1. INTRODUCTION
Globally, cancer ranks as the second-leading cause of mortality. Approximately 1.8 million people were diagnosed with cancer, and around 9.6 million people lost their lives in 2018. This burden keeps increasing, and it’s expected to escalate by two-fold in another two decades (World Health Organization, 2020).
Several investigations, including in vitro cell studies, clinical trials, animal models, and cohort studies, have authentically reported that omega-3 polyunsaturated fatty acids (PUFA) hold anticancer properties against various types of cancer. Omega 3 and omega 6 endocannabinoids (such as arachidonyl ethanolamide, docosahexaenoyl ethanolamide [DHEA], and eicosapentaenoyl ethanolamide [EPEA]) acting as agonists activate cannabinoid and other receptors, resulting in several signaling cascades. These signals are known to intervene in almost all-important stages of cancer development and establish anticancer effects by preventing the commencement of tumors, inhibiting and suppressing the rapid growth of tumors, and restraining the metastasis [1]. The antagonist effect of endocannabinoids was antiproliferative (inhibition of cancer cell growth) and was attained by either a combined process of autophagy [2], apoptosis, or arresting the cell cycle. Normally, both autophagy and cell cycle arrest will end with the induction of apoptosis (programmed cell death). Endocannabinoids were reported to exhibit anti-angiogenesis (preventing the formation of new blood vessels), anti-invasive, and anti-metastatic (inhibiting metastasis) properties [1]. These endocannabinoids do not elicit autophagy or pro-apoptotic activity in normal cells [2], which authenticates the use of omega-3 PUFA and their derived endocannabinoids as preventive and therapeutic agents as well as adjuvants in cancer therapy. Several studies have suggested that DHEA, EPEA, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) possess adjuvant potency in breast cancer therapy [3-5]. An in vitro study using Michigan Cancer Foundation-7 (MCF-7) breast cancer cell lines has demonstrated the ability of DHEA, EPEA, DHA, and EPA to elicit the expression of the PRARγ receptor and attenuate the AKI-mTOR pathway, resulting in inhibiting the proliferation of cancer cells by promoting autophagy [2]. Regular dosages of dietary DHA (120 mg) and EPA (180 mg) during chemotherapy in breast cancer patients have increased the antioxidant level, superoxide dismutase, glutathione reductase, and catalase activity in the patients’ blood profiles. Thus, PUFA acts as an adjuvant in battling post-effect chemotherapy [5].
Therefore, the in vitro anticancer potency of M. elongata lipids containing both omega 6 and omega 3 essential fatty acids such as gamma-linolenic acid (GLA), arachidonic acid (ARA), EPA, and DHA was investigated against MCF-7 cells.
2. MATERIALS AND METHODS
2.1. Isolation of Oleaginous Fungi
The oleaginous fungal isolate, Mortierella elongata (accession no. 402027), was isolated from the terrestrial soil of Nilgiris Hill, Western Ghats of Tamil Nadu, at 11.4007°N and 76.7358°E. In our earlier study, the total lipids were extracted using a low-toxicity solvent system with 3:2 v/v of n-hexane and isopropyl alcohol. The extracted total lipids were esterified, and fatty acid profiles were determined. The oleaginous fungus M. elongata was found to be a potential source of PUFA and was noted to produce the most essential fatty acids like omega 6 and omega 3, such as 0.79% of GLA, 1.24% of ARA, 1.24% of EPA, and 6.83% of DHA.
2.2. Anti-cancer Study
2.2.1. Cell culture
Human embryonic kidney cells (HEK) and human breast cancer cells (MCF-7) were procured from the National Centre for Cell Sciences, Pune, India. HEK and MCF-7 cells were seeded separately in T-25 flasks containing Dulbecco’s Modified Eagle’s Medium (DMEM) augmented with Balanced Salt Solution, l-glutamine (2 mM), and made up to contain 4-(2-hydroxyethyl)-piperazine ethane sulfonic acid (HEPES, 10 mM), sodium pyruvate (1 mM), fetal bovine serum (10% FBS, GIBCO, USA), and non-essential amino acids. Antibiotics such as penicillin and streptomycin of concentration (100 IU/100 μg) adjusted to 1 mL/l were used. Cells were incubated at 37°C in a 5% CO2 humidified incubator.
