1. INTRODUCTION
Piper sarmentosum Roxb. (Piperaceae) is a tropical plant that is commonly consumed as a vegetable and has medicinal properties in the South-east Asia region. It is a wild-growing herb with long creeping stems. This terrestrial herb has been used traditionally to treat many ailments and diseases. The leaves of this plant are alternate with heart-shaped and usually have a waxy surface in their young light green leaves. This plant has a pungent odor and the flower has a unisexual ovary [1-4]. Aerial parts of P. sarmentosum are consumed after cooking or boiling in water as a functional food [4,5].
Recently, many people have utilized herbal remedies daily. Piper species are used in a variety of traditional medicines. Especially in Indonesia, Malaysia, and the southern region in Thailand because of not harmful or unfavorable effects and also the cost is effectiveness. The leaves of the plant are commonly used to treat kidney stones and mitigate chest pain, indigestion, fever, and headache. The fruits and leaves are used as an expectorant, carminative, coughs, and muscle aches. In Indonesia, this plant is also used for asthma by chewing the rootlets with betel nut and swallowing the juice [2,4-6]. The previous study also mentioned that extracts of P. sarmentosum have shown various pharmacological activities such as antibacterial, antifungal, antioxidant, anti-cancer, antihypertensive, anti-tuberculosis, hepatoprotective, anti-inflammatory, antimalarial activity, fracture healing, and to prevent the vascular endothelial dysfunction caused by nicotine exposure and also has potential to be used in tissue regeneration [3,4,7-12]. P. sarmentosum contains bioactive compounds such as alkaloids (amide and pyrones), phenols, flavonoids, and tannins [4,13]. The previous study shows the phenolic compounds identified in the leaves and fruits of P. sarmentosum’s crude extract exhibited moderate to strong antibacterial activity against Pseudomonas fuscovaginae and Xanthomonas oryzae pv. [3]. The leaves of P. sarmentosum are also mentioned as a promising non-toxic antiparasitic agent against Trypanosoma evansi induced on mice [14].
Due to the high utilization value of this plant, another alternative is needed to reduce the exploitation of plants in nature just to get their secondary metabolite content. The therapeutic value of this plant by enhancing secondary metabolite content can be increased using tissue culture technology through callus culture. It is superior to the conventional method of propagation because of the high multiplication rate [15]. The induction of callus is more effective in meristematic organs [16]. The extract of cell suspension from the induction callus of B. aegypteaca using plant growth hormone (2,4-diclorophenoxyacetic acid [2,4-D] and NAA) has high anti-cancer activity. The previous study also mentioned that callus culture could be a promising method to obtain antimalarial secondary metabolites, especially by callus induction that has been conducted for seven Piper species such as Piper betle, Piper colubrinumm, Piper crocatum, Piper longum, Piper nigrum, Piper permucronatum, Piper solmsianum to obtain higher content of secondary metabolites with an additional plant growth hormones [16,17]. Secondary metabolites production can be increased using micropropagation which has the additional role of auxin and cytokinin as plant growth regulators. 2,4-Diclorophenoxyacetic acid (2,4-D) and benzylaminopurine (BAP) are two combinations of plant growth regulators that are often used and optimum in inducing callus to produce secondary metabolites [18,19]. In the previous study, information has not been found about callus induction from P. sarmentosum leaves using 2,4-D and BAP plant growth regulators. There is also no information about antioxidant and antimicrobial activities from methanol crude extract of P. sarmentosum’s callus. Hence, this study aimed to investigate the effect of different combination concentrations of 2,4-D and BAP on callus induction, profiles of secondary metabolites, and antimicrobial and antioxidant activities from P. sarmentosum leaves.
2. MATERIALs AND METHODS
2.1. Callus Culture
2.1.1. Plant material
Plant materials used in the current study were P. sarmentosum Roxb. obtained from Kayon Flower Market Surabaya, East Java, Indonesia. The leaves collected from the apical of plant as an explant. The leaves explants were soaked in water that had been added with liquid detergent for 10 min and then washed thoroughly with running tap water three times. The explants were treated with 70% alcohol solution for 6 min and 20% clorox solution for 5 min in aseptic condition. After that, the explants were washed with sterile dH2O three times to remove any traces of chemical solutions.
2.1.2. Medium preparation
MS medium supplemented with different combination concentrations of 2,4-Diclorophenoxyacetic acid (2,4-D) (0.5; 1.0; 1.5; 2.0; 2.5 mg/L) and BAP (0.5; 1.0; 1.5; 2.0; 2.5 mg/L) and control treatment with hormone-free in MS medium was prepared by adding 3% sucrose and 0.8% agar, totaling 26 treatments [Table 1]. The pH for the MS medium was maintained at 5.6–5.8 to autoclaving at 121oC for 15 min.
