1. INTRODUCTION
Nanotechnology is the most emerging field among the different branches of science including material science, chemistry, and biology [1]. The regular employment of nanotechnology for constructing nano-size products in the scientific field is rising [2]. In today’s era, nanoparticles (NPs) have been an eye-catching part for researcher having distinctive characteristics, such as plasticity, better thermal conductivity, catalytic reactivity, and boosting the potency of metals and alloys [3,4], and three major classifications of NPs are seen, (1) organic, (2) inorganic, and (3) carbon-based [5]. Physical, chemical, and biological methodologies are involved in the amalgamation, synthesis and disintegration of NPs. Despite that, the first two methods are quite exclusive, complex and hazardous for the surroundings because of the deadly compounds used as reducing agents [6]. The biological process for synthesizing NPs is less time-consuming, less expensive, and requires less energy [7]. Biological, morphological, and biochemical procedure aid to produce metal-based NPs like, bacterial reduction of metals by distinct plant portions, i.e., root, stem, leaf, and flower. Currently, more than 1000 commercial products containing NPs are available in the market. Cu, Ag, and Zn-based NPs are the most popular antibacterial agents out of all the numerous types of NPs, and they are also frequently used in agriculture [8]. Zinc based NPs are among the most accepted NPs in the nanoindustry, and are produced 10–100 times more over the other. The increased use and regular release undoubtedly lead to Zn-based NPs accumulating in the ecosystem.
Since 2.7 million years ago, oxygen-evolving photosynthesis has been adding oxygen (O2) to the Earth’s decreasing atmosphere. Reactive oxygen species (ROS), a byproduct of various metabolic activities, took part in accomplishing metabolization activities [9,10]. These principle signaling molecules enable cells to react swiftly to novel physiological stimuli and programming of plants activities. Across plants whole life cycle, ROS perform imperative job in biotic and abiotic stress signaling, interaction and combination of ecological incentives, and stress-mediated network, thus participating in the establishment of security method and plant resistance [11]. Thus, the initiation of a network is mediated by stress, all of which contribute to the development of security measures and plant resistance. Various studies have shown to the exposure of different environmental stresses including abiotic and biotic may cause plants to produce free radical scavengers and oxygen derivatives [12,13]. Stress signal and enzymatic regulation improve through free radicals, consolidate redox state and induces imperative participation of osmolytes [14-16]. The presence and function of respiratory burst oxidase homologues and NADPH oxidase are strongly predicated on this production, which accounts for 1–2% of the total oxygen (O2) usage in plants [17-19]. The products of oxidation are collectively referred to as ROS, and they mostly include the following radicals: Hydroxyl (OH), hydrogen peroxide (H2O2), singlet oxygen (1O2), perhydroxyl (HO2), and anion radical (O2−) [20,21]. Both forms of ROS are produced in nature at low levels throughout a variety of aerobic metabolic activities, such as photorespiration in peroxisomes, chloroplast, and mitochondrial electron chain [22-24]. Increased ion toxicity causes an oxidative burst by the production of ROS. Although ROS does not work as a stress signal, they are the secondary messengers that signal fundamental cell functions such as apoptosis, necrosis, and cell proliferation, thus regulating various functions in plants.
There are several factors (heavy metal, salinity, temperature, and dryness) that are known to alter the equilibrium between ROS production and its scavenging. In these situations, a few key criteria, such as the severity, duration of the stress, growth alteration, and the rate at which plants adapt to difficult circumstances, largely determine a plant’s capacity to tolerate [25]. Plants have developed a miscellaneous strategies to endure adverse environmental states like stress-responsive genes that encode their proteins needed for the initiation and control ROS to adapt to intriguing environmental conditions [26]. NPs activities due to the application of zinc oxide have been shown by [27] to conduct ROS scavenging capabilities, preventing oxidative damage in stressed plants. Numerous earlier kinds of research have demonstrated the use of NPs to lower ROS generation in plants under both natural and stressed environmental conditions [28-33]. Previous studies confirmed that NPs can regulate abiotic stress in various plant species by altering the hormonal levels, antioxidant enzymes activities, and gene expression in crop plants. Overall, scientists have concluded that minute concentration of NPs may start the ROS detoxification mechanism. Therefore, the inoculation of NPs has brightened the chance of crop cultivation in stressed crops. This article intended to increase our understanding of ROS production, signaling, and their function in plants to successfully handle abiotic stress. The impact and function of NPs in the ROS as well as the crosstalk between NPs and the ROS were discussed mainly focusing on the ROS as a biostimulant under abiotic stress.