2.2.2. Evaluation of cytotoxicity
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Hi-Media) analysis was carried out to evaluate the inhibitory concentration (IC50). Cells of density 1 × 104 cells/well were cultured in a 96-well plate and incubated for 48 h to attain 80% confluence. The spent medium was replaced with fresh medium without disturbing the monolayer cells. The cells were treated with various concentrations of M. elongata samples and incubated for 48 h at 37°C. After incubation, the spent medium was again removed with intense care without disturbing the adhered cells. An aliquot of 100 μL of DMEM and 100 μL of MTT dye were added to each well and allowed to be incubated for 4 h at 37°C. After the treatment, the supernatant was removed. To make formazan crystals soluble, 50 μL of DMSO was added and incubated for 10 min. Cells without treatment were taken as controls. The ELIZA multi-well plate reader (ROBONIK, India) was utilized to determine the optical density at 620 nm. The percentage viability of cells was calculated with the obtained OD value using the formula:
% of viability=[(OD value of exprimental sample)/(OD value of exprimental control)]*100
2.2.3. Morphological study
The opted breast cancer cells of density 1 × 105 cells/coverslip were fixed on the coverslip with 3:1 v/v of ethanol a acetic acid. Subsequently, the cells were treated with different concentrations of the test sample based on the IC50 values. Then the coverslips were mounted on the glass slide to determine the morphology of the treated and untreated cells. The morphological changes of the three monolayer cells were visualized and micro-graphed using a Nikon bright field microscope (Japan) at 20× magnification.
2.2.4. Analysis of apoptotic cell death by fluorescence microscopy
A nuclear staining dye blend was prepared by adding an equal proportion of 100 mg/mL of Acridine Orange (AO) and 100 mg/mL of Ethidium Bromide (EtBr) in deionized water. An aliquot of 1 μL of blended dye was mixed with 0.9 mL of cell suspension at a density of 1 × 105 cells/mL grown on the coverslip attached to a 6-well culture plate [6]. The cancer cells treated with various concentrations of M. elongata sample were processed by washing with PBS. Immediately, the cells were stained with 10 μL of AO/EtBr and incubated for 3 min. Subsequently, the cells were again washed with PBS and visualized under a fluorescence microscope (Nikon Eclipse, Inc., Japan) with an excitation filter of 480 nm. Besides, the cells were placed on a glass coverslip in six-well culture plates and treated with different concentrations of M. elongata samples for 24 h. After treatment, the cells were fixed with methanol and acetic acid (3:1) v/v. The fixed cells were washed with PBS and subsequently stained with 10 μL of 4,6-diamidino-2-phenylindole (DAPI) and incubated for 20 min in the dark. After incubation, the stained cells were observed under a fluorescence microscope (Nikon Eclipse, Inc., Japan) with an excitation filter of 480 nm [7]. The MCF-7 cells were placed on a glass coverslip in six-well culture plates and treated with different concentrations of M. elongata samples. After 24 h of treatment, the cells were washed 3 times with PBS. Then the cells were stained with 50 μL of propidium iodide (PI) at a concentration of 5 mg/mL and incubated for 10 min. After incubation, the stained cells were mounted and observed under a fluorescence microscope (Nikon Eclipse, Inc., Japan) with an excitation filter of 480 nm [8].
2.2.5. Determination of mitochondrial membrane potential (MMP)
The MCF-7 cells were placed on a glass coverslip in a six-well culture plate and treated with different concentrations of M. elongata samples. After 24 h of treatment, the cells were washed 3 times with PBS. Then the cells were stained with 2 μM of JC-1 dye and incubated at 37°C at 5% CO2 for 15–30 min. After incubation, the stained cells were washed and observed under a fluorescence microscope (Nikon Eclipse, Inc., Japan) [9].
2.2.6. Detection of apoptosis by annexin-V/fluorescein isothiocyanate (FITC) flow cytometer
MCF-7 cells at a density of 1 × 105 cells/ml were grown in a six-well culture plate. After 24 h of incubation in a 5% CO2 atmospheric incubator at 37°C, MCF-7 cells were treated with different concentrations of M. elongata samples for 24 h. Cells without treatment are considered control. After 24 h of treatment, the cells were harvested using trypsin, washed twice, and immediately stained using annexin V-FITC and PI. It was then incubated in the dark for 16 min at RT. Subsequently, binding buffer was added, and flow cytometry was carried out in duplicate with the BD FACS verse flow cytometer. Around 10,000 events were collected for all the experimental samples and controls. Fluorescent signal intensity was recorded and determined by Cell Quest and Modifit.
2.2.7. Determination of cell cycle
MCF7 cells at a density of 1 × 105 cells/ml were cultured in a six-well cell culture plate for 24 h at 37°C in a 5% CO2 atmospheric incubator. The cells were then treated with different concentrations of M. elongata samples. The untreated cells were considered a control. The treatment was then carried out for 24 h of incubation. After treatment, trypsin was used to harvest the cells, and 70% ethanol was used for fixation. Post-fixation, the cells were incubated at −20°C for 1 h. Subsequently, the cells were suspended in 0.5 mL of PBS supplemented with 50 μg/mL of PI and 100 μg/mL RNase. It is once again incubated for 30 min. A BD-FACS flow cytometer was used to analyze the cell cycle. The flow cytometer was done in duplicate. Around 10,000 events were collected, and fluorescent signals were recorded and determined by cell quest and modifit.