Table 1: Effect of different combination concentration of 2,4-D and BAP on callus induction time, fresh weight, and dry weight from Piper sarmentosum Roxb. leaves explants on MS medium for 6 weeks.
| Hormones (mg/L) | Callus induction time (days)* | Callus fresh weight (g)* | Callus dry weight (g)** | Callus color | |
|---|---|---|---|---|---|
| 2,4-D | BAP | ||||
| 0 | 0 | 0.000±0.000a | 0.000±0.000a | 0.000±0.000a | - |
| 0.5 | 0.5 | 14±0.972de | 0.174±0.047b | 0.039±0.011bc | Light brown |
| 0.5 | 1.0 | 13±0.928cd | 0.223±0.037d | 0.046±0.024bcd | Brownish |
| 0.5 | 1.5 | 10±0.500b | 0.262±0.048cdeg | 0.056±0.018bcde | Light brown |
| 0.5 | 2.0 | 14±0.866ef | 0.261±0.059cdef | 0.057±0.016bcdef | Light brown |
| 0.5 | 2.5 | 15±0.500fghj | 0.287±0.051cefi | 0.045±0.012bcd | Light brown |
| 1.0 | 0.5 | 15±0.782fgik | 0.236±0.034dh | 0.069±0.026ef | Brownish |
| 1.0 | 1.0 | 16±0.500ikm | 0.241±0.065cde | 0.048±0.016bcde | Light brown |
| 1.0 | 1.5 | 15±0.500fghj | 0.276±0.062cefhi | 0.052±0.013bcde | Light brown |
| 1.0 | 2.0 | 16±0.782lmn | 0.305±0.052efi | 0.061±0.019cdef | Light brown |
| 1.0 | 2.5 | 18±0.833o | 0.331±0.049i | 0.047±0.009bcde | Brownish |
| 1.5 | 0.5 | 18±0.866o | 0.260±0.085cdef | 0.051±0.023bcde | Light brown |
| 1.5 | 1.0 | 15±0.500ik | 0.259±0.049cdef | 0.057±0.023bcdef | Light brown |
| 1.5 | 1.5 | 16±0.527km | 0.284±0.049cefhi | 0.078±0.029f | Brownish |
| 1.5 | 2.0 | 14±0.527efg | 0.350±0.111fgi | 0.046±0.012bcde | Dark brown |
| 1.5 | 2.5 | 15±0.500ik | 0.288±0.076cefhi | 0.063±0.018def | Dark brown |
| 2.0 | 0.5 | 17±0.866l | 0.316±0.066fgi | 0.064±0.024def | Brownish |
| 2.0 | 1.0 | 17±0.866l | 0.297±0.060cefi | 0.056±0.024bcde | Light brown |
| 2.0 | 1.5 | 12±0.782c | 0.273±0.039cef | 0.061±0.018cdef | Dark brown |
| 2.0 | 2.0 | 14±0.866eh | 0.263±0.053cdef | 0.048±0.017bcde | Dark brown |
| 2.0 | 2.5 | 15±0.527km | 0.320±0.072fi | 0.042±0.009bcd | Dark brown |
| 2.5 | 0.5 | 16±0.500kn | 0.223±0.106bcd | 0.035±0.011b | Dark brown |
| 2.5 | 1.0 | 15±0.866gjk | 0.243±0.096bdef | 0.041±0.020bcd | Light brown |
| 2.5 | 1.5 | 15±0.866gjk | 0.284±0.122cdefi | 0.046±0.026bcd | Light brown |
| 2.5 | 2.0 | 21±8.544jklo | 0.206±0.114bcd | 0.050±0.043bcde | Light brown |
| 2.5 | 2.5 | 14±1.225efgi | 0.241±0.074cd | 0.036±0.020c | Light brown |
Value represents the mean±standard deviation of nine replicates.
* The same letter in same column are not significantly different by the Mann-Whitney’s test at 0.05% probability level and
** The same letter in same column are not significantly different by the Duncan’s multiple range test at 0.05% probability level. MS: Murashige and Skoog, BAP: Benzylaminopurine
2.1.3. Explant culture
Sterilized leaves explants were cut into 1 cm × 1 cm lengths and inoculated in MS medium with supplemented different combination concentrations of 2,4-D and BAP in sterile conditions. Three explants were cultured in each bottle, with nine replications for each treatment. All cultures were incubated at ±25°C under light conditions for 6 weeks.
2.2. Secondary Metabolites Extraction and Identification by Gas Chromatography-Mass Spectrometry (GC-MS)
2.2.1. Preparation of samples
Three treatments with the highest dry weight were selected to continue the extraction process. The dry callus in each treatment sample was then pulverized to form powder. The sample powder of P. sarmentosum callus was extracted with 5 mL of methanol solvent by maceration for 3 days at room temperature. Then, the methanol extract solution was filtered using filter paper and concentrated until the extract had a volume of 2 mL.
2.2.2. Equipment and chromatographic conditions
The sample (1 μL) was taken and injected into column type HP-5MS (30 m × 250 m × 0.25 μm, Agilent, USA) to be analyzed its the type of compounds and peak areal percentage (%) of compounds using GC-MS Agilent Technologies 7890A. Temperature of oven was set at 100oC for 2 min, then raised to 300°C for 1 min. Running time of each test extract was ± 24 min.
2.3. Determination of Antimicrobial Activity
Antimicrobial activity in this study is using the disk diffusion method. 25 μL of methanol extract was loaded to sterile discs (? = 6 mm) and placed on inoculated 100 μL microbes suspension and 15 mL Mueller-Hinton Agar medium in petri dish. For each concentration (0, 250, 500, 750, and 1000 ppm), three replicates were maintained [20]. The plates were incubated for 24 h (for bacteria) and 48 h (for fungi) at 37°C and zones of inhibition if any around the discs were measured in mm using caliper [21].