2. ROS AND ITS ROLE IN PLANTS
ROS production, which are naturally occurring by-products of cellular oxidative metabolism, is essential for controlling cell survival, cellular damage, differentiation, cell signaling, and the production of substances that cause inflammation[Table 1] [34,35]. ROS produces free radicals produces including singlet oxygen (1O2), peroxyl (RO2), carbonate (CO3-), alkoxyl (RO), superoxide (O2-), hydroxyl (HO), hydroperoxyl (HO2), and carbon dioxide radical (CO2-). The most persistent and prevalent ROS in plants is O2-, OH, and 1O2 [22]. This free oxygen is continuously produced through chloroplasts during the photosynthetic electron transport system (ETS) and is afterward eliminated by reduction and assimilation. In photosystem I and photorespiration, reduced components of the ETS reduce O2 to a superoxide radical [36]. O2 acts as a free radical with a reduced half-life because of superoxide dismutases (SODs) and eventually convert it to H2O2 rapidly [37]. Biologically relevant ROS also include non-radicals such as hypochlorous acid, nitric oxide, organic peroxides, peroxynitrite, peroxynitrate, peroxynitrous acid, H2O2, and ozone (O3) [38]. H2O2, a non-radical ROS product conveys ROS-mediated aquaporin membranes with greater stability and firmness than free radicals [37].
Table 1: Effects of ROS compartmentalization under abiotic stress.
| Different sites of ROS production and compatmentalization | Effects of ROS | Referencces |
|---|---|---|
| Mitochondrial | Electron transport chain is reduced, aging of leaves, higher carbonized proteins, H2O2 formation, Lipid peroxidation | [91,92] |
| Chloroplast | Increase ROS production, electron leakage, singlet oxygen generation | [93] |
| Peroxisome | Intracellular H2O2, glycolate oxidation, cellular redox homeostasis, physiological disorder | [94] |
| Plasma membrane | NADPH oxidase production, superoxide anion radical formation, mechanosensitive Ca2+ channel production | [95] |
| Cell wall | Peroxidase enzyme, electron transfer, ROS signaling, influence abiotic stressor | [96] |
| Nucleus | Gene expression, molecular oxygen reduction, redox potential hamper, | [97] |
| Apoplastic region | Cell surface, enzyme produces ROS, stomatal closure, programmed cell death | [50] |
| Cytosolic area | Oxidation procedure, diffusion, transport and leakage in ROS condition, APX overexpression | [98,99] |
ROS: Reactive oxygen species, APX: Ascorbate peroxidase
Numerous experiments have exhibited that exposure to a variety of environmental biotic or abiotic stressors can induce plants to develop both non-radical moderately reactive oxygen derivatives and highly reactive oxygen-free radicals [13]. This generation persuades the attainability and operational ability of NADPH oxidases and respiratory burst oxidase homologues [19]. It represents <1–2% of the plant’s overall O2 consumption [39]. Plants have developed sophisticated immune systems that can perceive pathogen transmission and activate an effective immune response through two separate but interdependent immune response stratums [40]. Pattern recognition receptors, built with extracellular conserved microbial- or pathogen-associated molecular patterns induce immunity in the first layer. Nucleotide-binding leucine-rich repeat receptors mediate the second layer [41].
ROS generated under unmitigated environmental circumstances cannot induce cellular impairment due to the production of stress-responsive genes [39]. Based on multiple pieces of evidences, it has been hypothesized that this degree of ROS production is related to a limited natural role in the developmental processes mediated by phytohormones such as auxins and cytokinins [10]. Oxidative stress is produced due to the excess genesis of ROS due to biotic stress also [Figure 1] [42]. Redox homeostasis to maintain a balanced biomolecules state in plants depends on ROS. Even though salicylic acid (SA) is thought to be the main ROS regulator, the underlying processes are rarely explored [43]. SA is indispensable in biotic stress management for preventing microbial growth, fungal diseases, and viral infections during HR in different pathosystems, including tobacco mosaic virus [44,45]. However, both the pathosystems and the source of ROS have an impact on the mechanism of SA regulation and obstruct ROS signaling [46]. Treatment with SA in Arabidopsis caused the PRRs to be regulated, which in turn caused ROS generation that was most likely At RBOHD-dependent [47].
 | Figure 1: Schematic representation of reactive oxygen species generation in chloroplast. [Click here to view] |
Several RBOHD isoforms promoters in Arabidopsis and rice containing SA-responsive cis-regulatory elements further validated the production [48]. However, under stressful environmental conditions, cellular ROS concentrations are excessively accelerated and reach levels that are greater than the antioxidant scavenging abilities that plants use to balance out excessive ROS generation [49]. This trait could lead to oxidative stress, protein, lipid, and nucleic acid damage in the membrane, eventually resulting cell death and dysfunction [42]. Increased ROS production is employed to increase the potency of damaging components in a genetically controlled process called abiotic stress-induced programmed cell death [50]. Natural stressors such as pathogen infection, heavy metals, heavy radiation, heat stress, salinity stress, and drought stress are just a few examples that could break the delicate balance between ROS creation and removal pathways [51]. Arsenic is one such hazardous metalloid that pollutes the environment and has severe effect on life on Earth. Arsenic is known to be harmful to plants and to induce a number of serious ailments in humans, even in trace amounts [52]. Studies show that the accumulation of As in cells increases the generation of ROS, such as O2 and H2O2, which creates oxidative stress in plants and results in impaired cellular metabolism, reduced plant development, and decreased yield [53]. Numerous crucial factors, such as the duration and intensity of the stress, cellular metabolic status, the level of ROS in the cells, and antioxidant capacity, are frequently consistent with plants response mechanism to the oxidative stress caused by high ROS concentrations [39].
