1. INTRODUCTION
Due to the modern era’s increased need for natural pigments, fungal pigments are gaining much attention due to their wide applications in many different industries such as beverages, textiles, cosmetics, painting, and pharmaceuticals. Colorants are substances that impart color to a substance. There has recently been a greater interest in creating colorants from natural sources worldwide as synthetic colorants are attributed to safety concerns [1]. Moreover, less synthetic colorants are used now due to the greater understanding of their toxic effects and long-term harmful effects. A crucial alternative for potential hazardous synthetic dyes are natural pigments synthesized as microbial metabolites. Microbial pigments are more popular than traditional pigments made from plants and animal skin because to their accessibility, economical extraction, and efficiency [2]. As a result, there is an increasing need for natural colorants, which has led to the emergence of investigations to explore the potential sources. Numerous studies have shown that the expense of manufacturing natural colorants is high because the microbial media is also expensive. Therefore, it is encouraged that exploration on pigment production using domestic and agro-industrial wastes as growth media be done as it results in cheaper costs and less environmental pollution by effectively utilizing waste materials [3].
Fungi belonging to different families, such as Chlorociboriaceae, Hyaloscyphaceae, Hymenochaetaceae, Polyporaceae, and Ophiostomataceae, are potent pigment producers and they secrete different secondary metabolites as pigments [1,4,5]. Secondary metabolites are a diverse group of biomolecules with various bioactivities. Pigments and other secondary metabolites are not involved directly in primary growth of fungus but play a major role in protecting the fungi from various adverse environmental factors. Numerous secondary metabolites produced by Fusarium spp., including gibberellins, carotenoids, and polyketides, have been extensively studied [6-8]. Two distinct groups of pigments are produced by Fusarium spp.- polyketides (e.g., Naphthoquinones, bikaverin and fusarins) and terpenoids (eg. carotenoids) [6]. Naphthoquinones are pigments from naphthalene that are members of the quinone family. They have a wide range of chemical structures and biological functions. Secondary metabolites of these compounds are present in higher plants, bacteria, and fungi. Fusarium solani is a broad species group that contains numerous saprophytic and endophytic species as well as some significant pathogenic fungi that are linked to human and animal diseases [9]. Secondary metabolites such as fusarubin and its derivatives (hydroxydihydrofusarubin, O-ethylfusarubin, O-ethylhydroxydihydrofusarubin and anhydrofusarubin, and javanicin), anthraquinones (e.g., bostrycoidin), aurofusarin, and bikaverin are reported in Fusarium solani. Fusarubin and their derivatives and bostrycoidin are found to possess several bioactivities such as antimicrobial, antitumor, antitubercular, antifungal, anticancer, and insecticidal activities [1,10-14]
Endophytic fungi are microorganisms that can live in the tissue of a host plant without causing any disease symptoms [15]. De Barry coined the word “endophyte” in 1866 to describe microorganisms that live within the healthy tissues of plants without producing disease. They live in intracellular spaces of host plant, producing bioactive natural compounds, medicines, and derivatives without harming or infecting their hosts [16]. Several earlier studies have reported that the fungal genera belonging to Fusarium, Talaromyces, Monascus, Penicillium, Aspergillus, and Trichoderma produce fungal pigments with antimicrobial activity against bacteria, fungus, and yeast [17-21]. Researchers have also reported that certain fungal pigments have shown promising antioxidant properties. Certain fungi such as Fusarium spp., Thermomyces spp., Penicillium spp., and Trichoderma spp. exhibit good antioxidant properties with promising applications in biomedical research [2,22]. Researchers have used a variety of techniques to investigate the cytotoxicity of fungal pigments, including Artemia lethality bioassay, CCK-8 (Cell counting kit-8) assay, and YTT (yeast toxicity test) [23]. The present study was done to screen the endophytic fungus, Fusarium solani, isolated from the stem of Alternanthera philoxeroides in Madiwala lake for pigment production. Green synthesis and characterization of fungal nanoparticles (NPs) were also standardized. The fungal pigment from Fusarium solani demonstrated good antimicrobial activity against various pathogens tested and also exhibited moderate antioxidant activity.