2.4. Determination of Antioxidant Activity
2,2-diphenyl-1-picrylhydrazyl (DPPH) solution was prepared beforehand and the stock solution of P. sarmentosum’s leaves callus methanol extract was diluted in several concentrations (100; 75; 50; 35; 25; 12.5; 10; 6.25 μg mL-1), each sample with a concentration which has been determined was repeated 2 times. The DPPH test used a 96 well microplate with each well added 200 μL of callus extract sample solution and 100 μL of DPPH solution. After that, it was incubated in a dark room for 1 h and continued with a UV-Vis microplate spectrophotometer (Thermo Fisher Scientific, USA) at a wavelength of 517 nm to determine the absorbance value at each of these concentrations [22,23]. The percentage of DPPH radical degradation from each extract concentration was calculated by the following formula:

Absorbance sample is the absorbance value of P. sarmentosum callus extract and absorbance blank is the absorbance value of the control blank which contains DPPH solution reagent only. Then, the result of the percentage inhibition (%I) was analyzed to determine the value of inhibitory concentration (IC50). The percentage inhibition was substituted in a linear equation; then, interpreted as IC50. The IC50 value is an expression of the antioxidant activity of the callus extract sample.
2.5. Data Analysis
The experiment of callus induction was carried out nine replications for each treatment. Qualitative data such as callus morphology and secondary metabolites profiles of P. sarmentosum were identified descriptively. Quantitative data such as callus induction time, fresh weight, and dry weight were analyzed by analysis of variance (ANOVA) followed by Duncan’s test and Mann–Whitney’s test for mean comparison. The experiment of antimicrobial activity was analyzed by measuring the zone of inhibition with three replications for each concentration, and the percentage of inhibition (%I) measured for antioxidant activity with two replications for each concentration.
3. RESULTS
3.1. Callus Induction
Callus was produced from cut ends of meristematic leaves of P. sarmentosum Roxb. after 6 weeks of culture. Induction of P. sarmentosum Roxb. achieved approximately 70% until 100% after 6 weeks of culture. The establishment of Piper cultures was frequently slow and difficult [24]. In the present study, compact callus was successfully established on all different combination concentrations of 2,4-D and BAP with various callus color, such as light brown, brownish, and dark brown [Figure 1]. MS medium with hormones free cannot induced callus.
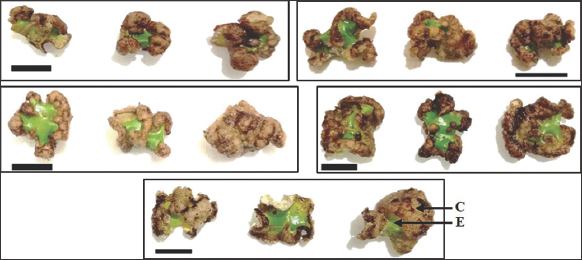 | Figure 1: Effect of different combination concentration of 2,4-D and BAP on callus morphology from Piper sarmentosum Roxb. leaves explants on MS medium for six weeks. (a.) 1.5 mg L-1 2,4-D + 1.5 mg L-1 BAP, (b.) 1.0 mg L-1 2,4-D + 0.5 mg L-1 BAP, (c.) 2.0 mg L-1 2,4-D + 0.5 mg L-1 BAP, (d.) 2.0 mg L-1 2,4-D + 2.0 mg L-1 BAP (dark brown callus), (e.) 2.5 mg L-1 2,4-D + 2.5 mg L-1 BAP (light brown callus). (a-c.) Brownish callus, C. Callus, and E. Explant. All callus were compact textured and explants were green except for control (brown explant and no callus). Bars = 1 cm. [Click here to view] |
Table 1 showed that the fastest mean of callus induction time was 0.5 mg/L 2,4-D and 1.5 mg/L BAP on 10 days, while the slowest mean of callus induction time was 2.5 mg/L 2,4-D and 2.0 mg/L BAP on 21 days. The treatment of combination concentration of 1.5 mg/L 2,4-D and 2.0 mg/L BAP showed the highest mean of callus fresh weight (0.350 g), while the lowest mean of callus fresh weight at the treatment of combination concentration of 0.5 mg/L 2,4-D and 0.5 mg/L BAP (0.174 g). The treatment of 1.5 mg/L 2,4-D and 1.5 mg/L BAP showed the highest mean of callus dry weight (0.078 g), while the lowest mean of callus dry weight at the treatment of 2.5 mg/L 2,4-D and 0.5 mg/L BAP (0.035 g).
Based on statistical data analysis, the mean of callus induction time, fresh weight, and dry weight with supplemented concentration of 2,4-D and BAP in MS medium did not show a significant difference. Three treatments with the highest dry weight were selected to continue the extraction process were 1.5 mg/L 2,4-D and 1.5 mg/L BAP; 1.0 mg/L 2,4-D and 0.5 mg/L BAP; 2.0 mg/L 2,4-D and 0.5 mg/L BAP at 0.078 g, 0.069 g and 0.064 g, respectively.
3.2. Secondary Metabolites Profile of Callus
The callus of P. sarmentosum that has been dried was then mashed to form a powder for extraction process with methanol solvent for 3 days. After that, the three selected methanol extracts were analyzed for secondary metabolites using GC-MS. In Table 2, it shows that there were many identified types of compounds. The combination concentration of 1.5 mg/L 2,4-D and 1.5 mg/L BAP contains 11 different compounds, which were dominated by cyanoacetamide (25.08%) and diisooctyl-phthalate (21.81%). The combination concentration of 1.0 mg/L 2,4-D and 0.5 mg/L BAP contains six different compounds, which were dominated by acetaldehyde (32.90%), 2-methoxyamphetamine (17.88%) and propanamide (16.72%). While the treatment of 2.0 mg/L 2,4-D and 0.5 mg/L BAP contains seven different compounds, which were dominated by myristicin (48.77%) and vanillin ethyl ether (39.22%). Myristicin and propanamide compounds were always identified in the three P. sarmentosum callus extracts, the content of myristicin appeared at the 9th min, while the content of propanamide appeared at the 15th min [Table 2].