2.2.8. Reactive oxygen species assay
MCF-7 cells at a cell density of 1 × 105 cells/mL were seeded and grown in a six-well culture plate for 24 h at 37°C in a 5% CO2 atmospheric incubator. After 24 h of incubation, the spent medium was replaced with fresh medium containing DMSO (0.25%, v/v) and different concentrations of M. elongata samples. It is then incubated for 6 h. Then, the cells were stained with 10 μM of DCFH-DA (2,7-dichlorofluorescein diacetate, Hi-Media). It was further incubated for 20 min at 37°C. The cells were then harvested and washed with medium without serum. The production of ROS was measured by flow cytometry for all the samples.
2.2.9. Statistics
All the in vitro cell culture studies were done in triplicate, and the statistical analysis was performed by SPSS software version 17.0. P < 0.05 was considered significant.
3. RESULTS AND DISCUSSION
The isolate, M. elongata (Accession No. OK402027), was isolated from the terrestrial soil of Nilgiris Hill, Western Ghats of Tamil Nadu, and was found to be a potent PUFA-producing oleaginous fungus. Several studies have reported the accumulation of PUFAs in Mortierella species, with special attention to M. elongata [10-12], as well as the anti-cancer activity of PUFAs such as ARA, gamma-linolenic acid, and EPA [13-18]. Several authors have specifically reported the anti-cancerous activity of PUFAs produced from Mortierella spp. [19-21].
3.1. Biocompatibility and Cytotoxic Effect of M. elongata Lipid
The biocompatibility and cytotoxic effects of the PUFA-enriched lipids extracted from M. elongata were investigated on normal HEK and MCF-7, respectively. The HEK cells were treated with various concentrations of total lipids. The MTT assay showed that M. elongata lipids had no cytotoxic effect on normal HEK cells. Thus, lipids from M. elongata were found to be compatible with human cell lines. The European Food Safety Authority (EFSA, 2008) has reported the safety of fungal oil from Mortierella spp.; it is not known to produce mycotoxin and is not pathogenic to mankind [22]. The cancerous cell line, MCF-7 cells, was treated with different concentrations ranging from 20, 40, 60, 80, and 100 μg/mL of M. elongata lipids and the reference drug, doxorubicin (Dox). The MTT assay indicated a significant and concentration-dependent cytotoxic effect against MCF-7 cells. The percentage inhibition of cell proliferation of the reference drug and lipids of M. elongata is graphically represented [Figure 1]. Based on the dose-dependent curve, the IC50 values of Dox and M. elongata lipids at 24 h were found to be 18 ± 0.4 and 28 ± 1.3 μg/mL, respectively. The National Cancer Institute, US, has recommended that the cytotoxicity of a compound be highly significant if the IC50 value is <20 μg/mL. Several studies have reported the anti-proliferative activity of PUFA-rich oil from various sources [23,24]. Palakurthi et al., have evaluated the IC50 value of EPA on 15 various cancer cell lines and reported 43.3 ± 3.6 μM against MCF-7 cells [25], and So et al., have reported the IC50 of DHA and EPA against LA-N-1 cells at 48 h to be 18 ± 1 μM and 35 ± 2 μM [26], which were found to be in line with the present study. Therefore, the following concentrations of 25, 50, and 100 μg/mL of M. elongata lipids were chosen for the experimental studies.
 | Figure 1: Anti-Proliferative activity of Mortierella elongata lipids on Michigan Cancer Foundation-7 cells. [Click here to view] |
3.2. The Morphological Variations of MCF-7
The anti-proliferative activity was further examined for morphological changes in the treated and untreated MCF-7 cells using an inverted light microscope. The most common variations, like cell detachment, cell shrinkage, and circular-shaped apoptotic bodies, were detected [Figure 2]. When compared to control cells, a decrease in the number of cells and an increase in the number of apoptotic bodies were observed in treated cells in a dose-dependent manner. The maximum number of apoptotic bodies were found in cells treated with 100 μg/mL of M. elongata lipids. Thus, the presence of these morphological changes confirmed the apoptotic activity of lipids from M. elongata. Similar morphological features of apoptosis were induced by Thamnidium elegans and Nannochloropsis salina lipid-derived salts against MCF-7 cells, as reported by Sayegh et al. [19].