2. MATERIALS AND METHODS
2.1. Isolation of Endophytic Fungus
All the reagents and chemicals used in this study were bought from HiMEDIA (India) and are of analytical grade. Endophytic fungi were isolated from the plant sample collected from Madiwala Lake, Bangalore, India (Lat N 120 54’ 3.492” Long E 770 37’ 4.0044”) and was washed with running tap water for 3–4 times. Under sterile conditions, the plant was dissected and the stem, leaf, and root were separated. The plant parts were then surface sterilized with 70% (v/v) ethanol, 1% (v/v) sodium hypochlorite, and sterile distilled water and subsequently air dried. Explants were prepared and placed on the PDA (Potato Dextrose Agar) media for the isolation of endophytic fungi. These cultures were incubated for about 1–2 weeks at room temperature (under dark conditions) following which the fungal colonies were purified by subculturing on SDA (Sabouraud dextrose agar) plates separately under the same parameters. The purified cultures were maintained at 4°C on SDA slants [2,15,16,24,25].
2.2. Molecular Identification and Characterization of Endophytic Fungus
The selected isolate’s morphology was studied employing both macroscopic and microscopic techniques. The size, growth pattern, and color of the colonies were observed to study their characteristics. Lactophenol cotton blue staining was used for microscopic analysis to determine the size, shape, and arrangement of the spores. The fungal isolate was subcultured in SDB (Sabouraud dextrose broth) for 10 days, following which the fungal DNA was isolated and checked for purity and yield using electrophoresis and spectrophotometry. Polymerase chain reaction (PCR) was used to amplify the ITS region fragment and subsequently, using the ITS1, 4 primers in the ABI 3730xl Genetic Analyzer’s BDT v3.1 Cycle sequencing kit, the amplicon was sequenced. Consensus sequence of this PCR product was made using aligner software and analyzed for nucleotide homology through BLAST software using NCBI GenBank. Based on nucleotide homology and maximum identity scores using ClustalW multiple alignment tool, the first ten sequences were selected and aligned following which the phylogenetic tree was constructed using MEGA 10 [26-28].
2.3. Screening for Fungal Pigment Production
A mycelial plug was cut from the 7 day old culture plate using sterilized scalpel, inoculated in 200 mL of SDB and incubated for 14 days. This fungal culture was then utilized subsequently for fungal pigment extraction.
2.4. Extraction of Fungal Pigment from Solid Media
For extraction of extracellular fungal pigments, the media (after fungal incubation) was cut into small pieces using a sterile blade, added into 40 mL of different solvents such as distilled water, acetone (99%), methanol (99.5%), and chloroform, and incubated at 200 rpm at 38–40°C for 1–2 h. It was subsequently filtered using a filter paper into a preweighed beaker and the contents were completely vaporized at room temperature for 1–2 days. The resultant extract was then dissolved in DMSO and kept at 4°C for subsequent studies. For extraction of intracellular fungal pigments, the fungal culture after incubation period was filtered using Whatman filter paper, washed with sterile distilled water and air-dried. The pigment was extracted using various solvents and separated using a separating funnel. The crude fungal pigment extract was concentrated and stored for further studies [29]. The fungal mycelia obtained after incubation was filtered with the aid of pre-weighed Whatman No. 1 paper. The mycelia were then air dried for 24 h. The estimated dry cell weight of the mycelium was then expressed as g/L [30].
2.5. Green Synthesis of Silver Nanoparticles from Fungal Culture
The fungal culture was incubated in SDB growth medium at room temperature for 15 days (in dark) following which it was filtered to obtain fungal biomass and filtrate. Under aseptic conditions, the fungal biomass was weighed and sterile water in the ratio of 1:10 (w/v) was added to it and kept for incubation for 72 h at 28°C. Subsequently, this was filtered and to the cell-free filtrate an equal volume of 1 mM of silver nitrate solution was added and kept for incubation at 28°C at 200 rpm and observed for formation of NP after 24, 48, and 72 h. This solution was then centrifuged for 15 min at 10,000 rpm and the pellet obtained was washed thrice repeatedly by centrifuging with sterile water at 10,000 rpm for 10 min each. The pellet finally obtained was dried at 40°C to get the powdered fungal AgNP [31-33].
2.5.1. UV–Visible spectrophotometry and Fourier transform infrared (FTIR) spectroscopy of fungal nanoparticles
Green synthesis of AgNP was performed following which they were characterized through UV–Visible Spectrophotometry and Fourier Transform Infrared (FTIR) Spectroscopy. Preliminary characterization of AgNP was performed using UV–Visible Spectrophotometry at wavelength between 200 and 800 nm. Biochemical composition of AgNP was further characterized using FTIR analysis (using Shimadzu IR Spirit with single reflection ATR accessory). This helped probe the functional groups, type of bonds, and chemical groups present in these AgNP.