In plants ROS-scavengers and non-enzymatic antioxidants such as ascorbate (AA), glutathione (GSH), carotenoids, -tocopherol, prolines, flavonoids, and phenolic chemicals play a role. These antioxidant enzymes include dehydroascorbate reductase (DHAR), catalase (CAT), SOD, glutathione reductase (GR), guaiacol peroxidase (GPX), monodehydroascorbate reductase (MDHAR), ascorbate peroxidase (APX), and low molecular mass antioxidants [54]. It is widely established that increased antioxidant enzyme or non-enzymatic antioxidant activity reduces the severity of oxidative stress-related damage in response to novel environmental stimuli [51]. For instance, it was shown that several conventional types of rice plants exposed to drought stress had an overall increase in the antioxidant enzymes APX, SOD, GPX, CAT, and GR [55]. Cu stress to Colobanthus quitensis (Kunth) Bartl, made it feasible to cause the upregulation of AA, GSH, phenolics, phytochelatins (as GSH oligomers), and sugars as non-enzyme-based antioxidants [56]. Before translating into adequate responses, ROS signals are first detected and processed by plants. The extent of alteration or modulation of potential signaling targets including transcriptional regulators, protein kinases, and stress-induced proteins depends on the oxidizing behavior of ROS aggregates. The ability of ROS to oxidise thiol groups and methionine residues in the protein to influence the protein redox status is noteworthy [57]. Thio- and gluta-redoxins are proteins can control cellular redox conditions through their reciprocal activation/deactivation or reversible oxidation/reduction [58]. It has been discovered that ROS-driven redox perturbations can activate quick adaptive responses by mitochondrial/chloroplastic retrograde signaling [59]. In addition, ROS can facilitate the retrograde signaling pathway from the plastid to the nucleus [39]. Therefore, the nucleus can accommodate the H2O2 produced in plastids at the consequence of triggering the expression of defense genes [60].
Limited studies have explored the interactions between ROS and other secondary messengers acting as a signal transduction cascade including Ca2+ and antimicrobial family derived reactive nitrogen species (RNS) [61]. Elevated amounts of oxidative potential cause them to react with the messengers of NO to form (non-) radical RNS products such as nitroxyl anion (NO), nitrate (NO3), nitrous acid (HNO2), nitrosonium cation (NO+), nitric dioxide (NO2), and ONOO [62]. These NOx species play a natural role in plant development, metabolism, stress signaling, and stomatal closure [63]. Depending on the concentration and subcellular microcompartment type, the interaction of ROS and RNS with antioxidant enzymes can have either favorable or detrimental effects on plant cells [64].
3. NPs AND ROS
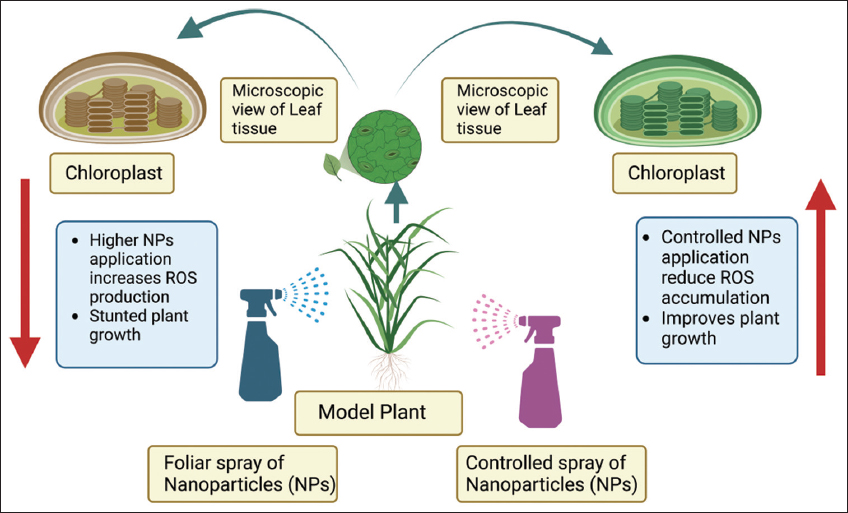
We scrutinized that the NPs contributed to oxidative stress by causing lipid peroxidation, a decrease in chlorophyll content, and the synthesis of GSSG [65]. Zn-based NPs persuaded free radicals development in Triticum aestivum, resulting in a rise MDA and down GSH amount and chlorophyll levels [66-68]. Kim et al. [69] demonstrated the toxic level of CuO-NPs on Cucumis sativus which exhibited a considerable enhancement of ROS. Oxidative stress caused by Cu-NPs has also been observed on Vigna radiata and T. aestivum grown on agar media and resulted in stunted seedling and shoot height [70]. The underlying mechanisms related to the generation of NP-generated ROS vary depending on the kind of NP, and the real cellular process relating to ROS production is yet unknown [34]. The higher formation of ROS by the stimulation of NPs exposure can induce oxidative stress and alter the all metabolic functioning of the plants and leads cell death and reduced growth [Figure 2]. Most of the NPs may incite the free-radicals facilitated toxicity through Fenton-type reactions (Huang et al., 2010). Since the primary result of NP-induced cellular harm or malfunction of cells is the result of ROS genesis [71]. Under biotic (fungal and bacterial) and abiotic (drought, salt, and cold) stress, (mitogen-activated protein kinase kinase kinase 1 [MEKK1]) and MAP3K are switch-on. MEKK1 is turn-on due to ROS formation. Production of ROS under different stresses start-up the MPK6 and MP3K cause diverse response.
