum. Polish J Natu Sci. 2019;34(6):87–105.
34. El-Badri AM, Batool M, Wang C, Hashem AM, Tabl KM, Nishawy E, et al. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol Environ Saf. 2021;225:112695. [CrossRef]
35. Sarkhosh S, Kahrizi D, Darvishi E, Tourang M, Haghighi-Mood S, Vahedi P, et al. Effect of zinc oxide nanoparticles (ZnO-NPs) on seed germination characteristics in two Brassicaceae family species: Camelina sativa and Brassica napus L. J Nanomaterials. 2022;2022:1892759. [CrossRef]
36. Kolenecík M, Ernst D, Komár M, Urík M, Šebesta M, Dobrocka E, et al. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica L.) under field conditions. Nanomaterials (Basel). 2019;9(11):1559. [CrossRef]
37. Ahmed B, Syed A, Rizvi A, Shahid M, Bahkali AH, Khan MS, et al. Impact of metal-oxide nanoparticles on growth, physiology and yield of tomato (Solanum lycopersicum L.) modulated by Azotobacter salinestris strain ASM. Environ Pollut. 2021;269:116218. [CrossRef]
38. López Valencia, OM, Johansen K, Aragón Solorio BJL, Li T, Houborg R, Malbeteau Y, et al. Mapping groundwater abstractions from irrigated agriculture: big data, inverse modelling, and a satellite–model fusion approach. Hydrol Earth Syst Sci. 2020;24:5251–77. [CrossRef]
39. Alnaser ZHA, Chowdhury SR, Razzak SA. Constructed wetlands for wastewater treatment in Saudi Arabia: opportunities and sustainability. Arab J Sci Eng. 2023;48:8801–17. [CrossRef]
40. Pandey SK, Singh H. A simple, cost-effective method for leaf area estimation. J Bot. 2011;2011:658240. [CrossRef]
41. Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140(2):315–22. [CrossRef]
42. Sullivan CY, Ross WM. Selection for drought and heat resistance in grain sorghum. In: Mussel H, Staples RC, editors. Chapter 17, Stress physiology in crop plants. New York (NY): John Willy and Sons; 1979. p. 263–81.
43. Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Planta. 1991;83(3):463–8. [CrossRef]
44. Chance B, Maehly AC. Assay of catalase and peroxidase. Methods Enzymol. 1955;2:764–75. [CrossRef]
45. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–87. [CrossRef]
46. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7. [CrossRef]
47. Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006;57(5):1025–43. [CrossRef]
48. Alipour ZT. The effect of phosphorus and sulfur nanofertilizers on the growth and nutrition of Ocimum basilicum in response to salt stress. J Chem Health Risks. 2016;6(2):125–31. [CrossRef]
49. Caliskan O, Kurt D, Temizel KE, Odabas MS. Effect of salt stress and irrigation water on growth and development of sweet basil (Ocimum basilicum L.). Open Agricult. 2017;2(1):589–94. [CrossRef]
50. Arshi A, Ahmad A, Aref IM, Iqbal M. Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. J Environ Biol. 2010;31(6):939–44. PMID: 21506479
51. Khan A, Khan AA, Samreen S, Irfan M. Assessment of sodium chloride (NaCl) induced salinity on the growth and yield parameters of Cichorium Intybus L. Nat Environ Pollut Technol. 2023;22 (2):845–52. [CrossRef]
52. Stavi I, Thevs N, Priori S. Soil salinity and sodicity in drylands: a review of causes, effects, monitoring, and restoration measures. Front Environ Sci. 2021;9:712831. [CrossRef]
53. Bhatt R, Asopa PP, Sihag S, Sharma R, Kachhwaha S, Kothari SL. Comparative three-way analysis of biochemical responses in cereal and millet crops under salinity stress. J Appl Biol Biotechnol. 2015;3(06):22–8. [CrossRef]
54. Hafez EM, Osman HS, Gowayed SM, Okasha SA, Omara AE-D, Sami R, et al. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy. 2021;11:676. [CrossRef]
55. Yasseen BT, Jurjee JA, Sofajy SA. Changes in some growth processes induced by NaCl in individual leaves of two barley cultivars. Indian J Plant Physiol. 1987;30:1–6.