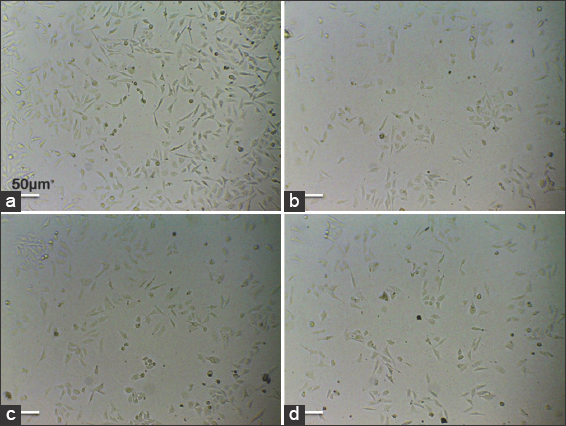 | Figure 2: Effects of Mortierella elongata lipids on cell morphology. The Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, and (c). 50 μg/ml, (d) 100 μg/mL. [Click here to view] |
3.3. Analysis of Apoptotic Cell Death by AO/EtBr, DAPI, and PI
AO/EtBr, a fluorescent staining technique, was performed to distinguish the live, apoptotic, and necrotic cells in MCF-7 cells treated with M. elongata lipids. The fluorescence micrograph of AO/EtBr-stained and untreated cells is represented in Figure 3. The untreated MCF-7 cells were largely and uniformly stained green in color. Among the treated cells, significant and adverse changes were observed in a dose-dependent manner. Based on the stages of apoptosis, the cells were found to get stained in a varied color pattern. The cells in the initial stages of apoptosis were stained pale green in color, and the cells in the later stages of apoptosis were found to get stained ununiformly with both pale orange and very mild green color [Figure 3b and c]. The necrotic cells were completely stained orange in color, which was observed in cells treated with 100 μg/mL of M. elongata lipids [Figure 3d]. This staining is evidence of apoptosis and is in line with the work reported by Chatterjee et al. [27] and Roy et al., [28], where the AO/EtBr staining of the ER + MCF-7 cells revealed the presence of apoptotic signals that were induced by the treatment with alpha-linolenic acid.
 | Figure 3: Induction of apoptosis by Mortierella elongata Lipids. Acridine orange/ethidium bromide staining of Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, and (c) 50 μg/mL, (d) 100 μg/mL. [Click here to view] |
DAPI, a nuclear-specific fluorescent staining technique, was carried out to elucidate the nuclear morphology of both untreated and 24-h-treated MCF-7 cells. Figure 4 represents the micrograph of DAPI-stained control and treated cells of MCF-7 cells. The untreated cells indicated a weak blue fluorescence with an intact nucleus. The treated cells have indicated a strong blue fluorescence with an increased number of nuclear fragmentations with irregular edges around the nucleus. The intensity of fluorescence was found to be higher in cells treated with 100 μg/mL of M. elongata lipids [Figure 4d]. This DAPI staining result is similar to the activity of leishmanial lipid (pLLD) on the growth of leukemic cell lines [27] and oleic acid against hepatocellular carcinoma [29].
 | Figure 4: Induction of apoptosis by Mortierella elongata Lipids. DAPI staining of Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, and (c) 50 μg/mL, (d) 100 μg/mL. [Click here to view] |
PI, a DNA-specific fluorescent staining technique, was performed to elucidate the nuclear morphology of apoptotic cells. Figure 5 represents the micrograph of PI-stained control and treated cells of MCF-7 cells. The untreated cells showed a weak red fluorescence with an intact nucleus. The treated cells showed a strong red fluorescence with an increased number of nuclear fragmentations with irregular edges around the nucleus. The intensity of fluorescence was found to be higher in cells treated with 100 μg/mL of M. elongata lipids [Figure 5d]. Thus, the apoptotic morphological changes elucidated by PI staining in this study are similar to the results observed in THP-I cells (Human Monocyte Leukemia cells) treated with oxidized DHA [30] and MCF-7 cells treated with punicic acid, an omega-5 fatty acid [31].
