1. INTRODUCTION
Tinospora cordifolia (Willd.) Hook. F. and Thoms., is a well-known medicinally important plant in Ayurveda which is commonly known as Giloy, Guduchi, Lat gulancha, Gurju, heart-leaved moonseed belonging to the family Menispermaceae. It is a large, deciduous, extensively spreading climbing shrub found in China and the Indian subcontinent such as Bangladesh, India, and Myanmar Sri Lanka [1,2]. In general, it is found in habitats in deciduous and dry forests. Conventionally, T. cordifolia is widely used as a medicine not only in tribal communities but also in modern countries. In the modern world, medicinally important plants are termed “Green Gold” due to their diverse biological applicability [3,4]. Most importantly, the T. cordifolia plant possess diverse ethnomedicinal values due to its significant application for the treatment of fever, gout, ulcer, rheumatism, diarrhea, old wound, burn, previous findings show that, this plant has anti-inflammatory, anti-allergic, anti-oxidant, anti-diabetic, anti-cancer, anti-microbial, immunomodulatory, anti-HIV, and anti-toxic activities [5]. This plant offers a veritable storehouse of biologically important alkaloids, flavonoids, glycosides, and phenolic compounds such as berberine, tinosporine, magnoflorine, cordifolioside, tinocordioside, and isocolumbin. [6].
At present, the plant faces immense threats primarily due to mindless destructions of their natural habitats by local people and pharmaceutical companies which are aggravated by its poor seed set and poor germination in natural condition. Vegetative propagation by stem cutting is also season-dependent [7]. That is why there is a need of standard protocol for a rise in seed germination of T. cordifolia seed. The germination behavior of T. cordifolia has not been well explored. Only two studies on germination of T. cordifolia reported until now [8,9]. An experimental results from the previous reports suggested that a moderate germination rate was achieved in T. cordifolia seed which is up to 57.5% [8]. Another study reported that control seeds show a decrease in germination as the seeds getting old. At 30oC temperature, fresh seeds exhibit 67% germination while the germination rate decrease at 31% within 6 months. The seeds of T. cordifolia, stored at a temperature of 20°C, exhibit an 80% germination rate, with the entire germination process spanning a duration of 62 days. Contrary to current findings, our GA treatment-based approach could support 100% seed germination within 10 days of sowing, which is remarkably better than earlier reports. Germination is a process where a plant grows within a seed or reactivation of the metabolic machinery of a seed resulting in the formation of a seedling. In general, seed germination depends on both internal and external factors such as water, oxygen, light, temperature, and hormones. Moreover, some seeds are extremely sluggish to germinate even in favorable environmental conditions due to losing germinability or remain in the dormant phase. With time, aged seeds start to lose their potential to germinate too. However, some seed germination enhancers can be used to prolong the ability of seeds to germinate. Gibberellins (GAs) and potassium nitrate (KNO3) are used to induce seed germination [10].
According to previous reports, GA3 and KNO3 are very much effective to enhance the germination of seeds [11]. The plant growth-promoting agent GA3 is a vital growth regulator for germination as well as for seedling growth. GA3 can enhance the synthesis of α-amylase and can hydrolyze the starch to glucose. It is a very crucial step for seed germination, as it provides nutrition to germinating seeds [12-14]. In addition, light enhances the efficacy of GA3 by stimulating the degradation pathway of phytochrome-interacting Factor 1, which is a negative regulator of GA3 synthesis. Besides the nutrient and light, water and oxygen play important metabolic functions promoting seed germination. It is reported that KNO3 increases water uptake by inducing changes in water potential. Moreover, the increase in the absorption of water results in the stimulation of respiration and auxin production during germination [15,16]. An earlier report shows that KNO3 promotes germination by increasing ambient oxygen levels as well as through reduction to ammonium ions which is essential for plant nutrients [17]. With regard to selection of GA3 and KNO3, it has been reported that the decline in germination in aged seeds of Plantago major was effectively halted by GA3 and KNO3 application [18]. The previous research found, that age of seed has direct correlation with the decline of seed germination frequency in P. lanceolata, which was successfully reduced by GA3 and KNO3 treatments [10].
This study is aimed to achieve enhanced seed germination of T. cordifolia (which loses viability within 90 days), within a short span of post-imbibition time with a reliable and economic protocol using GA3 and KNO3. To fulfill our objective, we have calculated the germination parameters (final germination percentage [FGP], mean germination time (MGT), coefficient velocity of germination, germination rate index [GRI]) seedling growth parameters (root length [RL], shoot length [SL], fresh weight [FW], dry weight [DW], vigor index [VI]), and recorded changes in biochemical profile (seed protein, alpha-amylase, and soluble sugar) related to germination event by investigating the effect of GA3 and KNO3 treatment on the seed germination. This study also helps in sustainable use of T. cordifolia seeds to extend further research and propagation.
2. MATERIALS AND METHODS
2.1. Collection of Seeds
Red-colored ripe fruits of T. cordifolia were collected from Raghunathpur (23° 32’ 19.0716’’ N and 86° 40’ 24.7908” E) Purulia, West Bengal, India. Then thoroughly washed in fresh running water. Later on, the skin and pulp were removed completely to get clean and mature seeds.