56. Pitann B, Schubert S, Mühling KH. Decline in leaf growth under salt stress is due to an inhibition of H+ pumping activity and increase in apoplastic pH of maize leaves. J Plant Nutr Soil Sci. 2009;172:535–43. [CrossRef]
57. Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 2009;27(1):84–93. [CrossRef]
58. Zribi L, Fatma G, Fatma R, Salwa R, Hassan N, Nejib RM. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci Hort. 2009;120:367–72. [CrossRef]
59. Hayat S, Yadav S, Wani AS, Irfan M, Ahmad A. Response of tomato to two possible modes of salinity stress – A comparative analysis. J Soil Salinity Water Quality. 2010;2(2):84–90.
60. Hayat S, Mir BA, Wani AS, Hasan SA, Irfan M, Ahmad A. Screening of salt tolerant genotypes of Brassica juncea based on photosynthetic attributes. J Plant Interact. 2011;6:53–60. [CrossRef]
61. Akram NA, Ashraf M. Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L.) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pak J Bot. 2011;43:521–30.
62. Tolay I. The impact of different Zinc (Zn) levels on growth and nutrient uptake of Basil (Ocimum basilicum L.) grown under salinity stress. PLoS One. 2021;16(2):e0246493. [CrossRef]
63. Iyengar ERR, Reddy MP. Photosynthesis in high salt tolerant plants. In: Pesserkali M, editor. Handbook of photosynthesis. Baten Rose (LA): Marshal Deker; 1996. p. 56–65.
64. Machado RMA, Serralheiro RP. Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3(2):30. [CrossRef]
65. Petropoulos SA, Levizou E, Ntatsi G, Fernandes Â, Petrotos K, Akoumianakis K, et al. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017;214:129–36. [CrossRef]
66. Mehta P, Jajoo A, Mathur S, Bharti S. Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem. 2010;48(1):16–20. [CrossRef]
67. Noreen Z, Ashraf M, Akram NA. Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci. 2010;196:273–85. [CrossRef]
68. Eisa S, Hussin S, Geissler N, Koyro HW. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Australian J Crop Sci. 2012;6(2):357–68.
69. Ahmad P, Hakeem KUR, Kumar A, Ashraf M, Akram NA. Salt induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). African J Biotech. 2012;11(11):2694–703. [CrossRef]
70. Megdiche W, Hessini K, Gharbi F, Jaleel CA, Ksouri R, Abdelly C. Photosynthesis and photosystem-2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica. 2008;46:410–9.
71. Shu S, Guo SR, Sun J, Yuan LY. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol Plant. 2012;146(3):285–96. [CrossRef]
72. Alabdallah NM, Alzahrani HS. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J Biol Sci. 2020;27(11):3132–7. [CrossRef]
73. Mazhar Z, Akhtar J, Alhodaib A, Naz T, Zafar MI, Iqbal MM, et al. Efficacy of ZnO nanoparticles in Zn fortification and partitioning of wheat and rice grains under salt stress. Sci Rep. 2023;13(1):2022. [CrossRef]
74. Pang CH, Wang BS. Oxidative stress and salt tolerance in plants. In: Lüttge U, Beyschlag W, Murata J, editors. Progress in botany. Berlin: Springer; 2008. p. 231–45.
75. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–30. [CrossRef]
76. Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot. 2002;53(372):1283–304. [CrossRef]
77. Sharma P, Jha AB, Dubey RS. Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Pessarakli M, editor. Handbook of plant and crop stress. 3rd ed. Florida: CRC Press; 2010. p. 89–138.
78. Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol. 2009;166(16):1764–74. [CrossRef]
79. Ashraf MA, Ashraf M, Ali Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: leaf lipid peroxidation and phenolic contents. Pak J Bot. 2010;42:559–66.
80. Santos MA, Camara R, Rodriguez P, Glaparols I, Torne JM. Influence of exogenous maize callus subjects to salt stress. Plant Cell Tissue Organ Cult. 1996;47:59–65.
81. Jain M, Mathur G, Koul S, Sarin NB. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep. 2001;20:463–8. [CrossRef]
82. Sabir P, Ashraf M, Akram NA. Accession variation for salt tolerance in proso millet (Panicum miliaceum L.) using leaf proline content and activities of some key antioxidant enzymes. J Agron Crop Sci. 2011;197(5):340–7. [CrossRef]
83. Adil M, Bashir S, Bashir S, Aslam Z, Ahmad N, Younas T, et al. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front Plant Sci. 2022;13:932861. [CrossRef]
84. Ali B, Saleem MH, Ali S, Shahid M, Sagir M, Tahir MB, et al. Mitigation of salinity stress in barley genotypes with variable salt tolerance by application of zinc oxide nanoparticles. Front Plant Sci. 2022;13:973782. [CrossRef]
85. Rakgotho T, Ndou N, Mulaudzi T, Iwuoha E, Mayedwa N, Ajayi RF. Green-synthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agriculture. 2022;12(5):597. [CrossRef]
86. Singh A, Sengar RS, Rajput VD, Minkina T, Singh RK. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture. 2022;12(7):1014. [CrossRef]
87. Ciriello M, Formisano L, Kyriacou M, Soteriou GA, Graziani G, De Pascale S, et al. Zinc biofortification of hydroponically grown basil: stress physiological responses and impact on antioxidant secondary metabolites of genotypic variants. Front Plant Sci. 2022;13:1049004. [CrossRef]
88. Singh A, Sengar RS, Shahi UP, Rajput VD, Minkina T, Ghazaryan KA. Prominent effects of zinc oxide nanoparticles on roots of rice (Oryza sativa L.) grown under salinity stress. Stresses. 2022;3(1):33–46. [CrossRef]
89. Amira MS, Qados A. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J Saudi Soc Agri Sci. 2011;10:7–15. [CrossRef]
90. Rahman MM, Hossain M, Hossain KFB, Sikder MT, Shammi M, Rasheduzzaman M, et al. Effects of NaCl-salinity on tomato (Lycopersicon esculentum Mill.) plants in a pot experiment. Open Agricult. 2018;3:578–85. [CrossRef]
91. Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, et al. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem. 2021;161:122–30. [CrossRef]
92. Lacerda JS, Martinez HE, Pedrosa AW, Clemente JM, Santos RH, Oliveira GL, et al. Importance of zinc for arabica coffee and its effects on the chemical composition of raw grain and beverage quality. Crop Sci. 2018;58:1360–70. [CrossRef]
93. Marschner H. Marschner’s mineral nutrition of higher plants. Academic Press; 2011.
94. Rout GR, Das P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. In: Lichtfouse, E., Navarrete M, Debaeke P, Véronique S, Alberola C, editors. Sustainable agriculture. Dordrecht: Springer Netherlands; 2009. p. 873–84.
95. Zhou P, Adeel M, Shakoor N, Guo M, Hao Y, Azeem I, et al. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: an overview. Nanomaterials (Basel). 2020;11(1):26. [CrossRef]
96. Kalteh M, Alipour ZT, Ashraf S, Marashi Aliabadi M, Falah Nosratabadi A. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J Chem Health Risks. 2014;4(3):49–55. [CrossRef]
97. Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem. 2014;33(11):2429–37. [CrossRef]
98. Haghighi M, Afifipour Z, Mozafarian M. The effect of N-Si on tomato seed germination under salinity levels. Int J Env Sci. 2012;6:87-90.