Table 2: Secondary metabolites profiles identified in methanol extract of Piper sarmentosum Roxb.’s callus.
| Retention time (min) | Name of compounds | Peak area (%) | Activity |
|---|---|---|---|
| 1.5 mg/L 2,4-D+1.5 mg/L BAP | |||
| 4.404 | Acetaldehyde | 7.48 | Antimicrobial and antioxidant [35] |
| 8.682 | Benzenemethanamine | 3.18 | Antimicrobial [38] |
| 9.110 | Chloracetamide | 3.75 | Antimicrobial [39] |
| 9.571 | Myristicin | 8.94 | Antimicrobial and antioxidant [40] |
| 10.115 | 1-Methylheptylamine | 9.86 | Antimicrobial [41] |
| 11.305 | Phenylpropanolamine | 2.32 | Antioxidant [42] |
| 11.824 | 1,4-Dimethylpentylamine | 5.69 | - |
| 13.278 | Cyanoacetamide | 25.08 | Antioxidant [43] |
| 13.706 | 2-Aminononadecane | 4.67 | Antimicrobial and antioxidant [44] |
| 15.621 | Propanamide | 7.22 | Antioxidant [45] |
| 22.205 | Diisooctyl-phthalate | 21.81 | Antimicrobial and antioxidant [46] |
| 1.0 mg/L 2,4-D+0.5 mg/L BAP | |||
| 4.330 | Acetaldehyde | 32.90 | Antimicrobial and antioxidant [35] |
| 8.682 | 2,4-Dimethylamphetamine | 13.90 | - |
| 9.571 | Myristicin | 5.44 | Antimicrobial and antioxidant [40] |
| 12.797 | 2-Methoxyamphetamine | 17.88 | Antimicrobial [47] |
| 15.610 | Propanamide | 16.72 | Antioxidant [45] |
| 20.344 | (Tetrahydroxycyclopentadienone) tri carbonyliron (0) | 13.16 | - |
| 2.0 mg/L 2,4-D+0.5 mg/L BAP | |||
| 5.541 | 1-Methylcaprolactam | 3.82 | - |
| 8.677 | Caryophyllene | 3.17 | Antimicrobial and antioxidant [48] |
| 9.566 | Myristicin | 48.77 | Antimicrobial and antioxidant [40] |
| 10.216 | 1,4-Benzenedicarboxylic acid | 0.78 | Antimicrobial and antioxidant [49] |
| 15.235 | Vanillin ethyl ether | 39.22 | Antioxidant [50] |
| 15.600 | Propanamide | 3.52 | Antioxidant [45] |
| 18.551 | 1,1-Cyclobutanedimethanamine | 0.73 | - |
BAP: Benzylaminopurine
3.3. Antimicrobial Activity of Callus and Leaves Extract
The results of the antimicrobial activity test between the callus extract and the leaves of P. sarmentosum had differences [Figure 2]. In inhibiting the growth of Candida albicans and Escherichia coli, it was shown that the leaf extract was better at inhibiting their growth than the callus extract. At a concentration of 1000 ppm, P. sarmentosum leaf extract inhibited the highest C. albicans growth with an average inhibition zone of 11.70 mm. In inhibiting the growth of E. coli, the concentration of 1000 ppm of leaf extract was also among the highest at an average inhibition zone of 16.60 mm. Whereas in inhibiting the growth of S. aureus, callus extract was better at inhibiting its growth for the best concentration being 1000 ppm callus extract of P. sarmentosum (11.60 mm).
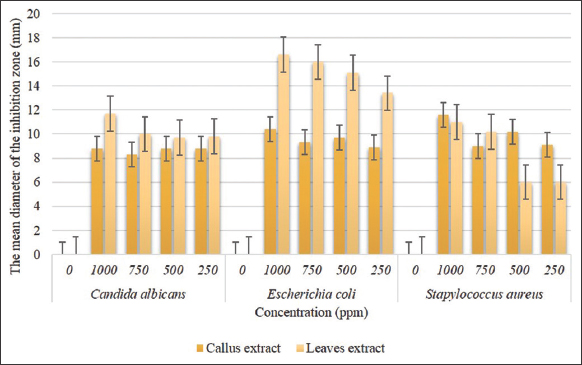 | Figure 2: The mean diameter of the inhibition zone produced by callus and leaves extracts against various pathogen. [Click here to view] |
3.4. Antioxidant Activity of Callus and Leaves Extract
Figure 3 shows that there is a difference in the antioxidant activity between P. sarmentosum leaves extract and callus compared to silymarin as a standard. The P. sarmentosum leaves extract had an IC50 value of 340.161 μL/mL which was included in the category of very weak antioxidant activity, while the IC50 value of the P. sarmentosum callus extract was 26.709 μL/mL which was included in the category of very strong antioxidant activity.