2.2. Preparation of T. cordifolia Seeds to Study Germination Pattern
Mature and dried seeds (7, 30, 60, and 90 days old) were left for imbibition in distilled water for 24 h. There month old seeds were subjected to chemical treatment with GA3 (Sigma-Aldrich, BioReagent, suitable for plant cell culture, purity ≥ 90%) and KNO3 (Sigma-Aldrich, BioReagent, suitable for plant cell culture) singly applied at different concentrations (100, 300, and 500 ppm). Surface sterilization was performed with 0.1% mercuric chloride for 60 s and subsequently washed with sterilized distilled water. The sterilization procedure has been done in a sterilized chamber.
2.3. Experimental Set Up
All experimental sets with three replicas were prepared in each petri plate (9 cm) containing 10 seeds. Optimal germination conditions were determined at room temperature with 75% relative humidity. All experiments were repeated for 3 times. Germination data were collected every 24 h time interval for 10 days after sowing (DAS) the seeds and the data of seedling growth were also collected on 20 DAS. Soluble sugar and protein content were recorded just after 24 h imbibition and amylase activity was recorded at 0 h, 24 h, and 48 h after imbibition.
2.4. Calculation of Germination Parameters
Following germination parameters were used in the present study to assess the seed germination pattern of T. cordifolia.
FGP: Final No. of seeds germinated in a seed lot ×100. The higher the FGP value, the greater the seed population germination [19].
MGT: MGT= ∑f.x/∑f, f = Seeds germinated on day x. The lower the MGT, the faster a population of seeds germinated [20].
Coefficient of Velocity of Germination (CVG): N1 + N2 +···+ Nx/100 × N1T1 +···+ Nx Tx; N = No. of seeds germinated each day, T = No. of days from seeding corresponding to N. Jones and Sanders [21].
GRI: G1/1 + G2/2 +···+ Gx/x; G1 = Germination percentage × 100 at the 1st day after sowing, G2 = Germination percentage × 100 at the 2nd day after sowing. Higher GRI values indicate higher and faster germination [22].
VI: Seedling length × Germination FGP [23].
2.5. Measurement of Total Protein Content
The protein content was measured by following the method described by Lowry et al. In this experiment, 500 mg tissue was used and homogenized in 10 mL of sodium phosphate buffer (0.5 M, pH = 8). Then the tissue was centrifuged at 10,000 rpm for 10 min at 4°C. Next, the phosphate buffer was used to dilute the supernatant, and 1 mL of this solution was combined with 5 mL of alkaline copper solution (50 mL of 2% sodium carbonate in 0.1 (N) sodium hydroxide added to 1 mL of 0.5% copper sulfate pentahydrate in 1% sodium potassium tartrate). After incubation at room temperature for 10 min, the mixture was added to the 0.5 mL of Folin-Ciocalteau reagent. Finally, the blue color was measured at 660 nm after 30 min of incubation in the dark at room temperature with a spectrophotometer (Lasany microcomputer Single Beam Visible Spectrophotometer LI-721). The concentration was calculated using a BSA standard curve [24].
2.6. Measurement of Soluble Sugar Content
The number of carbohydrates (soluble) was calculated using the protocol as described by McCready et. al., (1950) with appropriate modifications. At first, 100 mg of samples were homogenized with 80% ethanol and boiled for 1 min, before being centrifuged at 6000 rpm for 10 min. For soluble carbohydrates, the supernatant was collected and dried over a watch glass at room temperature. To remove the chlorophyll, the dried samples were washed several times with pet ether. Next to the washing of dried samples, it was dissolved in 10 mL of 80% ethanol. Subsequently, 1 mL of ethanolic sample solution was mixed with 5 mL of freshly produced pre-chilled 0.2% anthrone reagent. After 30 min of incubation in the dark, the blue-green color was detected with a spectrophotometer (Lasany microcomputer Single Beam Visible Spectrophotometer LI-721) at 620 nm. The glucose-based standard curve was used to calculate the carbohydrate concentration [25].
2.7. Measurement of Amylase Activity
The amylase activity was evaluated based on the standard method by Miller (1959) with some modifications. In this experiment, 100 mg tissue was homogenized with ice-cold 1 mL of 10 mM CaCl2 solution and incubated at room temperature for 3 h. Then centrifuged at 10000 rpm for 10 min at 4°C. Subsequently, 0.1 mL of the supernatant solution was mixed with 1 mL of 1% w/v of the starch solution following the addition of 0.9 mL of 0.1 M sodium acetate buffer (at pH = 4.7). The reaction mixture was incubated for 30 min at 37°C. The reaction was then quenched by the addition of 2 mL dinitro salicylic acid and 1 mL of 40% sodium potassium tartrate solution and boiled for 20 min. After cooling, the sample in running tap water and the released sugar (reduced) was measured using a spectrophotometer at 520 nm, using glucose as a standard [26].
2.8. Statistical Analysis
Analysis of variance (one-way analysis of variance [ANOVA]) and standard deviation was performed by MS Excel 2016. Multiple means of comparison were determined by analysis of variance (one-way ANOVA) using the post hoc Tukey test for each germination parameter. Then, auto-scaling normalization of germination parameter data was done to remove unwanted analytical variation, to correct inter and intra batch variability, and reduce the influence of outliers. The Pearson correlation was performed to observe correlation between FGP and the rest of the germination parameters on the normalized data obtained from germination parameters. All statistical analyses were performed with Metaboanalyst version 5.0.