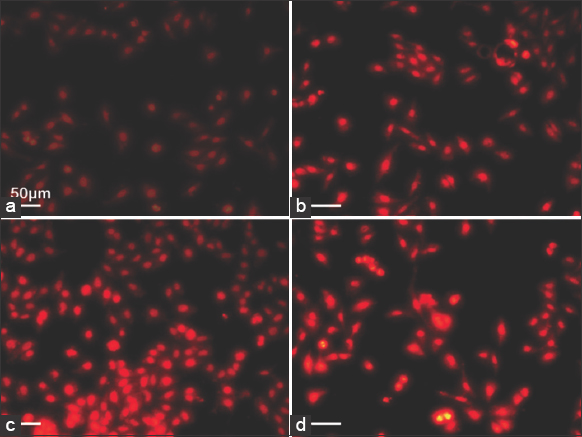 | Figure 5: Induction of apoptosis by Mortierella elongata lipids. Propidium iodide Staining of Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, (c) 50 μg/mL, and (d) 100 μg/mL. [Click here to view] |
3.4. Determination of MMP
The JC-1 stain enters and accumulates in the mitochondria of healthy cells and forms J aggregates immediately due to energized and negatively charged mitochondria. In the case of apoptotic cells, the J aggregates are not formed due to a loss of electrochemical potential, and hence the JC-1 stain remains green in color. Figure 6 represents the assessment of mitochondrial depolarization using JC-1 dye. The control cells showed strong green-colored fluorescence [Figure 6a]. The treated cells revealed a weak fluorescence [Figure 6d]. A decrease in the green (530 nm) fluorescence intensity by exposure to lipids from M. elongata indicates the depolarization or disruption of the mitochondrial membrane. Thus, JC-1 acts as an indicator of MMP and indicates the loss of membrane potential after treatment with M. elongata lipids. This mitochondrial dysfunction by M. elongata is in line with the activity of PUFA-rich lipids from various sources [27,28].
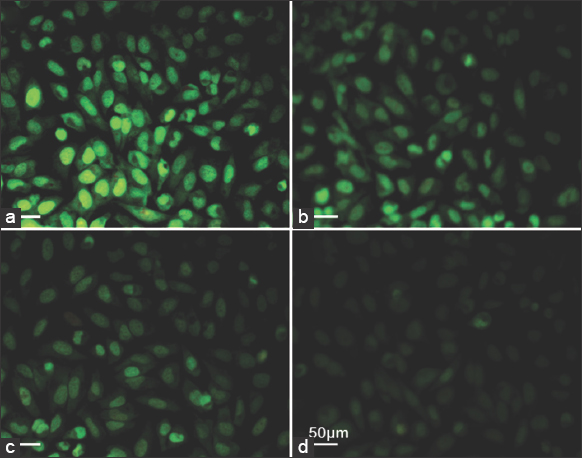 | Figure 6: Effects of Mortierella elongata lipids on mitochondrial membrane potential. The Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, (c) 50 μg/mL, and (d) 100 μg/mL. [Click here to view] |
3.5. Quantification of Apoptotic Stages by Annexin V-FITC
The mode of cell death by apoptosis was further confirmed, and their apoptotic phases were quantified by flow cytometric assay using Annexin V-FITC and the PI staining method. The flow cytometric results are represented in Figure 7. The treatment of different doses of lipids from M. elongata on MCF-7 cancer cells for 24 h has resulted in a significant decrease in the percentage of live cells, while the percentage of apoptotic and necrotic cells was found to be increased when compared to the untreated cells. The MCF-7 cells treated with 25 μg/mL of M. elongata lipids indicated 31.73% of apoptotic cells and 5.98% of necrotic cells. Treatment with 50 μg/mL has resulted in 43.60% and 13.10% of apoptotic and necrotic cells, whereas 100 μg/mL has resulted in 57.36% of apoptotic and 14.10% of necrotic cells. Thus, the antiproliferative effect of M. elongata lipids was found to be dose-dependent. The induction of apoptosis by M. elongata lipids is consistent with the previous reports, which revealed the anti-cancer potency of PUFA-rich lipids from different sources against various cancer cell lines. Several researchers have quantified the apoptotic cells induced by the treatment with PUFA using annexin V-FITC and PI stains, and their results were in line with the present findings [26,32,33].