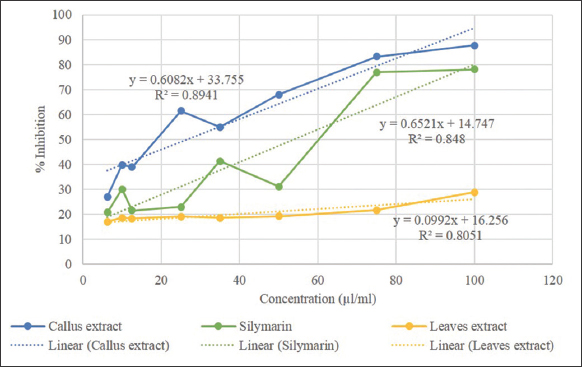 | Figure 3: Graph plot percentage inhibition against concentration showing DPPH free radical scavenging activity of leaves methanol extract and callus methanol extract compared with silymarin (standard). [Click here to view] |
4. DISCUSSION
The plant growth regulators was supplemented on MS medium induced the growth rate of explant cultured cells. Based on this study, the formation of P. sarmentosum Roxb. callus induced at 10 days to 21 days after planting. It indicated that the different combination concentration of 2,4-D and BAP in P. sarmentosum callus were not too significantly different. Except for the treatment 0.5 mg/L 2,4-D and 1.5 mg/L BAP (10 days) was significantly different from the other treatment, while the slowest mean of callus induction time was 2.5 mg/L 2,4-D and 2.0 mg/L BAP (21 days). In general, callus P. sarmentosum induced on 14 days, 15 days, and 16 days. The results were in accordance with previous research on callus induction of P. retrofractum Vahl. with additional various combination of 2,4-D and BAP showed the average time in inducing callus on 15 days after planting [25]. But also, this respone was possibly due to different combinations of plant growth regulators applied to MS medium in addition to physiological condition of respective explants [18]. Due to strong activities to stimulate dedifferentiation cell process, organogenesis, and maintain callus growth, 2,4-D is a plant growth regulator mostly used on callus culture [26]. An increased in the concentration of 2,4-D added to MS medium can also inhibit callus proliferation [27]. In the previous study, the treatment of 0.5 mg/L 2,4-D and 1.0 mg/L BAP showed the fastest mean of callus induction time of Piper betle L. Var. Nigra leaves at 7.25 days [18]. Another study showed that the combination concentration of 13.50 μM 2,4-D and 4.50 μM BAP was the fastest treatment to callus induced of Hymenocallis littoralis at 15 days [28]. The application of 2,4-D and BAP in various concentrations also showed the fastest callus induction time (5–7 days) in various plant explants [29-31].
The combination concentration of 1.5 mg/L 2,4-D and 2.0 mg/L BAP showed the highest mean of callus fresh weight at 0.350 g, while the highest mean of callus dry weight was the combination concentration of 1.5 mg/L 2,4-D and 1.5 mg/L BAP at 0.078 g. In the current study, the difference in the value of callus fresh weight and dry weight is probably due to the presence of more water content in the combination concentration of 1.5 mg/L 2,4-D and 2.0 mg/L BAP than in the combination concentration of 1.5 mg/L 2,4-D and 1.5 mg/L BAP, the highest dry weight of P. sarmentosum’s callus in this treatment indicates a best combination concentration for the production of secondary metabolite content. In the previous study, the various combination concentration of 2,4-D and BAP also showed the optimum dry weight for producing secondary metabolites in various plants, such as the combination concentration of 0.5 mg/L 2,4-D and 2.0 mg/L BAP at 0.0670 g callus dry weight in Piper betle L. Var. Nigra leaves and the treatment of 1.5 mg/L 2,4-D and 2.0 mg/L BAP at 0.108 g callus dry weight in Justicia gendarussa leaves [18,26]. Callus morphology of P. sarmentosum showed a variety of callus colors with a compact texture. The colors that appeared after explants in culture for 6 weeks were light brown, brownish, and dark brown [Table 1 and Figure 1]. In the present study, there are different colors of callus which is caused various hormone treatments which clearly indicate the influence of different plant growth regulators on determining callus morphology [32].
The combination concentration of 1.5 mg/L 2,4-D and 1.5 mg/L BAP (0.078 g); 1.0 mg/L 2,4-D and 0.5 mg/L BAP (0.069 g); 2.0 mg/L 2,4-D and 0.5 mg/L BAP (0.064 g) were the best three treatments for callus dry weight. After the identification of secondary metabolite compounds using GC-MS, each treatment showed the number and variety of different compounds [Table 2]. Myristicin and propanamide compounds were always identified in the three P. sarmentosum callus extracts. In previous study explained that Myristicin compound is the highest potent inhibition against R. solani and B. oryzae with half maximal inhibitory concentration (IC50) of 0.69 mmol/L [33]. Myristicin compound in extract methanol of P. sarmentosum also can exhibited strong antifeedant [34]. Propanamide compounds were previously reported to have antibacterial and antioxidant activity in other study [35,36]. Myristicin compound was also identified in the ethanol extract of P. sarmentosum leaves in Malaysia [37].
In Figure 2 shows that P. sarmentosum leaves and callus extracts have varying antimicrobial activity at each treatment concentration except for the 0 ppm extract treatment. In this study, it was seen that the growth of S. aureus was inhibited by the best concentration of 1000 ppm of P. sarmentosum callus extract (11.60 mm), while the growth of C. albicans and E. coli were inhibited best by the concentration of 1000 ppm of P. sarmentosum leaves extract with a diameter of inhibition zone 11.70 mm and 16.60 mm, respectively. The difference in inhibitory ability is due to differences in the total and type of secondary metabolite compounds between callus extract and leaves extract of P. sarmentosum. Table 2 also shows that the callus extract of P. sarmentosum contains a myristicin compound, while the methanol leaves extract of P. sarmentosum contains an elemicin compound [51]. Myristicin and elemicin are phytochemical compounds that have antimicrobial, antioxidant, and antiviral activities. These two compounds can inhibit the synthesis process in microbial cells so that their growth is hampered and die. Figure 2 also shows the concentration of 250–1000 ppm; both extracts were able to against the growth of three types of pathogen microbes. It shows that the callus and leaves extracts of P. sarmentosum are effective against C. albicans, E. coli, and S. aureus. A previous study also stated that methanol extract of C. dactylon rhizome with a concentration of 1000 ppm could best inhibit the growth of E. coli, B. cereus, and P. aeruginosa with an average diameter of the inhibition zone 16.80 mm, 18.30 mm, and 12.80 mm, respectively [52].