3. RESULTS AND DISCUSSION
Seeds of the T. cordifolia are creamy or brown in color. The size of the seed is 5–6 cm. Shape of the seeds is subglobose, covered by a rough and semi-heard seed coat. Seed coat exhibits a longitudinal ridge at the opposite side of micropyle. During germination, seed coat breaks through the longitudinal ridge area.
3.1. Germination Pattern of T. cordifolia Seed
In this present experiment [Figure 1], freshly collected untreated seeds show a gradual decline in germination rate as the seeds getting old until 3 months. 7-day-old seeds register a maximum germination percentage (83.33 ± 5.7) among untreated seed sets. For 3-month-old seed set, there was a sharp fall in germination rate (13.33 ± 5.7). One month and 2-month-old seeds show moderate to low germination ability (66.66 ± 5.7 and 33.33 ± 5.7, respectively). Hence from the above result, it is clear that the seeds of T. cordifolia did not show any dormancy but exhibit a gradual decrease in germination percentage within 3 months.
 | Figure 1: Germination response of Tinospora cordifolia seed. (a) 7 days old seeds, (b) 30 days old seeds, (c) 60 days old seeds and (d) 90 days old seeds showing germination. Each plate contains 10 seeds. [Click here to view] |
3.2. Germination Parameters
Germination is a pre-program developmental response of a seed that takes place at a particular time, although each seed under treatment responds at different time intervals. Thus, it is clear that only the final germination percentage is not a sufficient parameter to reflect all germination events, rather it requires consideration of other germination parameters too. The aspects, FGP, MGT, CVG, and GI were used in this study to compare the germination capacities of 3-month-old T. cordifolia seeds under various treatments and untreated seed sets.
3.2.1. FGP
FGP is an important parameter for seed germination which reflects the viability of a seed lot. In this study, the experimental results of 3 months old T. cordifolia seed germination show that the treatment with 300 and 500 ppm of GA3 gives the maximum (100 ± 0) germination percentage within 10 DAS [Figure 2 and Table 1]. While KNO3 treated seeds result in a very low-to-moderate germination percentage (26.66 ± 5.77–30 ± 0). On the contrary, the control seed sets that are soaked with water do not provide any observable result. Thus, the obtained data suggest that the germination percentage under the treatment with high concentrations of GA3 (300 and 500 ppm) is significantly high, but it gives a relatively low germination percentage (76.66 ± 15.27) when the treatment concentration of 100 ppm GA3 is relatively low. Overall, the results clearly suggest that 300 and 500 ppm of GA3 provide the maximum rate of germination percentage, while, the untreated (water-soaked) seed fail to yield germination (13.33 ± 5.77).
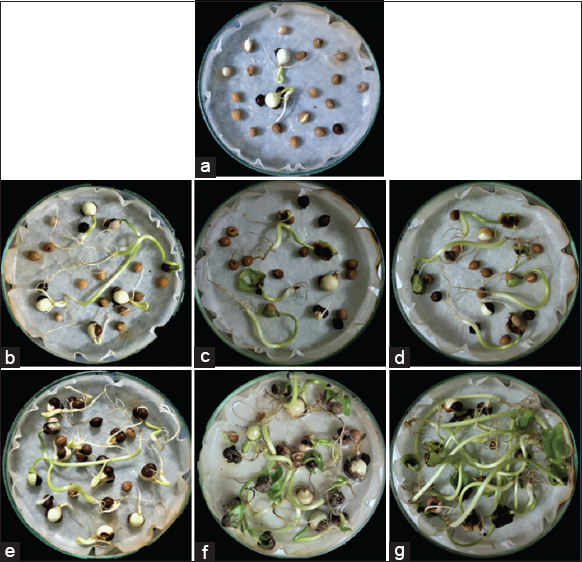 | Figure 2: Germination response of 90 days old Tinospora cordifolia seeds (20 seeds in each petriplate) showing (a) control set, (b) 100 ppm potassium nitrate (KNO3), (c) 300 ppm KNO3, (d) 500 ppm KNO3, (e) 100 ppm GA3, (f) 300 ppm GA3, (g) 500 ppm GA3. [Click here to view] |
Table 1: Calculation of various germination parameters from the seed (3 months old) germination experiments.
| Germination parameters | FGP (%) | MGT (D) | GRI | CVG |
|---|---|---|---|---|
| Control | 13.33±5.77a | 7.00±2.00a | 2.17±2.06a | 15.13±4.50a |
| KNO3 | ||||
| 100 ppm | 30±0ab | 6.40±0.17ab | 4.60±0.20ab | 15.52±0.45ab |
| 300 ppm | 26.66±5.77abc | 5.33±0.57abc | 5.23±1.3abc | 18.88±1.92abc |
| 500 ppm | 30±0abc | 5.4±0.72abcd | 5.63±0.80abc | 18.57±2.51abcd |
| GA3 | ||||
| 100 ppm | 76.66±15.27 | 5.30±0.78abcde | 15.23±5.68 | 18.88±3.12abcde |
| 300 ppm | 100±0d | 5.00±0.20abcdef | 22.60±2.11d | 20.02±0.80abcdef |
| 500 ppm | 100±0d | 4.83±0.23abcdef | 22.70±1.25d | 20.71±0.96d |
FGP: Final germination percentage, MGT (D): Mean germination time (D), GRI: Germination rate index, CVG: Coefficient of velocity of germination, KNO3: Potassium nitrate, GA3: Gibberellic acid, data are means of three replications±SE. Columns with different letters are significantly different at P<0.05.