2.5.2. X-ray diffraction (XRD) analysis of fungal nanoparticles
AgNP synthesized from MEFAphS1 were further characterized to check the morphological and crystalline nature of these particles using XRD with wide ranging Bragg angles 2θ and scanning rate of 30–80 at 0.041/min with 2 s time constant.
2.5.3. Antimicrobial activity of fungal nanoparticles
Agar well diffusion method was used to examine the antibacterial activity of green synthesized AgNP against Gram-negative (Vibrio harveyi, Klebsiella spp., E. coli) and Gram-positive (Streptococcus spp., Bacillus spp.) bacteria. The overnight culture of the test organisms was inoculated in nutrient agar plates and wells were made using a sterile gel puncture, into which 40 μL of the green synthesized AgNP each were loaded. DMSO and ampicillin were taken as the negative and positive controls, respectively. Zone of inhibition (ZOI) was measured 24 h after incubation [34,35].
2.6. Optimization of Fungal Pigment Production
2.6.1. Optimization of pH in fungal growth media
The optimum pH for fungal pigment production was determined by culturing the fungi in SDA fungal growth media at different pH (ranging from pH of 4, 6, 7, 8, and 10) and incubating at room temperature (under dark conditions) for about 14–16 days. To determine the optimal pH for pigment production, the optical density of the culture filtrates was measured. The dry weight of the mycelium was also recorded [36].
2.6.2. Optimization of carbon sources in fungal growth media
With a view to study the effect of various carbon sources on the production of the pigment, Sabouraud dextrose broth was prepared with different carbon sources. By substituting alternative carbon sources at the same concentration for dextrose (40 g/L), the impact of those sources on pigment synthesis was calculated. The tested carbon sources include sucrose, maltose, lactose, and starch [37]. The medium containing dextrose was used as a control. Fungal spores were inoculated into each flask and incubated for 2 weeks at 30°C at a pH of 6. The dry weight and optical density values were measured [36-38].
2.6.3. Solid state fermentation
Different substrates such as orange peel, chikku peel, sugarcane bagasse, corn cob, and wheat bran substrate were washed thoroughly, dried, and powdered. Media comprising 20 g of each substrate was mixed with 8 mL of salt solution containing MgSO4.7H2O (1g/L), FeSO4.7H2O (0.5g/L), KH2PO4 (2 g/L), NaCl (1 g/L), and (NH4)2SO4 (2 g/L) and 70% moisture content with pH 6. 1 mL of fungal suspension was added to this media and incubated for 14 days at room temperature. After the incubation period, the pigmented substrates were completely dried and these dried substrates were finely ground and extracted using distilled water in a shaking incubator. The contents were then filtered and centrifuged and the supernatant was preserved at 4°C for further analysis [39-42].
2.7. Antioxidant Activity
The antioxidant activity was assessed based on the free radical scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH). The fungal pigment and fungal AgNPs of varying concentrations (25 μg/mL to 200 μg/mL) were taken and made up to 1mL using methanol and 1 mL of 1 mM of DPPH solution was added. The resulting mixtures were incubated at room temperature (under dark conditions) for 30 min followed by recording the absorbance at 517 nm to measure the reduction of DPPH. Methanol and 1 mM of DPPH reagent served as blank and negative control, respectively. Ascorbic acid solutions with the same concentrations (25 μg/mL–200 μg/mL) were used as the standard. The percentage of DPPH scavenging activity was calculated and expressed as radical scavenging activity (RSA) as follows [17,32,43].
 |
2.8. Cytotoxicity Studies
2.8.1. Antimicrobial activity of fungal pigment
The antimicrobial activity of crude fungal pigment extract was tested by agar well diffusion method against Streptococcus sp., Bacillus sp., Klebsiella sp., E. coli, and Vibrio harveyi. The nutrient agar plates were prepared and the test organisms were inoculated in each plate followed by boring of wells in these agar plates using a sterile cork borer. Into each of these wells, 40 and 10 μL of the crude fungal pigment extract was added. DMSO and ampicillin were taken as the negative and positive controls, respectively. The zone of inhibition (ZOI) was determined 24 h after incubation at 37°C [44].