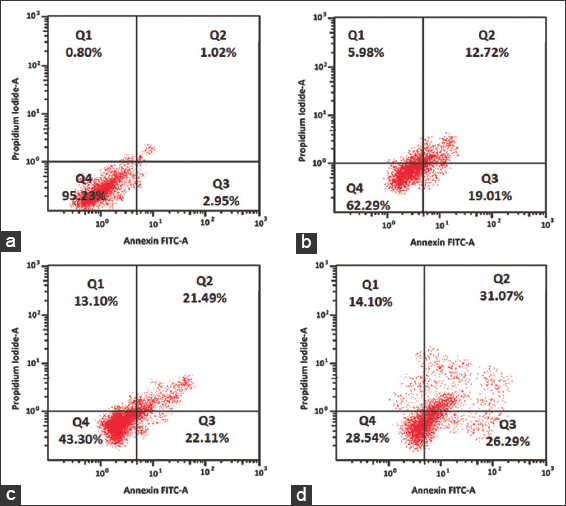 | Figure 7: Analysis of apoptosis through Annexin V –fluorescein isothiocyanate flow cytometry. Michigan Cancer Foundation-7 cells treated with various concentration of Mortierella elongata lipids for 24 h. (a) untreated, (b) 25 μg/mL, (c) 50 μg/mL, and (d) 100 μg/mL. [Click here to view] |
3.6. Effect of M. elongata Lipids on Cell Cycle Regulation
The involvement of PUFA-rich lipids of M. elongata in arresting the cell cycle progression was evaluated by flow cytometry using PI stain. The flow cytometric results are represented in Figure 8. The percentage of cell population in MCF-7 treated with different concentrations of M. elongata lipids was found to accumulate more in the G1 phase and decrease in the M phase when compared to the control [Figure 9]. The cell population in the G1 phase was found to be 40.11% in 25 μg/mL of treated cells, 45.11% in 50 μg/mL of treated cells, and 54.49% in 100 μg/mL of treated cells. The percentage of cell population in the G1 phase increases with respect to the increase in concentration of lipids. The percentage of cells in S phase was found to be slightly increased in 25 and 50 μg/mL treated cells in comparison with control, whereas S phase was significantly decreased to 18.59% in the cells treated with 100 μg/mL. Thus, the flow cytometric method suggested that M. elongata lipids trigger the G1 cell cycle arrest in a concentration-dependent manner, accompanied by a decrease in the percentage of cells at the S phase. Several reports have revealed that PUFA-rich lipids from varied sources could deploy their anti-cancer activity against various cancer cell lines through triggering cell cycle arrest at the G1 phase in a concentration- and time-dependent manner [25-27]. EPA and DHA were known to down-regulate the levels of cyclin-dependent kinase-2, cyclin E, cyclin D1, and cyclin G1 proteins in a concentration-dependent manner, which could possibly be attributed to their ability to trigger the cell cycle arrest at the G1 phase [25,26].
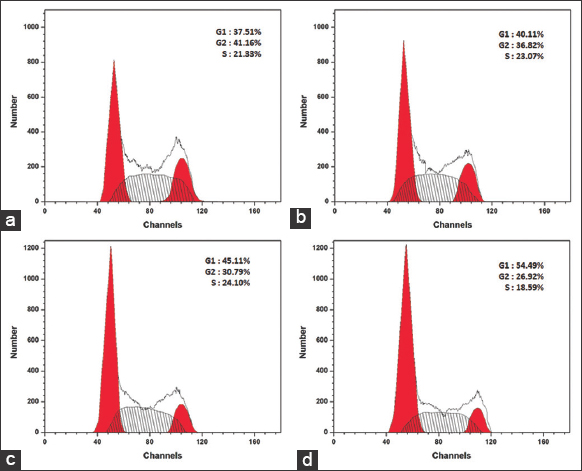 | Figure 8: Cell cycle analysis by flow cytometry. The Michigan Cancer Foundation-7 cells treated with various concentration of Mortierella elongata lipids treated for 24 h. (a). untreated, (b) 25 μg/mL, (c) 50 μg/mL, and (d) 100μg/mL. [Click here to view] |
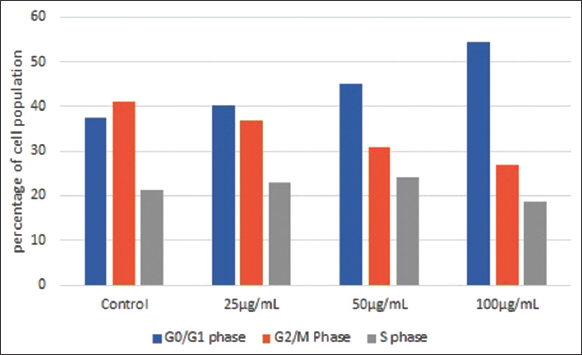 | Figure 9: Effect of Mortierella elongata lipids on cell cycle progression. [Click here to view] |
3.7. Effect of M. Elongata Lipids on the Generation of ROS
ROS are well known for their involvement in apoptosis-mediated cell death. Therefore, the production of ROS was determined in the MCF-7 cells treated with three different concentrations of M. elongata lipids for 24 h. The non-fluorescent dye DCF-DA got oxidized in the presence of ROS and resulted in the formation of DCF, a fluorescent molecule that was measured at 530 nm. The generation of intracellular ROS was determined by flow cytometry. The histogram of cytometric analysis is represented in Figure 10, which indicates the significant difference in the levels of intracellular ROS generation in MCF-7 cells treated with M. elongata lipids compared to the untreated cells. A gradual increase in ROS generation in the treated MCF-7 cells was observed with an increase in the concentration of M. elongata lipids. The percentage of DCF-positive cells in the MCF-7-treated cells was 32.45, 38.75, and 45.68% at 25, 50, and 100 μg/mL concentrations, respectively, which was significantly higher than the control with 21.76%. The effect of M. elongata lipids on ROS generation was found to be dose-dependent. The increased generation of ROS in cancer cells mediates apoptosis, and the results were in line with previous studies [27,34,35]. Oono et al., (2020) revealed the anticancer effect of EPA on PC3 cells through increased production of ROS [35], and Chatterjee et al., (2015) have demonstrated that pLLD induces apoptosis via increased generation of ROS by regulating the MAPK pathway [27]. Thus, flow cytometric analysis using DCF-DA has confirmed the anti-cancerous activity of M. elongata lipids via apoptosis.