3.2.2. MGT
MGT specifies the day when maximum germination occurs but does not notify the time span or percentage of germination. Thus, lower MGT values represent faster germination of a seed population or vice versa. The experimental data show that 100 ppm of KNO3 treated seeds (3 months old) gives the highest value of MGT (6.40 ± 0.17 days) while 500 ppm of GA3 treated sets gives the lowest value of MGT (4.83 ± 0.23) among all the treated seed sets [Table 1]. On the other hand, the control set shows a maximum MGT value (7.00 ± 2.00 days). The result indicates that the maximum number of untreated seeds germinated by 7.00 ± 2.00 days whereas, 500 ppm of GA3 treated seeds germinate in the highest number in a considerable shorter time (4.83 ± 0.23) day. Overall, as with other parameters, GA3 provides the best result among the other treated condition.
3.2.3. CVG
Among the germination parameters, CVG gives an indication of the rapidity of germination. Theoretically, the highest CVG maybe 100 only when all seeds germinated on the 1st day. The present experimental data of 3 months old T. cordifolia seed germination indicates that among the GA3-treated seeds, 500 ppm gives the best CVG value (20.71 ± 0.96), while 100 ppm GA3-treated seeds show least CVG value (18.88 ± 3.12) [Table 1]. However on the contrary, KNO3-treated seeds show low to moderate results (15.52 ± 0.45–18.57± 2.51). Control set shows least CVG (15.13 ± 4.50) among all experimental sets. The control set shows a significant difference with 500 ppm GA3 treated seeds where rest of the treated seed sets show non-significant difference with the control set [Table 1].
3.2.4. GRI
GRI is the measure of the germination percentage every single day. Hence, the higher the GRI value, the faster will be the germination rate. In our experiment, GA3-treated seeds with concentrations of 300 and 500 ppm show the maximum GRI values (22.60 ± 2.11 and 22.70 ± 1.25, respectively) [Table 1]. Whereas, 100 ppm of GA3 gives a decrease in GRI values (15.23 ± 5.68). From the result, it is clear that in the case of a high concentration of GA3 has no difference in GRI values of T. cordifolia seeds. On the other hand, in the case of KNO3-treated seeds, there is a sharp fall in the GRI values. KNO3-treated seeds show extremely low values of GRI (4.60 ± 0.20–5.63 ± 0.80). In untreated seed sets, GRI value is the lowest (2.17 ± 2.06) among all seed sets [Table 1].
T. cordifolia seeds (3 months old) reached up to 100% when treated with 300 and 500 ppm of GA3, while the 100 ppm of GA3 resulted in a slightly low germination percentage [Table 1]. On the other hand, all the KNO3-treated seeds (100, 300, and 500 ppm) exhibit poor germination percentage and the untreated seed set shows unsatisfactory results. In addition, other seed germination parameters such as CVG and GRI, also shows tremendous result at 300 and 500 ppm of GA3. However, the MGT values were not distinguishable among all the treated seed sets (KNO3 and GA3), except for 100 ppm of KNO3 and the control seed set. Overall, the results clearly indicate that the higher concentration (500 ppm) of GA3 helps in germination and increases the rate of germination in T. cordifolia seeds [Table 1]. The previous reports state that GA3 can enhance seed germination in a bifunctional manner, firstly by inducing the growth potential of an embryo and secondly by enhancing the hydrolytic enzymes [27]. Therefore, in this study, the increased germination in T. cordifolia seeds under the influence of GA3 may be associated with enhanced enzyme activities as well as improved nutrient contribution. Thus, it can be concluded that the presence of a high concentration (500 ppm) of GA3 is the prime driving force behind the enhanced germination capacity of 3-month-old T. cordifolia seeds.
3.3. Seedling Growth Parameters
Seedling growth and development during germination totally depend on energy stored in the seed. For a successful seedling establishment, seedling growth is a crucial factor. Following growth parameters of 20-day-old seedlings were used to choose a healthy one for further experiment [Figure 3 and Table 2].
 | Figure 3: Growth response of 20 days old Tinospora cordifolia seedling: C – Control set, K1 – 100 ppm potassium nitrate (KNO3), K3 - 300 ppm KNO3, K5 – 500 ppm KNO3, G1 – 100 ppm GA3, G3 – 300 ppm GA3, G5 – 500 ppm GA3. [Click here to view] |
Table 2: Seed sets with different germination parameters used to study seedling growth after germination.
| Growth parameters | SL (cm) | RL (cm) | FW (g) | DW (g) | VI |
|---|---|---|---|---|---|
| Control | 3.80±1.18a | 2.8±0.18a | 0.24±0.07a | 0.01±0.00a | 81.33±7.76 |
| KNO3 | |||||
| 100 ppm | 5.6±0.55ab | 2.1±0.44ab | 0.02±0.00a | 0.39±0.08ab | 232.00±22.5a |
| 300 ppm | 5.33±0.76abc | 5.20±2.25abc | 0.03±0.00b | 0.47±0.02abc | 235.00±20.55a |
| 500 ppm | 7.4±2.8abcd | 6.63±0.59c | 0.03±0.00bc | 0.48±0.02acd | 422.00±54.52 |
| GA3 | |||||
| 100 ppm | 7.66±0.81abcde | 2.70±1.11c | 0.02±0.00bc | 0.31±0.07acde | 766.00±38.07 |
| 300 ppm | 8.33±2.55abcdef | 6.60±1.61cd | 0.03±0.00d | 0.48±0.01cdef | 1516.00±47.25b |
| 500 ppm | 8.56±0.25bcdef | 6.3±1.43cd | 0.06±0.00d | 0.54±0.06df | 1463.0±40.41b |
SL: Shoot length, RL: Root length, FW: Fresh weight, DW: Dry weight, VI: Vigor index. Data are means of three replications±SE. Columns with different letters are significantly different at P<0.05.