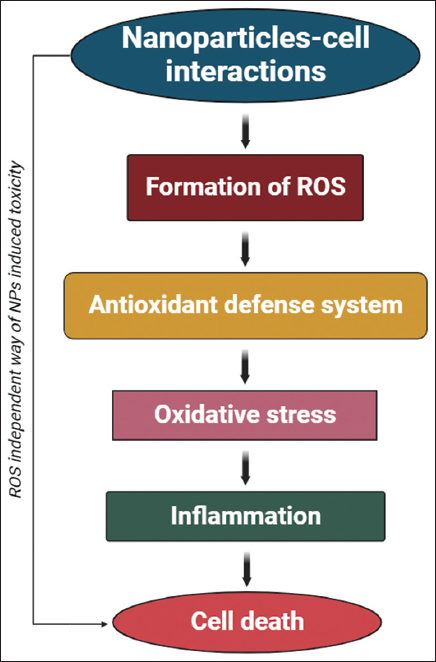 | Figure 2: A schematic representation of nanoparticles-mediated reactive oxygen species generation leading to cell death. [Click here to view] |
Plants establish an antioxidant defense system to scavenge the excessive ROS to combat oxidative stress which performs as an adaptive response mechanism [72,73]. The cellular amount of ROS and physio-biochemical states are tightly controlled by diverse detoxifying enzymes, including CAT, glutathione peroxidase (GPX), SOD as well as a variety of antioxidants, including flavonoids, ascorbic acid, GSH, and Vitamin E. ROS is produced as intermediates under various physiobiochemical states [51,74]. In unfavorable conditions, ROS accumulations also enhance, and with the assistance of precise signal transduction pathways, they contribute to the plant’s defense mechanism [42,75].
The increased amount of ROS genesis during stress conditions within the cell, membrane damage occurs along with DNA, RNA, and protein synthesis can result in cell death through oxidative stress [76,77]. As a result of changes in metabolic activity and as a subtype of the abiotic stress response signal transduction network, ROS are produced as the primary cause of ROS in abiotic stress [Figure 3] [78].
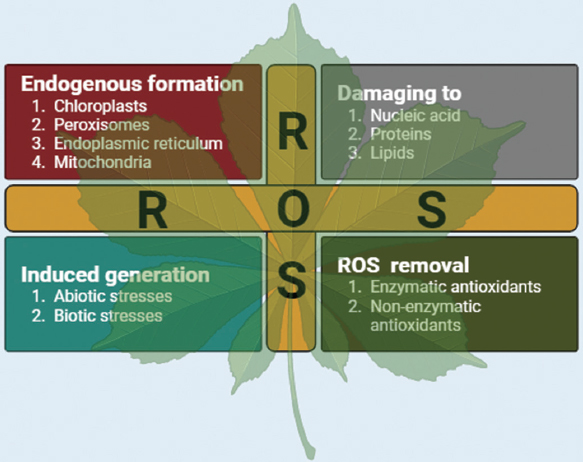 | Figure 3: Schematic representation of reactive oxygen species formation, its effects and removal. [Click here to view] |
4. BIOSTIMULANT FOR REGULATION OF ROS IN PLANTS UNDER ABIOTIC STRESS
Plants under stress conditions especially under abiotic stress (salinity, drought, heat, water, metal, chilling, and temperature) faces various deteriorating conditions to survive. Plants had developed diverse internal mechanisms and defensive criteria to survive in the stressful conditions [79]. Some of them are immune stimulation, molecular metabolism, and especially biostimulant. Biostimulant promotes defense mechanisms, improve growth, yield, climate, and stress resilience [80]. Plant interaction with stimulants reveals reactions with enzymes, antioxidants, metabolic fluxes, and cellular physiology [81]. Crop health improvement through minimizing ROS production of various plants is performed by proper application of biostimulant [82]. Although more scientific ground is required for properly understanding the functions of biostimulant during stressful conditions, Yakhin et al. [83] suggested comparatively detailed information about the functions of biostimulant which help provide nutrients to plants, assimilation, soil health improvement, etc.