 | Figure 10: Effect of Mortierella elongata lipids on reactive oxygen species generation. The Michigan Cancer Foundation-7 cells treated with various concentration of M. elongata lipids treated for 24 h. (a) untreated, (b) 25 μg/mL, (c) 50 μg/mL, and (d) 100 μg/mL. [Click here to view] |
4. CONCLUSION
The present study has clearly revealed the anti-proliferative potency of M. elongata lipids against MCF-7 cells. The anti-proliferative activity was found to be associated with the induction of apoptosis and cell cycle arrest, along with other hallmarks of apoptosis such as increased generation of ROS and loss of MMP. The anticancer activity of M. elongata lipids was found to be more significant in a concentration-dependent manner. The anti-proliferative activity of the sample was directly proportional to its concentration. Thus, M. elongata lipids inhibit proliferation through apoptosis and cell cycle arrest at the G1 phase. This in vitro anti-cancer study could be further taken for in vivo studies in the future.
5. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
6. FUNDING
There is no funding to report.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Brown I, Cascio MG, Rotondo D, Pertwee RG, Heys SD, Wahle KW. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog Lipid Res 2013;52:80-109. [CrossRef]
2. Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARg activation in MCF 7 breast cancer cells. J Cell Physiol 2013;228:1314-22. [CrossRef]
3. Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, et al. Cannabinoid receptor-dependent and-independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and-negative prostate cancer cell lines. Carcinogenesis 2010;31:1584-91. [CrossRef]
4. Brown I, Lee J, Sneddon AA, Cascio MG, Pertwee RG, Wahle KW, et al. Anticancer effects of n-3 EPA and DHA and their endocannabinoid derivatives on breast cancer cell growth and invasion. Prostaglandins Leukot Essent Fatty Acids 2020;156:102024. [CrossRef]
5. Mansara P, Ketkar M, Deshpande R, Chaudhary A, Shinde K, Kaul-Ghanekar R. Improved antioxidant status by omega-3 fatty acid supplementation in breast cancer patients undergoing chemotherapy:A case series. J Med Case Rep 2015;9:148. [CrossRef]
6. Thangam R, Suresh V, Rajkumar M, Vincent JD, Gunasekaran P, Anbazhagan C, et al. Antioxidant and in vitro anticancer effect of 2-pyrrolidinone rich fraction of Brassica oleracea var. capitata through induction of apoptosis in human cancer cells. Phytother Res 2013;27:1664-70. [CrossRef]
7. Saengkhae C, Premsuriya Y, Srivibool R, Praiboon J. Sensitization of human carcinoma of nasopharynx cells to doxorubicin and induction of apoptosis by Sargassum baccularia lipophilic fraction. Walailak J Sci Technol 2015;12:515-25.
8. Al-Assaf AH. Chemopreventive effect of corosolic acid in human hepatocellular carcinoma cells. Afr J Biotechnol 2013;12:2733-42.