3.3.1. SL
According to theory, SL is increased if treated with GA3. In this experiment, GA3 treatments show the highest SL among all sets [Figure 3 and Table 2]. While 300 and 500 ppm GA3 treatment gives the longest shoot (8.33 ± 2.55 and 8.56 ± 0.25 cm respectively) and 100 ppm GA3 treatment shows a slightly smaller length (7.66 ± 0.81 cm) of the shoot [Table 2]. The SLs (7.40 ± 2.8 cm) of the seed set treated with 500 ppm KNO3 are almost the same with 100 ppm GA3. Whereas 100 and 300 ppm KNO3 soaked seed set results in smaller SLs compared to other sets except for the control set. The control set gives the least SL among all. From the above result, it is clear that GA3 treatment promotes SL [Table 2].
3.3.2. RL
As similar to SL, RL is also influenced by GA3 treatment [Table 2 and Figure 3]. RLs are greater in GA3-treated sets. The highest RL (6.60 ± 1.61 cm) shows 300 ppm GA3 [Table 2]. Minimum RL is shown by 100 ppm KNO3 (2.10 ± 0.44 cm), followed by 100 ppm GA3 (2.70 ± 1.11 cm), surprisingly these two RLs are smaller than the control set [Table 2].
3.3.3. FW
The GA3-treated T. cordifolia seeds with 500 ppm concentration yields the highest FW (0.54 ± 0.06 g) [Table 2]. At concentrations of 100 and 300 ppm of GA3, the resulting fresh weight is lower, measured at 0.31 ± 0.07 g and 0.48 ± 0.01 g, respectively, in comparison to the fresh weight observed at 500 ppm GA3 as indicated in Table 2. Among KNO3 treated seed sets, 300 and 500 ppm yield the highest (0.47 ± 0.02 and 0.48 ± 0.02 g, respectively) FW compared to 100 ppm (0.39 ± 0.08 g) [Table 2]. The lowest FW (0.24 ± 0.07g) among all sets showed by the control set. From the above result, it is clear that a sharp difference in FW lies between untreated and treated concentration treated sets).
3.3.4. DW
According to DW, 500 ppm gives the best result (0.06 ± 0.00 g) while 100 and 300 ppm GA3 show lesser FW (0.02 ± 0.00 and 0.3 ± 0.00 g, respectively) [Table 2]. Among KNO3 treated samples, a little variation occurs in DW (0.02 ± 0.00 to 0.03± 0.00 g). Thus, no notable changes in DW were found except only slightly high DW among seed lots at higher GA3 concentrations (500 ppm) [Table 2].
3.3.5. VI
Seed vigor is an important quality parameter to assess the germination and viability of a seed lot. In ISTA Congress, 1977, seed vigor was well defined, as “The sum total of those properties of the seed which determine the level of activity and performance of the seed or seed lot during germination and seedling emergence.” Here seeds treated with 300 ppm GA3 shows the best VI (1516 ± 47.25) among all germinating seed set, and 100 and 500 ppm GA3 soaked seeds exhibit VI of 766 ± 38.07 and 1463 ± 40.41, respectively [Table 2]. Among KNO3-treated seeds 500 ppm KNO3 gives the best VI (422 ± 54.52). The rest of the KNO3-treated seeds show moderate VI (232 ± 22.5–235 ± 20.55). Thus, GA3 positively impacts VI until 300 ppm, which declines thereafter (500 ppm) [Table 2]. The control set shows the least VI 81.33 ± 7.76. Seedling growth is represented by FW, DW, RL, SL, and VI [Table 2]. Among those parameters, FW, DW, and SL show the highest result for 500 ppm GA3. In the case of RL 500 ppm KNO3, 300 and 500 ppm GA3 show the highest length among all treatments. The rest of the treatments show very small RLs except 300 ppm KNO3. As is a combined parameter of germination percentage and seedling height thus, it is an important parameter for the successful establishment of a seedling. In the present study, VI is highest when seeds were treated with 300 ppm GA3. GA3 with 500 ppm concentration also gives a significant result but lower concentration of GA3 shows moderate VI. All concentration of KNO3 gives low to moderate VI where the control set gives a negligible result compare to other treatments. According to Fagge and Mangal, (2011), the effect of GA3 application on the growth and seedling establishment of Bougainvillaea glabra (paper flower), Ixora coccinea (jungle geranium), and Rosa chinensis (Chinese rose) revealed that 100 mg/L GA3 improved root establishment, growth, and development in all tested species [28]. Arteca et al., used four concentrations of GA3 (0.05, 0.5, 5.0, or 50 mg/L) on seven Pelargonium (geranium) cultivars and discovered that a raise in GA3 concentration results, relative growth rate of all examined samples were increased [29]. Similarly in our experiment, a higher concentration of GA3 offers gives better results for seedling growth compared to other treatments.