Salinity stress in lettuce is induced by improving reluctant metabolites like sterols, terpenes, etc. by using protein based biostimulant [84]. Tomato (Solanum lycopersicum) plants under salt stress were also observed using seaweed-based biostimulant, which accelerated proline and antioxidant contents in plants [85]. Vegetal biopolymer in melon under stress improves growth conditions, root growth, photosynthetic activity, and hormonal interaction [86]. Drought-resistant protein-based biostimulant derivatives in tomato mitigate oxidative stress; reduce hormonal imbalance, and excess level enzyme content [87]. Calcium-treated rice (Oryza sativa) plants stimulate MDA, LOX, MDHAR, and GR contents tremendously where Mn-treated plants show reduced amount of MDA, DHA, improves SOD, MDHAR in rice [88]. Biochar application in Brassica chinensis and Spinacia oleracea lead to MDA reduction, APX increases internally [89]. GABA co-treatment in Brassica juncea L. cv. BARI Sharisha-11 contributed similarly to biochar treatment and accelerated enzymatic functions [90].
5. CONCLUSION AND FUTURE PERSPECTIVE
This review has summarized the wealth of data on the ROS generation due to higher accumulation of NPs. Elevated amount of NPs increased the level of MDA and lipid peroxidation which leads to oxidative stress. Enhancement of ROS leads in reduced morpho-physiological attributes and crop yield. NPs altered the polyunsaturated fatty acids, injured cell membrane accessibility and disturbed cell shape, harm protein and DNA, finally cell death. To decrease the formation of ROS is helpful in sustainable crop production and crop health. Biostimulant is helpful to control the production of ROS under abiotic stress conditions, increase defense performance, growth indices, crop yield, and abiotic stress resilience. In future, effects of NPs on the production of ROS under omics, metabolomics, and transcriptomics level could be explored.
6. ACKNOWLEDGMENTS
The study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1444). TM and VDR acknowledge the financial support by the Ministry of Science and Higher Education of the Russian Federation (no.FENW-2023-0008).
7. AUTHORS’ CONTRIBUTIONS
MF, PA, SH, and VDR: Conceptualization, SA and SHT: Investigation, AF and SHT: Resources, MF, ANY, and SA: Writing—original draft preparation, PA and SMA: Writing—review and editing, TM: Visualization, SH: Supervision.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the sources of data provided in this manuscript have duly been referred in the references which are freely available in public domain.
11. Publisher’s Note
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Pirtarighat S, Ghannadnia M, Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostructure Chem 2019;9:1-9. [CrossRef]
2. Castillo-Henríquez L, Alfaro-Aguilar K, Ugalde-Álvarez J, Vega-Fernández L, de Oca-Vásquez GM, Vega-Baudrit JR. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020;10:1763. [CrossRef]
3. Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, et al. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 2021;161:122-30. [CrossRef]
4. Faizan M, Faraz A, Hayat S, Bhat JA, Yu F. Zinc oxide nanoparticles and epibrassinolide enhanced growth of tomato via modulating antioxidant activity and photosynthetic performance. Biocell 2021;45:1081. [CrossRef]
5. Ealia SA, Saravanakumar MP. A review on the classification, characterisation, synthesis of nanoparticles and their application. Proc IOP Conf Ser Mater Sci Eng 2017;263:32019. [CrossRef]
6. Ahmad N, Sharma S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain Chem 2012;2:141-7. [CrossRef]
7. Koul A, Kumar A, Singh VK, Tripathi DK, Mallubhotla S. Exploring plant-mediated copper, iron, titanium, and cerium oxide nanoparticles and their impacts. In:Nanomaterials in Plants, Algae, and Microorganisms. Netherlands:Elsevier;2018. 175-94. [CrossRef]
8. Hazarika A, Yadav M, Yadav DK, Yadav HS. An overview of the role of nanoparticles in sustainable agriculture. Biocatal Agric Biotechnol 2022;43:102399. [CrossRef]
9. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363-83. [CrossRef]
10. Mhamdi A, Van Breusegem F. Reactive oxygen species in plant development. Development 2018;145:dev164376. [CrossRef]
11. Noctor G, Lelarge-Trouverie C, Mhamdi A. The metabolomics of oxidative stress. Phytochemistry 2015;112:33-53. [CrossRef]
12. Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, et al. Antioxidant defense responses:Physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 2009;31:427-36. [CrossRef]
13. Sewelam N, Kazan K, Schenk PM. Global plant stress signaling:Reactive oxygen species at the cross-road. Front Plant Sci 2016;7:187. [CrossRef]
14. Kido EA, Ferreira Neto JR, Silva RL, Belarmino LC, Bezerra Neto JP, Soares-Cavalcanti NM, et al. Expression dynamics and genome distribution of osmoprotectants in soybean:Identifying important components to face abiotic stress. BMC Bioinform 2013;14 Suppl 1:S7. [CrossRef]
15. Kumar K, Kumar M, Kim SR, Ryu H, Cho YG. Insights into genomics of salt stress response in rice. Rice (N Y) 2013;6:27. [CrossRef]
16. Jalil SU, Ansari MI. Physiological role of gamma-aminobutyric acid in salt stress tolerance. In:Salt and Drought Stress Tolerance in Plants. Netherlands:Springer;2020. 337-50. [CrossRef]
17. Bhattacharjee S. Reactive oxygen species and oxidative burst:Roles in stress, senescence and signal transducation in plants. Curr Sci 2005;89:1113-21.