2.8.2. Brine shrimp cytotoxicity test
Brine shrimps (Artemia salina) eggs were purchased and kept for hatching (3–5 g/L) in salt water at 30 ppt salinity under a constant supply of light and aeration for 48 h. Stock solutions of fungal pigment extracts of the required concentration were prepared by dissolving them in 0.1% (v/v) DMSO. Different concentrations of working solutions of the pigment extracts (20–100 μg/mL) were then made from this stock solution. 100 μL of each of the working solutions was added to each well of a microtiter plate. DMSO and salt water at 30 ppt salinity were used as the controls, respectively. Ten Artemia nauplii were introduced into each microtiter well using a micropipette and were incubated under a light source for 24 h. The number of dead nauplii was then counted after incubation and the percentage of mortality was calculated [45,46].
3. RESULTS AND DISCUSSION
3.1. Isolation and Identification of Endophytic Fungus
Several endophytic fungal isolates were screened for pigments production, of which MEFAphS1 which produced reddish orange pigment and isolated from the stem part of the Alternanthera philoxeroides was selected for the study. The obtained endophytic fungus was cultured in SDA media and subcultured for further studies. The colony characteristics of this fungus were white cottony in nature. The hyphae obtained were initially hyaline and mycelium was yellowish-white, which became reddish-brown after 1–2 weeks. There was a characteristic brownish-red appearance in the center [Figure 1]. These preliminary characteristics were studied for the initial identification of the endophytic fungus. Subsequent fungal identification was performed using lactophenol cotton blue staining and molecular sequencing of the ITS regions of this endophytic fungus. Based on preliminary fungal staining and molecular identification, the fungal endophytic culture which was labeled as MEFAphS1 was found to be Fusarium solani [Figure 2]. The genetic sequence was submitted to NCBI GenBank and can be found under the accession number OM866265, labeled as MEFAphS1.
 | Figure 1: Fungal culture of Fusarium solani: (a) front view of fungal culture on SDA growth media, (b) rear view of fungal culture on SDA growth media, and (c and d) lactophenol cotton blue staining depicting the fungal spores and mycelium. [Click here to view] |
 | Figure 2: The phylogenetic tree of the isolated strain MEFAphS1 and other fungal relatives based on neighbor-joining analysis of ITS sequences. [Click here to view] |
3.2. Extraction of Fungal Pigment
Fungal pigment was extracted from Fusarium solani fungal culture 14 days after the incubation. The initial verification of fungal pigment production was determined by observing a change in the color of the growth media, which shifted from a light yellow hue to a reddish-brown shade. This broth culture was filtered using Whatman filter paper and biomass was dried to estimate its dry weight. The broth filtrate containing the extracellular fungal pigment was stored at 4 °C for further studies.
3.3. Green Synthesis of Silver Nanoparticles from Fungal Culture
In this research, silver NPs were synthesized using the fungus Fusarium solani. The formation of AgNP was visualized with a change in color from light to dark brown within 24 h of addition of silver nitrate. Mahdieh et al. (2012) have reported biosynthesis of crystalline AgNP by Spirulina platensis in aqueous system [47]. Clarance et al. (2020) have reported the environmentally friendly synthesis of gold NPs using an endophytic strain of Fusarium solani ATLOY – 8, which was isolated from the plant Chonemorpha fragrans with potential anticancer and pharmaceutical applications [48].
3.3.1. UV–visible spectrophotometry and Fourier transform infrared (FTIR) spectroscopy of fungal nanoparticles
The UV–Visible spectra of the fungal extract of Fusarium solani treated with AgNO3 showed a peak at 419 nm indicating the reduction of AgNO3 into AgNP [Figure 3a].
 | Figure 3: Characterization of silver nanoparticles from Fusarium solani: (a) UV–visible Spectrum depicting the peak obtained corresponding to AgNP, (b) FTIR spectrum depicting the functional groups detected in AgNP, and (c) XRD analysis of AgNP. [Click here to view] |
The FTIR spectrum gives the information about the functional groups present in the synthesized AgNP, as shown in Figure 3b. The data provide the possible biomolecules responsible for the reduction of silver and synthesis of AgNP and also stabilizing them [49]. Eleven peaks were observed in FTIR spectrum and few were 3258.75 cm–1 and 1399 cm–1 associated with O-H stretching in carboxylic acid; 2920.31 cm–1 and 2851.76 cm–1 attributes to C-H stretching in alkane and 1636.51 cm–1 band assigned to the N-H bending and carbonyl stretching in amide linkage of proteins [50].