In Figure 3, it can be seen that the antioxidant activity of P. sarmentosum callus extract is the best compared to P. sarmentosum leaves extract and silymarin as standards in this assay. The IC50 value of callus extract is 26.709 μL/mL which is very strong in its antioxidant activity compared to leaves extract which has an IC50 value of 340.161 μL/mL which is very weak in its antioxidant activity. This test shows that callus induction of P. sarmentosum with the addition of growth regulators such as 2,4-D and BAP can increase the production of secondary metabolites which play a role in increasing antioxidant activity such as myristicin and propanamide compounds which are also antioxidants [35,36]. Other studies stated that the myristicin compound in Daucus pumilus was successfully increased through the micropropagation method and thus its antioxidant activity became stronger [53]. Another study also shows that Saraca asoca callus in various extracts has better scavenging ability than extract of S. asoca in vivo leaves with an IC50 value of 38.79 μL/mL [54]. The antioxidant activity of extracts is influenced by the content of natural polyphenols such as flavonoids, phenolics, tannins, and saponins [55].
5. CONCLUSION
This study concluded that the different combination concentrations of plant growth regulators 2,4-D and BAP affected callus induction and profile of secondary metabolites of P. sarmentosum Roxb. leaves explants. The combination concentration of 2.0 mg/L 2,4-D and 0.5 mg/L BAP was the best treatment in inducing callus because the highest myristicin compound was identified, so it can be used to produce callus for secondary metabolite production. Myristicin and propanamide compounds were identified in three methanol extracts of P. sarmentosum callus, and that callus extract had the highest dry weight. The morphology of callus grown during this study was compact in various colors, such as light brown, brownish, and dark brown. The best treatment of P. sarmentosum callus extract had better antioxidant and antimicrobial activities than P. sarmentosum leaf extract.
6. ACKNOWLEDGMENTS
The authors thankfully acknowledge the Annual Consideration Activity Plan, Faculty of Science and Technology, Universitas Airlangga, in the 2023 fiscal year, for financial support (PDU 2023).
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data are available with the authors and shall be provided on request.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Nirwana SI, Suhana MR, Fadziyah MA, Farihah HS, Fairus A, Alfakri MN. Piper sarmentosum improves bone structure and biomechanical strength of rats given excess glucocorticoid. Br J Pharm Res 2012;2:168-87. [CrossRef]
2. Seyyedan A, Yahya F, Kamarolzaman MF, Suhaili Z, Desa MN, Khairi HM, et al. Review on the ethnomedicinal, phytochemical and pharmacological properties of Piper sarmentosum:Scientific justification of its traditional use. TANG (Humanit Tradit Med) 2013;3:1-32.
3. Syed Ab Rahman SF, Sijam K, Omar D, Abd Wahab MZ. Identification of phenolic compounds and evaluation of antibacterial properties of Piper sarmentosum Roxb. Against rice pathogenic bacteria. Malays J Microbiol 2016a;12:475-84.
4. Syed Ab Rahman SF, Sijam K, Omar D. Piper sarmentosum Roxb:A mini review of ethnobotany, phytochemistry and pharmacology. J Anal Pharm Res 2016b;2:00031.
5. Hussain K, Hashmi FK, Latif A, Ismail Z, Sadikun A. A review of the literature and latest advances in research of Piper sarmentosum. Pharm Biol 2012;50:1045-52. [CrossRef]
6. Thent ZC, Das S. Piper sarmentosum maintains blood pressure and morphological integrity of liver in type 1 diabetic rats. Int J Pharm Med Biol Sci 2015;4:24-8.
7. Fadze NF, Ugusman A, Aminuddin A, Zakaria Z, Nordin NA. Piper sarmentosum reduces blood pressure in dexamethasone-induced hypertensive rats. Int J Cardiol 2017;249:S1-5.
8. Shi YN, Liu FF, Jacob MR, Li XC, Zhu HT, Wang D, et al. Antifungal amide alkaloids from the aerial parts of Piper flaviflorum and Piper sarmentosum. Planta Med 2017;83:143-50.
9. Wang DF, Zhou LL, Zhou HL, Hou GY, Zhou X, Li W. Effects of Piper sarmentosum extract on the growth performance, antioxidant capability and immune response in weaned piglets. J Anim Physiol Anim Nutr (Berl) 2017;101:105-12. [CrossRef]
10. Hematpoor A, Paydar M, Liew SY, Sivasothy Y, Mohebali N, Looi CY, et al. Phenylpropanoids isolated from Piper sarmentosum Roxb. Induce apoptosis in breast cancer cells through reactive oxygen species and mitochondrial-dependent pathways. Chem Biol Interact 2018;279:210-8. [CrossRef]
11. Salleh MF, Aminuddin A, Hamid AA, Salamt N, Japar Sidik FZ, Ugusman A. Piper sarmentosum Roxb. Attenuates vascular endothelial dysfunction in nicotine-induced rats. Front Pharmacol 2021;12:667102. [CrossRef]
12. Abidin IZ, Johari AN, Ariffin ZZ, Yazid MD, Dyari HR, Ariffin SH. Cytotoxic and osteoblast differentiation induction properties of crude polar extract of Piper sarmentosum leaves:Cytotoxicity and differentiation effect of P. sarmentosum. J Trop Life Sci 2023;13:231-8. [CrossRef]
13. Ugusman A, Zakaria Z, Hui CK, Nordin NA, Mahdy ZA. Flavonoids of Piper sarmentosum and its cytoprotective effects against oxidative stress. EXCLI J 2012;11:705-14.