3.4. Measurement of Biochemical Parameters
It is a well-known fact that during the germination of a seed, primary metabolites such as the number of soluble sugars, proteins, and some enzymes like amylase, and protease are increased in the imbibed seeds that help in the germination [15]. In addition, the chemicals which help in the germination of a seed also enhance the number of primary metabolites as well as promote activation in germinating seeds. In this present study, we have measured the number of soluble sugars, proteins, and amylase activity to investigate the germinating capacities of KNO3 and GA3 in the germination of T. Cordifolia seeds [Figure 4].
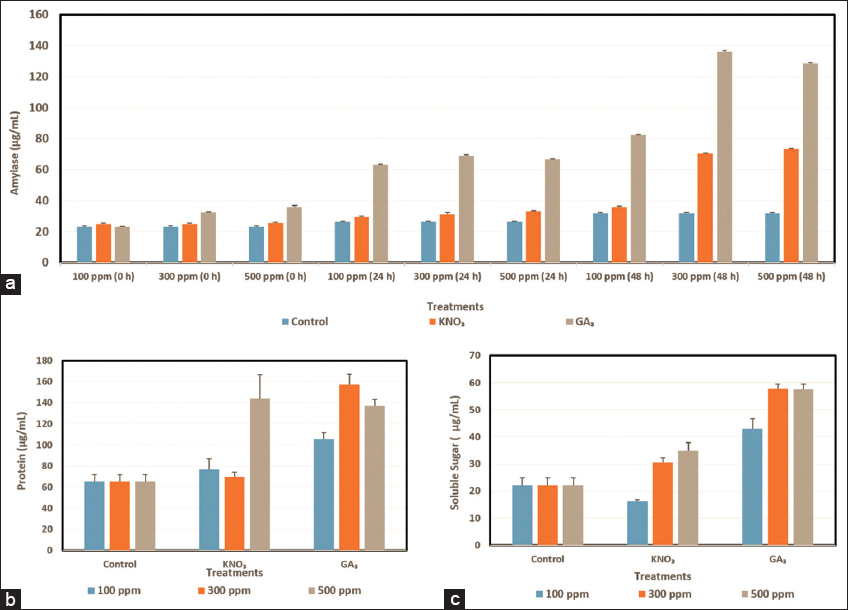 | Figure 4: Quantification of different biochemical parameters in the germinating seeds of Tinospora cordifolia under untreated and treated with different concentrations of potassium nitrate and GA3 (100, 300, and 500 ppm). (a) Quantification of amylase activities under time intervals (0, 24, and 48 h). (b) Quantification of protein content at 48 h, and (c) estimation of soluble sugars at 48 h. [Click here to view] |
3.4.1. Amylase activity
During seed germination GAs are transported to the aleurone layer and release α-amylase. Amylase in the seeds is crucial for hydrolyzing starch, present in seed endosperm, and converting it into soluble sugars during seed germination, which in turn supplies energy for the seed germination and formation of roots and shoots [30]. In this experiment, amylase activity in all experimental sets increases as time reaches germination [Figure 4a]. As T. cordifolia seeds germinate after 72 h of imbibition, amylase exhibits its highest activity at 48 h and the lowest just after the completion of imbibition period in all seed sets. Among all experimental sets, 300 ppm of GA3 gives the best result (135.99 ± 0.87) for amylase activity beside 500 ppm GA3 also shows a satisfactory result (128.55 ± 0.50). Whereas, 300 and 500 ppm KNO3 give moderate results (70.33 ± 0.33 and 73.22 ± 0.38, respectively) for amylase activity at 48 h.
3.4.2. Total protein content
During seed development and maturation, plants produce and store proteins in protein storage vacuoles. These storage proteins are released during seed germination to provide nutrients for seedling growth. Large amounts of storage proteins are accumulated in the seeds of high plants throughout seed development and seed maturation, which are then released to give the necessary components and energy for seed germination and early seedling growth (Wang et al. 2007). Proteins increase during the germination of a seed under the influence of KNO3 (Lara et al. 2014). In this study, we have observed that the amount of protein content is higher (105.66 ± 5.80 to 157.33 ± 9.4 μg/mL) in the KNO3 treated sets [Figure 4b]. GA3-treated seeds (69.66 ±10.06–79.00 ± 6.08 μg/mL), as well as untreated seeds (65.33 ± 6.50 μg/mL) (control), show lower protein content in this present experiment of T. Cordifolia seeds. Among all the treatments including KNO3 and GA3, the KNO3 treated seeds (500 ppm) exhibit a maximum amount of protein content (157.33 ± 9.4 μg/mL) [Figure 4b].