99. Sabaghnia N, Janmohammad M. Effect of nano-silicon particles application on salinity tolerance in early growth of some lentil genotypes. Ann UMCS Biol. 2015;69:39–55. [CrossRef]
100. Mukarram M, Petrik P, Mushtaq Z, Khan MMA, Gulfishan M, Lux A. Silicon nanoparticles in higher plants: uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ Pollut. 2022;310:119855. [CrossRef]
101. Mukarram M, Khan MMA, Kurjak D, Lux A, Corpas FJ. Silicon nanoparticles (SiNPs) restore photosynthesis and essential oil content by upgrading enzymatic antioxidant metabolism in lemongrass (Cymbopogon flexuosus) under salt stress. Front Plant Sci. 2023;14:1116769. [CrossRef]
102. Csonka LN, Hanson AD. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. [CrossRef]
103. Yancey PH. Compatible and counteracting solutes. In: Strange K, editor. Cellular and molecular physiology of cell volume regulation. Boca Raton (FL): CRC Press; 1994. p. 81–109.
104. Laware SL, Raskar S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int J Curr Microbiol App Sci. 2014;3(7):749–60.
105. Kumar A, Singh K, Verma P, Singh O, Panwar A, Singh T, et al. Effect of nitrogen and zinc nanofertilizer with the organic farming practices on cereal and oil seed crops. Sci Rep. 2022;12(1):6938. [CrossRef]
106. Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Minaswamy V, Raja Reddy K, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr. 2012;35(6):905–27. [CrossRef]
107. Saxena R, Tomar RS, Kumar M. Exploring nanotechnology to mitigate abiotic stress in crop plants. J Pharmaceut Sci Res. 2016;8(9):974–80.
108. Rossi L, Fedenia LN, Sharifan H, Ma X, Lombardini L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol Biochem. 2019;135:160–6. [CrossRef]
109. Awan S, Shahzadi K, Javad S, Tariq A, Ahmad A, Ilyas S. A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J Saudi Soc Agricul Sci. 2021;20(1):18–24. [CrossRef]
110. Srivastav A, Ganjewala D, Singhal RK, Rajput VD, Minkina T, Voloshina M, et al. Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants (Basel). 2021;10(12):2556. [CrossRef]
111. Fathi A, Zahedi M, Torabian S. Effect of interaction between salinity and nanoparticles (Fe.O. and ZnO) on physiological parameters of Zea mays L. J Plant Nutr. 2017;40(19):2745–55. [CrossRef]
112. Hezaveh TA, Pourakbar L, Rahmani F, Alipour H. Interactive effects of salinity and ZnO nanoparticles on physiological and molecular parameters of rapeseed (Brassica napus L.). Commun Soil Sci Plant Anal. 2019;50(6):698–715. [CrossRef]
113. Gaafar R, Diab R, Halawa M, Elshanshory A, El-Shaer A, Hamouda M. Role of zinc oxide nanoparticles in ameliorating salt tolerance in soybean. Egypt J Bot. 2020;60(3):733–47. [CrossRef]
114. Kiferle C, Ascrizzi R, Martinelli M, Gonzali S, Mariotti L, Pistelli L, et al. Effect of Iodine treatments on Ocimum basilicum L.: biofortification, phenolics production and essential oil composition. PLoS One. 2019;14(12):e0226559. [CrossRef]
115. Alamery AA, Ahmed NA. Effect of biofertilizers and zinc nano particles on growth, yield and oil percentage of sunflower (Helianthus annuus L.). Plant Archives. 2020;20(2):4648–52.
116. Khan A, Khan AA, Irfan M, Sayeed Akhtar M, Hasan SA. Lead-induced modification of growth and yield of Linum usitatissimum L. and its soil remediation potential. Int J Phytoremediation. 2023;25(8):1067-76. [CrossRef]