14. Baba MS, Hassan ZA. Piper sarmentosum leaf as a promising non-toxic antiparasitic agent against Trypanosoma evansi-induced mice. Malays J Microsc 2019;15:46-60.
15. Deventhiran M, Wyson WJ, Mohamed MS, Jaikumar K, Saravanan P, Anand D. In vitro propagation and comparative phytochemical analysis of wild plant and micropropagated Cleome rutidosperma DC. Int J Pharmacogn Phytochem Res 2017;9:253-7.
16. Sherif SS, Emara NA. Anticancer activity of Balanitis aegyptiaca extract on human hepatoma cells and prostate cell line culture. Int J Pharmtech Res 2016;9:53-64.
17. Putri NS, Noli ZA. Callus culture as the method in providing antimalarial compounds of Piper genus. J Environ Sci Sustain Soc 2021;10:8-11.
18. Junairiah, Purnomo, Utami ESW, Ni'matuzahroh, Sulistyorini L. Callus induction of Piper betle L. Var Nigra using 2,4-Dichlorofenoxyacetic acid and 6-Benzil aminopurin. Biosaintifika 2018;10:588-96.
19. Santos MR, Guimarães MC, Paz ES, Magalhães GM, Souza CA, Smozinski CV, et al. Induction and growth pattern of callus from Piper permucronatum leaves. Rev Bras Plantas Med 2016;18:142-8. [CrossRef]
20. Junairiah, Fatimah, Nurhariyati T, Zuraidassanaaz NI. Antioxidant, antimicrobial activity and phytochemical screening of Syzygium cumini L. Leaves in tropical region from Surabaya, East Java, Indonesia. Asian J Plant Sci 2023;22:104-12. [CrossRef]
21. Ahmed GS, Coskun US. Investigation of antibacterial and antifungal activity of Saussurea costus root extracts. An Acad Bras Cienc 2023;95:e20230059.
22. Tamuly C, Hazarika M, Bora J, Gajurel PR. Antioxidant activities and phenolic content of Piper wallichii (Miq.) Hand-Mazz. Int J Food Proper 2014;17:309-20. [CrossRef]
23. Prieto JM. Procedure:Preparation of DPPH radical, and antioxidant scavenging assay. DPPH Micro Protoc 2012;7-9.
24. Balbuena TS, Santa-Catarina C, Silveira V, Kato MJ, Floh EI. In vitro morphogenesis and cell suspension culture establishment in Piper solmsianum C. DC. (Piperaceae). Acta Bot Bras 2009;23:274-81. [CrossRef]
25. Junairiah, Arofah J, Manuhara YSW, Nurhariyati T, Ni'matuzahroh. Effect of 2,4-D and BAP on callus induction of Piper retrofractum Vahl. Res J Pharm Technol 2021;14:1390-4. [CrossRef]
26. Wahyuni DK, Andriani P, Ansori AN, Utami ES. Callus induction of gendarussa (Justicia gendarussa) by various concentration of 2,4-D, IBA, and BAP. Biosaintifika 2017;9:402-8.
27. Damayanti F, Indrianto A, Sasongko AB, Fajarina S, Prabowo BH, Iskandar A, et al. Variation of 2,4-dichlorophenoxyacetic acid (2,4-D) concentration on kaffir lime callus growth as raw material for cell suspension. AIP Conf Proc 2020;2260:030012. [CrossRef]
28. Sundarasekar J, Anthony JJ, Murugaiyah V, Subramaniam S. Preliminary responses of 2,4-D and BAP on callus initiation of an important medicinal-ornamental Hymenocallis littoralis plants. J Med Plant Res2012;6:2088-93.
29. Andaryani S, Samanhudi, Yunus A. Effect of BAP and 2,4-D on callus induction of Jatropha curcas in vitro. Cell Biol Dev 2019;3:56-65.
30. Mayerni R, Satria B, Wardhani DK, Chan SR. Effect of auxin (2,4-D) and cytokinin (BAP) in callus induction of local patchouli plants (Pogostemon cablin Benth.). IOP Conf Ser Earth Environ Sci 2020;583:012003. [CrossRef]
31. Muthi'ah A, Sakya AT, Setyawati A, Samanhudi, Rahayu M. Callus induction of Calotropis gigantea using BAP and 2,4-D in vitro. IOP Conf Ser Earth Environ Sci 2023;1177:012021. [CrossRef]
32. Kumar GP, Subiramani S, Govindarajan S, Sadasivam V, Manickam V, Mogilicherla K, et al. Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Biotechnol Rep (Amst) 2015;7:72-80. [CrossRef]
33. Chanprapai P, Chavasiri W. Antimicrobial activity from Piper sarmentosum Roxb. Against rice pathogenic bacteria and fungi. J Integr Agric 2017;16:2513-24. [CrossRef]
34. Qin W, Huang S, Li C, Chen S, Peng Z. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera:Hispidae). Pestic Biochem Physiol 2010;96:132-9. [CrossRef]
35. Rassem HH, Nour AH, Yunus RM. Analysis of bioactive compounds for jasmine flower via gas chromatography-mass spectrometry (GC-MS). Malays J Fundam Appl Sci 2018;14:198-201. [CrossRef]
36. Shankar S, Settu S, Segaran G, Sundar RD, Ravi L. Phytochemical constituents of Dracaena mahatma leaves and their anti-bacterial, anti-oxidant and anti-inflammatory significance. Biotechnol Res Innov 2018;2:1-8. [CrossRef]
37. Ibrahim MA, Nasir MH, Azahari NF. Cytotoxicity, antioxidant activity and chemical constituents of Piper sarmentosum ethanolic extract. Int J Allied Health Sci 2020;4:1257-65.