18. Kadota Y, Shirasu K, Zipfel C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol 2015;56:1472-80. [CrossRef]
19. Stankovic-Valentin N, Melchior F. Control of SUMO and ubiquitin by ROS:Signaling and disease implications. Mol Aspects Med 2018;63:3-17. [CrossRef]
20. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002;7:405-10. [CrossRef]
21. Swanson S, Gilroy S. ROS in plant development. Physiol Plant 2010;138:384-92. [CrossRef]
22. Foyer CH. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 2018;154:134-42. [CrossRef]
23. Del Río LA, López-Huertas E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol 2016;57:1364-76.
24. Bogatek R, Gniazdowska A. ROS and phytohormones in plant-plant allelopathic interaction. Plant Signal Behav 2007;2:317-8. [CrossRef]
25. Yamasaki H, Ogura MP, Kingjoe KA, Cohen MF. d-Cysteine-induced rapid root abscission in the water fern Azolla pinnata:Implications for the linkage between d-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants (Basel) 2019;8:411. [CrossRef]
26. Nadarajah KK. ROS homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 2020;21:5208. [CrossRef]
27. García-López JI, Niño-Medina G, Olivares-Sáenz E, Lira-Saldivar RH, Barriga-Castro ED, Vázquez-Alvarado R, et al. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants (Basel) 2019;8:254. [CrossRef]
28. Faizan M, Faraz A, Hayat S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J Plant Biochem Biotechnol 2020;29:553-67. [CrossRef]
29. Faizan M, Faraz A, Mir AR, Hayat S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J Plant Growth Regul 2021;40:101-15. [CrossRef]
30. Faizan M, Sehar S, Rajput VD, Faraz A, Afzal S, Minkina T, et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants (Basel) 2021;10:2254. [CrossRef]
31. Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S. Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 2020;39:641-55. [CrossRef]
32. Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019;214:269-77. [CrossRef]
33. Rizwan M, Ali S, Zia Ur Rehman M, Adrees M, Arshad M, Qayyum MF, et al. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ Pollut 2019;248:358-67. [CrossRef]
34. Dayem AA, Hossain MK, Lee SB, Kim K, Saha SK, Yang GM, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 2017;18:120. [CrossRef]
35. Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 2016;17:327. [CrossRef]
36. Jan B, Bhat TA, Sheikh TA, Wani OA, Bhat MA, Nazir A, et al. Agronomic bio-fortification of rice and maize with iron and zinc:A review. Int Res J Pure Appl Chem 2020;21:28-37. [CrossRef]
37. Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, et al. Reactive oxygen species in plants:From source to sink. Antioxidants (Basel) 2022;11:225. [CrossRef]
38. Wu H, Yin JJ, Wamer WG, Zeng M, Lo YM. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J Food Drug Anal 2014;22:86-94. [CrossRef]
39. Zandi P, Schnug E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology (Basel) 2022;11:155. [CrossRef]
40. Lu Y, Tsuda K. Intimate association of PRR-and NLR-mediated signaling in plant immunity. Mol Plant Microbe Interact 2021;34:3-14. [CrossRef]
41. Lukan T, Coll A. Intertwined roles of reactive oxygen species and salicylic acid signaling are crucial for the plant response to biotic stress. Int J Mol Sci 2022;23:5568. [CrossRef]
42. Huang H, Ullah F, Zhou DX, Yi M, Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 2019;10:800. [CrossRef]
43. Saleem M, Fariduddin Q, Castroverde CD. Salicylic acid:A key regulator of redox signalling and plant immunity. Plant Physiol Biochem 2021;168:381-97. [CrossRef]