3.4.3. Total soluble sugar content
As GA3 helps in the hydrolysis of starch present in seeds, by releasing α-amylase, GA3 shows the best result (43.13 ± 3.49–57.55 ± 1.89) for the production of soluble sugar [Figure 4c]. In this present study, among all experimental sets, 300 ppm GA3 treated seeds produce the highest amount of soluble sugar (57.78 ± 1.73), followed by 500 ppm GA3 (57.55 ± 1.89). On the other hand, KNO3 produces a much lower amount of soluble sugar (16.14 ± 0.62–34.89 ± 3.05). Surprisingly, 100 ppm KNO3 shows poor soluble sugar production (16.14 ± 0.62) even lower than the untreated set (22.17 ± 2.70). The hydrolysis of endosperm starch into soluble sugars, which fuels the growth of roots and shoots, occurs during cereal seed germination as a result of the activity of the enzyme α-amylase in the aleurone layer. The previous physiological and biochemical studies have shown that the aleurone layer expresses α-amylase in the manner that the embryo starts producing active GA, and then the GAs are transferred from the embryo to the aleurone layer. GAs activates the production of α-amylase which is released into the endosperm from the aleurone layer to catalyze the hydration reaction of stored starch [30].
3.5. Statistical Analysis
The statistical analysis using ANOVA following the Tukey test (P < 0.05) exhibits clear significant difference for GA3-treated seeds with both KNO3-treated and control sets. However, for the 300 and 500 ppm of GA3-treated seeds, the difference in FGP is also non-significant (P < 0.05). The control set of CVG shows a significant difference with 500 ppm GA3-treated seeds while the rest of the treated seed sets show non-significant difference with the control set. According to the MGT, no significant (P < 0.05) difference was found between control and treated seed sets but for GRI the control seed sets show a significant difference (P < 0.05) with GA3 treated seed sets and an insignificant difference with KNO3 treated seed sets [Table 1]. All parameters except SL (FW, DW, RL, and VI) of the seedling growth parameter and all the biochemical parameters show a significant difference (P < 0.05) between control and 500 ppm GA3-treated sets of seeds [Table 2]. Correlation analysis between FGP had a significantly positive correlation with some germination parameters, growth, and biochemical parameters whereas a negative correlation with others [Figure 5]. From the Pearson correlation, it is clear that FGP is strongly correlated with GRI (r = 0.9667).
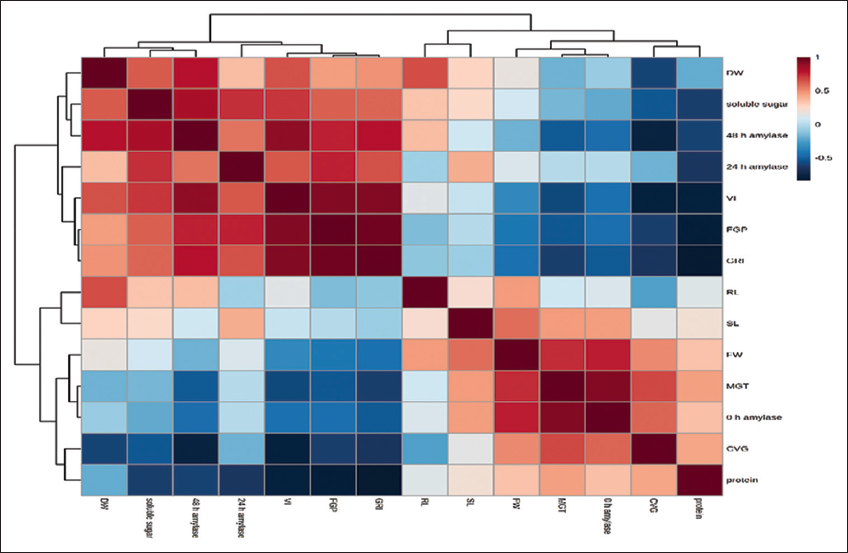 | Figure 5: Heat map correlations among the germination parameters of Tinospora cordifolia seed. Each square represents the Pearson correlation coefficient between the germination parameters of the column with that of row. [Click here to view] |
VI (r = 0.92139) and 48 h amylase content (r = 0.76362) followed by soluble sugar, 24 h amylase content (r = 0.7599), and DW (r = 0.46249). Where protein content of seedling and CVG show a strong negative correlation with FGP (r = 0.61555). A negative correlation exists between FGP and MGT, FW, RL, and amylase content at 0 h (r = 0.52799, 0.41021, 0.13881, and 0.45027, respectively) [Figure 5].
The possible explanation of the findings points toward a strong positive correlation of synthesis of assayed biochemicals that decidedly regulate seed germination and seedling growth. The positively correlated biochemicals such as soluble sugar and amylase content at 24 and 48 h boost germination as well as increase the DW of seedlings and VI. During germination, amylase hydrolyses starch to soluble sugar which gives energy to seeds to germinate. SL, RL, and FW were negatively correlated with FGP and GRI but positively correlated with protein content. It may be concluded that protein content does have not such an effect on germination percentage and per-day germination but it has a significantly positive effect on SL, RL, and FW of T. cordifolia.
4. CONCLUSION
Like many medicinal plants, the scarcity of T. cordifolia plant is mainly due to mindless harvesting by pharmaceutical companies. This problem is compounded by the pathetic germinability of its seeds due to dramatic loss of viability (13.3% only) even before 3 months of seed maturation. In this backdrop, this study successfully establishes an economic and effective protocol for improved germination of T. cordifolia seeds. Simple treatment with GA3 (300, 500 ppm) not only helps to achieve 100% seed germination but provides excellent seedling health. This protocol offers practical scope for conservation and sustainable harvesting of viable healthy seeds of T. cordifolia, which is supreme importance for drug manufacturers as well as to ecologists.