9. Sivandzade F, Bhalerao A, Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc 2019;9:e3128. [CrossRef]
10. Cheng MH, Walker TH, Hulbert GJ, Raman DR. Fungal production of eicosapentaenoic and arachidonic acids from industrial waste streams and crude soybean oil. Bioresour Technol 1999;67:101-10. [CrossRef]
11. Stredansky M, Conti E, Salaris A. Production of polyunsaturated fatty acids by Pythium ultimum in solid-state cultivation. Enzyme Microb Technol 2020;26:304-7. [CrossRef]
12. Deshpande S, Patil T, Alone S, Duragkar N. Microbial conversion of plant based polyunsaturated fatty acid (PUFA) to long chain pufa and its identification by gas chromatography. J Biotechnol Biomater 2013;S13:6. [CrossRef]
13. Das UN. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett 1991;56:235-43. [CrossRef]
14. Sagar PS, Das UN. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins Leukot Essent Fatty Acids 1995;53:287-99. [CrossRef]
15. Ramesh G, Das UN. Effect of cis-unsaturated fatty acids on Meth-A ascitic tumour cells in vitro and in vivo. Cancer Lett 1998;123:207-14. [CrossRef]
16. Das UN, Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis 2011;10:159. [CrossRef]
17. Dai J, Shen J, Pan W, Shen S, Das UN. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis 2013;12:71. [CrossRef]
18. Meng H, Liu Y, Zhai Y, Lai L. Optimization of 5-hydroxytryptamines as dual function inhibitors targeting phospholipase A2 and leukotriene A4 hydrolase. Eur J Med Chem 2013;59:160-7. [CrossRef]
19. Sayegh F, Elazzazy A, Bellou S, Moustogianni A, Elkady AI, Baeshen MN, et al. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann Microbiol 2016;66:937-48. [CrossRef]
20. Baldeweg F, Warncke P, Fischer D, Gressler M. Fungal biosurfactants from Mortierella alpina. Org Lett 2019;21:1444-8. [CrossRef]
21. Abu-Elghait M, Hasanin M, Hashem AH, Salem SS. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles:Characterization, antibiofilm and biocompatibility. Int J Biol Macromol 2021;175:294-303. [CrossRef]
22. European Food Safety Authority (EFSA). Safety of 'fungal oil from Mortierella alpina'-scientific opinion of the panel on dietetic products, nutrition and allergies. EFSA J 2008;6:770. [CrossRef]
23. Al-Hwaiti MS, Alsbou EM, Abu Sheikha G, Bakchiche B, Pham TH, Thomas RH, et al. Evaluation of the anticancer activity and fatty acids composition of “Handal”(Citrullus colocynthis L.) seed oil, a desert plant from south Jordan. Food Sci Nutr 2021;9:282-9. [CrossRef]
24. Vilakazi H, Olasehinde TA, Olaniran AO. Chemical characterization, antiproliferative and antioxidant activities of polyunsaturated fatty acid-rich extracts from Chlorella sp. S14. Molecules 2021;26:4109. [CrossRef]
25. Palakurthi SS, Fluckiger R, Aktas H, Changolkar AK, Shahsafaei A, Harneit S, et al. Inhibition of translation initiation mediates the anticancer effect of the n-3 polyunsaturated fatty acid eicosapentaenoic acid. Cancer Res 2000;60:2919-25.
26. So WW, Liu WN, Leung KN. Omega-3 polyunsaturated fatty acids trigger cell cycle arrest and induce apoptosis in human neuroblastoma LA-N-1 cells. Nutrients 2015;7:6956-73. [CrossRef]
27. Chatterjee N, Das S, Bose D, Banerjee S, Jha T, Das Saha K. Lipid from infective L. donovani regulates acute myeloid cell growth via mitochondria dependent MAPK pathway. PLoS One 2015;10:e0120509. [CrossRef]
28. Roy S, Rawat AK, Sammi SR, Devi U, Singh M, Gautam S, et al. Alpha-linolenic acid stabilizes HIF-1 a and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017;8:70049. [CrossRef]
29. Giulitti F, Petrungaro S, Mandatori S, Tomaipitinca L, De Franchis V, D'Amore A, et al. Anti-tumor effect of oleic acid in hepatocellular carcinoma cell lines via autophagy reduction. Front Cell Dev Biol 2021;9:629182. [CrossRef]
30. Iuchi K, Ema M, Suzuki M, Yokoyama C, Hisatomi H. Oxidized unsaturated fatty acids induce apoptotic cell death in cultured cells. Mol Med Rep 2019;19:2767-73. [CrossRef]
31. Gbayisomore O, Klausner H, Phelan SA. Punicic acid inhibits proliferation and induces apoptosis in human MCF-7 breast cancer cells. Int J Cancer Clin Res 2023;10:179. [CrossRef]
32. Avula CR, Zaman AK, Lawrence R, Fernandes G. Induction of apoptosis and apoptotic mediators in balb/C splenic lymphocytes by dietary n- 3 and n- 6 fatty acids. Lipids 1999;34:921-7. [CrossRef]
33. Wang CC, Liu WB, Cao XF, Huang YY, Wang X, Xiao K, et al. Excess DHA induces cell cycle arrest by activating the P53/cycling pathway in blunt snout bream (Megalobrama amblycephala). Front Mar Sci 2020;7:286. [CrossRef]
34. D'Eliseo D, Velotti F. Omega-3 fatty acids and cancer cell cytotoxicity:Implications for multi-targeted cancer therapy. J Clin Med 2016;5:15. [CrossRef]
35. Oono K, Ohtake K, Watanabe C, Shiba S, Sekiya T, Kasono K. Contribution of Pyk2 pathway and reactive oxygen species (ROS) to the anti-cancer effects of eicosapentaenoic acid (EPA) in PC3 prostate cancer cells. Lipids Health Dis 2020;19:15. [CrossRef]