44. Calil IP, Fontes EP. Plant immunity against viruses:Antiviral immune receptors in focus. Ann Bot 2017;119:711-23. [CrossRef]
45. Baebler Š, Witek K, Petek M, Stare K, Tušek-Žnidaric M, Pompe-Novak M, et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J Exp Bot 2014;65:1095-109. [CrossRef]
46. Liu Y, He C. Regulation of plant reactive oxygen species (ROS) in stress responses:Learning from AtRBOHD. Plant Cell Rep 2016;35:995-1007. [CrossRef]
47. Yi SY, Shirasu K, Moon JS, Lee SG, Kwon SY. The activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition. PLoS One 2014;9:e88951. [CrossRef]
48. Chang YL, Li WY, Miao H, Yang SQ, Li R, Wang X, et al. Comprehensive genomic analysis and expression profiling of the NOX gene families under abiotic stresses and hormones in plants. Genome Biol Evol 2016;8:791-810. [CrossRef]
49. Nath M, Bhatt D, Prasad R, Gill SS, Anjum NA, Tuteja N. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front Plant Sci 2016;7:1574. [CrossRef]
50. Petrov V, Hille J, Mueller-Roeber B, Gechev TS. ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 2015;6:69. [CrossRef]
51. Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2014;2:53. [CrossRef]
52. Kandhol N, Aggarwal B, Bansal R, Parveen N, Singh VP, Chauhan DK, et al. Nanoparticles as a potential protective agent for arsenic toxicity alleviation in plants. Environ Pollut 2022;300:118887. [CrossRef]
53. Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020;10:240. [CrossRef]
54. Talbi S, Romero-Puertas MC, Hernández A, Terrón L, Ferchichi A, Sandalio LM. Drought tolerance in a saharian plant Oudneya africana:Role of antioxidant defences. Environ Exp Bot 2015;111:114-26. [CrossRef]
55. Nahar S, Vemireddy LR, Sahoo L, Tanti B. Antioxidant protection mechanisms reveal significant response in drought-induced oxidative stress in some traditional rice of Assam, India. Rice Sci 2018;25:185-96. [CrossRef]
56. Contreras RA, Pizarro M, Köhler H, Sáez CA, Zúñiga GE. Copper stress induces antioxidant responses and accumulation of sugars and phytochelatins in Antarctic Colobanthus quitensis (Kunth) Bartl. Biol Res 2018;51:48. [CrossRef]
57. Waszczak C, Akter S, Jacques S, Huang J, Messens J, Van Breusegem F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J Exp Bot 2015;66:2923-34. [CrossRef]
58. Waszczak C, Carmody M, Kangasjärvi J. Reactive oxygen species in plant signaling. Annu Rev Plant Biol 2018;69:209-36. [CrossRef]
59. Cui F, BroschéM, Shapiguzov A, He XQ, Vainonen JP, LeppäläJ, et al. Interaction of methyl viologen-induced chloroplast and mitochondrial signalling in Arabidopsis. Free Radic Biol Med 2019;134:555-66. [CrossRef]
60. Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM. Photosynthesis-dependent H(2)O(2) transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun 2017;8:49. [CrossRef]
61. Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017;63:70-96. [CrossRef]
62. Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA. When bad guys become good ones:The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 2016;7:471. [CrossRef]
63. PiterkováJ, LuhováL, NavrátilováB, SedlárováM, PetrivalskýM. Early and long-term responses of cucumber cells to high cadmium concentration are modulated by nitric oxide and reactive oxygen species. Acta Physiol Plant 2015;37:1-12.
64. Kohli SK, Khanna K, Bhardwaj R, Abd Allah EF, Ahmad P, Corpas FJ. Assessment of subcellular ROS and NO metabolism in higher plants:Multifunctional signaling molecules. Antioxidants (Basel) 2019;8:641. [CrossRef]
65. Pirasteh-Anosheh H, Saed-Moucheshi A, Pakniyat H, Pessarakli M. Water Stress and Crop Plants. United States:John Wiley and Sons;2016.