5. ACKNOWLEDGMENT
The authors also thankfully acknowledge The University of Burdwan for infrastructural facilities. We acknowledge Dr. Ritesh Pal for his assistance in the manuscript preparation.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ghosh S, Saha S. Tinospora cordifolia:One plant, many roles. Anc Sci Life 2012;31:151-9. [CrossRef]
2. Sharma P, Dwivedee BP, Bisht D, Dash AK, Kumar D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019;5:e02437. [CrossRef]
3. Garg M, Agarwal P, Bora A, Sood A, Pradhan R. A systematic review on the bioactive compounds and health benefits of Tinospora cordifolia. Pharm Innov J 2022;11:1987-91.
4. Pandey V, Vaishya JK, Balakrishnan P, Nesari TM. Nutritional aspects of Tinospora cordifolia (Giloe). Med Plants 2020;12:158-60. [CrossRef]
5. Arora A. Tinospora cordifolia:A magical wand with immense medicinal applications. Plant Arch 2021;21:143-7. [CrossRef]
6. Kumar P, Kamle M, Mahato DK, Bora H, Sharma B, Rasane P, et al. Tinospora cordifolia (Giloy):Phytochemistry, ethnopharmacology, clinical application and conservation strategies. Curr Pharm Biotechnol 2020;21:1165-75. [CrossRef]
7. Mangal M, Sheoryan A, Mangal AK, Kajla S, Choudhury A, Dhawan A. Biotechnological advances in Tinospora cordifolia (Willd.) Miers Ex Hook. F. and Thoms:Overview of present status and future prospects. Vegetos 2012;25:182-91.
8. Warrier RR, Singh GB, Sivalingam R, Anandalakshmi R, Sivakumar V. Fruit chromticity:A maturity index in Tinospora Cordifolia. Int J Integr Biol 2008;3:118-22.
9. Anilkumar C, Chitra CR, Bindu S, Rajkumar G. Seed storage behavuior of Tinospora cordifolia. Seed Res 2016;44:102-6.
10. Sarihan EO, Ipek A, Khawar KM, Atak M, Gurbuz B. Role of GA3 and KNO3 in improving the frequency of seed germination in Plantago lanceolata l. Pak J Bot 2005;37:883-7.
11. Yang LE, Peng DL, Li ZM, Huang L, Yang J, Sun H. Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers 2020;42:168-73. [CrossRef]
12. Mrva K, Wallwork M, Mares DJ. Alpha-amylase and programmed cell death in aleurone of ripening wheat grains. J Exp Bot 2006;57:877-85. [CrossRef]
13. Nasri F, Koshesh Saba M, Ghaderi N, Mozafari AA, Javadi T. Improving germination and dormancy breaking in Alstromeria ligtu hybrid seeds. Trakia J Sci 2014;12:38-46.
14. Richards DE, King KE, Ait-Ali T, Harberd NP. How gibberellin regulates plant growth and development:A molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 2001;52:67-88. [CrossRef]
15. Bewley JD, Black M. Seed physiology of development and maturation. In:Seeds. US:Springer;1994. [CrossRef]
16. Kang JS, Choi YW, Son BG, Lee YJ, Ahn CK, Choi IS, et al. Effect of osmotic priming and solid matrix priming to improved seed vigor and early growth of pepper and tomato seeds. J Life Sci 2003;13:433-40. [CrossRef]
17. Arc E, Galland M, Godin B, Cueff G, Rajjou L. Nitric oxide implication in the control of seed dormancy and germination. Front Plant Sci 2013;4:346. [CrossRef]
18. Saruhan N, Kadioglu A, Durmus N. Alleviation of seed dormancy in Plantago major. Israel J Plant Sci 2002;50:177-9. [CrossRef]
19. Scott SJ, Jones RA, Williams WA. Review of data analysis methods for seed germination. Crop Sci 1984;24:1192-9. [CrossRef]
20. Orchard T. Estimating the parameters of plant seedling emergence. Seed Sci Technol 1977;5:61-9.
21. Jones KW, Sanders DC. The influence of soaking pepper seed in water or potassium salt solutions on germination at three temperatures. J Seed Technol 1987;11:97-102.
22. Esechie HA. Interaction of salinity and temperature on the germination of Sorghum. J Agron Crop Sci 1994;172:194-9. [CrossRef]
23. Abdul-Baki AA, Anderson JD. Vigor determination in soybean seed by multiple criteria. Crop Sci 1973;13:630-3. [CrossRef]
24. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [CrossRef]
25. McCready RM, Guggolz J, Silviera V, Owens HS. Determination of starch and amylose in vegetables. Anal Chem 1950;22:1156-8. [CrossRef]
26. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 1959;31:426-8. [CrossRef]
27. Gupta R, Chakrabarty SK. Gibberellic acid in plant:Still a mystery unresolved. Plant Signal Behav 2013;8:e25504. [CrossRef]
28. Fagge AA, Manga AA. Effect of sowing media and gibberellic acid on the growth and seedling establishment of Bougainvillea glabra, Ixora coccinea and Rosa chinensis. 2:Root characters. Bayero J Pure Appl Sci 2012;4:155-9. [CrossRef]
29. Arteca RN, Schlagnhaufer CD, and Arteca JM. Root applications of gibberellic acid enhance growth of seven Pelargonium cultivars. HortScience 1991;26:555-6. [CrossRef]
30. Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The alpha-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol 2002;128:1264-70. [CrossRef]