38. Beulah GG, Soris PT, Mohan VR. GC-MS determination of bioactive compounds of Dendrophthoe falcata (L.F) Ettingsh:An epiphytic plant. Int J Health Sci Res 2018;8:261-9.
39. Bogdanovic A, Lazic A, Grujic S, Dimkic I, Stankovic S, Petrovic S. Characterisation of twelve newly synthesised N-(substituted phenyl)-2-chloroacetamides with QSAR analysis and antimicrobial activity tests. Arh Hig Rada Toksikol 2021;72:70-9.
40. Gupta AD, Bansal VK, Babu V, Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J Genet Eng Biotechnol 2013;11:25-31. [CrossRef]
41. Yun H, Hwang BY, Lee JH, Kim BG. Use of enrichment culture for directed evolution of the Vibrio fluvialis JS17 omega-transaminase, which is resistant to product inhibition by aliphatic ketones. Appl Environ Microbiol 2005;71:4220-4. [CrossRef]
42. Zhao T, Sun L, Wang Z, Nisar T, Gong T, Li D, et al. The antioxidant property and a-amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT Food Sci Technol 2019;111:252-9. [CrossRef]
43. Krishnan KG, Kumar CU, Lim WM, Mai CW, Thanikachalam PV, Ramalingan C. Novel cyanoacetamide integrated phenothiazines:Synthesis, characterization, computational studies and in vitro antioxidant and anticancer evaluations. J Mol Struct 2020;1199:127037. [CrossRef]
44. Sharma A, Noda M, Sugiyama M, Ahmad A, Kaur B. Production of functional buttermilk and soymilk using Pediococcus acidilactici BD16 (alaD+). Molecules 2021;26:4671. [CrossRef]
45. Alhusadi NA, Turgutalp B, Deniz I, Acar ET, Sipahi H, Yarim M, et al. Synthesis, antimicrobial, antioxidant and molecular docking studies on novel 6-methoxybenzothiazole-piperazine derivatives with propanamide chain. Curr Top Med Chem 2020;20:1733-41. [CrossRef]
46. Djebbah FZ, Al-Dhabi NA, Arasu MV, Belyagoubi L, Kherbouche F, Abdelouahid DE, et al. Isolation and characterisation of Streptomyces sp. Strain GLD25 with antimicrobial and antioxidant effects from Gueldaman cave (GLD1), Akbou-Algeria. J King Saud Univ Sci 2022;34:101719. [CrossRef]
47. Rapando JW, Ngugi MP, Muturi M, Ogutu JO. Phytochemical composition and antibacterial activities of the ethyl acetate leaf extract of Ocimum basilicum. Res Square 2020;19.
48. Dahham SS, Tabana YM, Iqbal MA, Ahamed MB, Ezzat MO, Majid AS, et al. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene b-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015;20:11808-29. [CrossRef]
49. Naqvi SF, Khan IH, Javaid A. Hexane soluble bioactive components of Chenopodium murale stem. Pak J Weed Sci Res 2020;26:425-32. [CrossRef]
50. Oladimeji OH, Njinga S, Abdullahi ST. Evaluation of antioxidant activity of obtained derivatives of vanillin. J Pharm Res Sci Tech 2022;6:17-25. [CrossRef]
51. Junairiah, Nurhariyati T, Zuraidassanaaz NI. Phytochemical in the methanol extract of Piper sarmentosum. Ecol Environ Conserv 2020;26:S123-6.
52. Savadi S, Vazifedoost M, Didar Z, Nematshahi MM, Jahed E. Phytochemical analysis and antimicrobial/antioxidant activity of Cynodon dactylon (L.) Pers. Rhizome methanolic extract. J Food Qual 2020;2020:1-10. [CrossRef]
53. Arafa AM, Abdel-Ghani AE, El-Dahmy SI, Abdelaziz S. Micropropagation, myristicin production enhancement, and comparative GC-MS analysis of the n-hexane extracts of different organs of Daucus pumilus (Gouan), family Apiaceae. J Pharm Bioallied Sci 2020;12:324-36. [CrossRef]
54. Vignesh A, Selvakumar S, Vasanth K. Comparative LC-MS analysis of bioactive compounds, antioxidants and antibacterial activity from leaf and callus extracts of Saraca asoca. Phytomed Plus 2022;2:100167. [CrossRef]
55. Ghatak A, Nair S, Vajpayee A, Chaturvedi P, Samant S, Soley K, et al. Evaluation of antioxidant activity, total phenolic content, total flavonoids, and LC-MS characterization of Saraca asoca (Roxb.) De.Wilde. Int J Adv Res 2015;3:318-27.