66. Nieder R, Benbi DK, Reichl FX. Soil Components and Human Health. Germany:Springer;2018. [CrossRef]
67. Aarti PD, Tanaka R, Tanaka A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol Plant 2006;128:186-97. [CrossRef]
68. Panda SK, Chaudhury I, Khan MH. Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol Plant 2003;46:289-94. [CrossRef]
69. Kim S, Lee S, Lee I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut 2012;223:2799-806. [CrossRef]
70. Lee WM, An YJ, Yoon H, Kweon HS. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum):Plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 2008;27:1915-21. [CrossRef]
71. Giordo R, Nasrallah GK, Al-Jamal O, Paliogiannis P, Pintus G. Resveratrol inhibits oxidative stress and prevents mitochondrial damage induced by zinc oxide nanoparticles in zebrafish (Danio rerio). Int J Mol Sci 2020;21:3838. [CrossRef]
72. Fatma M, Iqbal N, Sehar Z, Alyemeni MN, Kaushik P, Khan NA, et al. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants (Basel) 2021;10:1216. [CrossRef]
73. Hasanuzzaman M, Bhuyan MH, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress:Revisiting the crucial role of a universal defense regulator. Antioxidants (Basel) 2020;9:681. [CrossRef]
74. Suzuki N, Mittler R. Reactive oxygen species and temperature stresses:A delicate balance between signaling and destruction. Physiol Plant 2006;126:45-51. [CrossRef]
75. Singh H, Bhat JA, Singh VP, Corpas FJ, Yadav SR. Auxin metabolic network regulates the plant response to metalloids stress. J Hazard Mater 2021;405:124250. [CrossRef]
76. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277-84.
77. Pang CH, Wang BS. Oxidative stress and salt tolerance in plants. In:Progress in Botany. Netherland:Springer;2008. 231-45. [CrossRef]
78. Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J 2017;90:856-67. [CrossRef]
79. Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun 2012;3:926. [CrossRef]
80. Fleming TR, Fleming CC, Levy CC, Repiso C, Hennequart F, Nolasco JB, et al. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann Appl Biol 2019;174:153-65. [CrossRef]
81. Tugizimana F, Steenkamp PA, Piater LA, Labuschagne N, Dubery IA. Unravelling the metabolic reconfiguration of the post-challenge primed state in Sorghum bicolor responding to Colletotrichum sublineolum infection. Metabolites 2019;9:194. [CrossRef]
82. Rouphael Y, Colla G. Toward a sustainable agriculture through plant biostimulants:From experimental data to practical applications. Agronomy 2020;10:1461. [CrossRef]
83. Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in plant science:A global perspective. Front Plant Sci 2016;7:2049. [CrossRef]
84. Lucini L, Rouphael Y, Cardarelli M, Canaguier R, Kumar P, Colla G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci Hortic 2015;182:124-33. [CrossRef]
85. El Arroussi H, Benhima R, Elbaouchi A, Sijilmassi B, El Mernissi N, Aafsar A, et al. Dunaliella salina exopolysaccharides:A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J Appl Phycol 2018;30:2929-41. [CrossRef]
86. Lucini L, Rouphael Y, Cardarelli M, Bonini P, Baffi C, Colla G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front Plant Sci 2018;9:472. [CrossRef]
87. Paul K, Sorrentino M, Lucini L, Rouphael Y, Cardarelli M, Bonini P, et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front Plant Sci 2019;10:493. [CrossRef]
88. Rahman A, Mostofa MG, Nahar K, Hasanuzzaman M, Fujita M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Brazil J Bot 2016;39:393-407. [CrossRef]
89. Kamran M, Malik Z, Parveen A, Zong Y, Abbasi GH, Rafiq MT, et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J Environ Manag 2019;250:109500. [CrossRef]
90. Vargas JT, Rodríguez-Monroy M, Meyer ML, Montes-Belmont R, Sepúlveda-Jiménez G. Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ Exp Bot 2017;136:85-93. [CrossRef]
91. Quan L, Zhang B, Shi W, Li H. Hydrogen peroxide in plants:A versatile molecule of the reactive oxygen species network. J Integr Plant Biol 2008;50:2-18. [CrossRef]
92. Bartoli CG, Gómez F, Martinez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 2004;55:1663-9. [CrossRef]
93. You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 2015;6:1092. [CrossRef]
94. Das P, Nutan KK, Singla-Pareek SL, Pareek A. Oxidative environment and redox homeostasis in plants:Dissecting out significant contribution of major cellular organelles. Front Environ Sci 2015;2:70. [CrossRef]
95. Glyan'ko AK, Ischenko AA. Structural and functional characteristics of plant NADPH oxidase:A review. Appl Biochem Microbiol 2010;46:463-71. [CrossRef]
96. Kolupaev YE, Karpets YV. Participation of reactive oxygen species in formation of induced resistances of plants to abiotic stressors. In:Handbook on Reactive Oxygen Species (ROS):Formation Mechanisms, Physiological Roles and Common Harmful Effects. New York:Nova Science Publishers;2013. 109-35.
97. Sharma P, Jha A, Dubey RS, Pessaraklim RO. Oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012;2012:1-26. [CrossRef]
98. Zhang Z, Zhang Q, Wu J, Zheng X, Zheng S, Sun X, et al. Gene knockout study reveals that cytosolic ascorbate peroxidase 2 (OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS One 2013;8:e57472. [CrossRef]
99. Zarepour M, Kaspari K, Stagge S, Rethmeier R, Mendel RR, Bittner F. Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol Biol 2010;72:301-10. [CrossRef]