3.3.2. X-ray diffraction (XRD) analysis of fungal nanoparticles
The crystallinity of the synthesized AgNP was examined by XRD. Figure 3c depicts the XRD pattern of the green synthesized AgNPS obtained. The spectrum showed three distinct peaks at 77, 44.6, and 38.38° attributes to the 311, 200, and 111 planes, respectively. The XRD peaks obtained in our study are in good agreement with the earlier reports [51]. The AgNPs were found to possess a face centered cubic structure when compared to the reference standard of the Joint Committee of Powder Diffraction Standard (JCPDS file number 04-0783). The presence of other peaks observed could possibly be due to the presence of organic compounds [52,53].
3.4. Fungal Pigment Production
3.4.1. Optimization of fungal pigment production
After 14 days of incubation, maximum yield of the fungal pigment was observed. The fresh weight of biomass was found to be 17 g and dry weight after 48 h was found to be 7 g. As depicted in Figure 4, the fungal growth was found to be optimum at pH 7, which is reported to be favorable for fungal growth and pigment production. For fungal growth and pigment production, dextrose was found to be the most suitable carbohydrate source [Figure 4]. Fusarium solani was able to grow in all carbohydrate sources except lactose possibly due to inability of proper lactose utilization. Menezes and coworkers conducted optimization studies to enhance the production of red pigment production from the fungus Fusarium solani BRM054066. They identified that a submerged fermentation system at 200 rpm with a glucose concentration of at least 20 g/L resulted in optimal conditions red pigment production [9].
 | Figure 4: pH and carbon source optimization for maximum pigment production by endophytic Fusarium solani. [Click here to view] |
3.4.2. Solid state fermentation (SSF)
The SSF of various substrates was performed and the pigmented substrates were dried 14 days after incubation, finely powdered to increase the surface area, and extracted using water. Orange peel showed the highest absorbance followed by wheat bran and sugarcane bagasse [Figure 5]. There was no pigment production and decreased growth in corn cob substrate possibly due to the presence of non-starch polysaccharides glucuronoarabinoxylans that are difficult to degrade [54]. Sugarcane bagasse also showed least value as it is rich in non-starch polysaccharide and has a substantial amount of tannin that may prevent fungal growth [54,55]. Statistical value of p < 0.05 shows that there has been a significant difference between the different substrates used.
 | Figure 5: Absorbance values recorded across different substrates used in SSF using Fusarium solani. [Click here to view] |
3.5. Antioxidant Activity
The antioxidant properties of the fungal pigment and the green synthesized AgNP from Fusarium solani was determined by DPPH assay. The standard ascorbic acid showed highest antioxidant properties followed by green synthesized AgNP and fungal pigment, as depicted in Figure 6. The IC50 of fungal pigment was observed at 104.25 μg/mL. RSA of the pigment ranged between 13.11 and 27.33% and of AgNP ranged between 67.62 and 77.41%. Menezes et al. (2020) have reported that the red pigment produced by Fusarium solani BRM054066 showed an antioxidant activity at a concentration of 24 μg/mL, by scavenging 50% of the DPPH radicals [9]. Khan et al. have documented the antioxidant activity of compounds obtained from F. solani using the DPPH scavenging method. The findings showed that bostrycoidin displayed considerable antioxidant activity (IC50 = 1.6 μg/mL), surpassing the positive control BHA (Butylated hydroxyanisole), trolox and ascorbic acid, with IC50 values of 1.2, 1.3, and 1.5, respectively. Fusarubin and anhydrofusarubin exhibited antioxidant activity with IC50 values of 34.8 μg/mL and 12.4 μg/mL, respectively [56].
 | Figure 6: Radical scavenging activity (RSA) of fungal pigment crude extract of Fusarium solani. [Click here to view] |
3.6. Cytotoxicity Studies
3.6.1. Antimicrobial activity of fungal pigment and fungal nanoparticles
Antibacterial activity of the green synthesized AgNP was probed using agar well diffusion assay by testing it against both Gram-positive and Gram-negative microorganisms [Table 1]. In this present study, mycosynthesized AgNPs exhibited the highest antimicrobial activity against both Gram-positive and negative bacterial organisms. The highest antibacterial activity was obtained against E. coli (25 mm) followed by Bacillus spp., (21 mm) and Vibrio harveyi and Klebsiella spp. (20 mm). The lowest activity was observed against Streptococcus spp. (14 mm). The force of electrostatic attraction between silver ions that are positively charged and the negatively charged bacterial cell walls create pores on the membrane increasing AgNP permeability, producing reactive oxygen species and inhibiting replication through silver ion release [57]. It was observed that AgNP was most effective against Gram-negative bacteria that can be attributed to the presence of thin cell wall, which is absent in Gram-positive bacteria that reduce the AgNP penetration into cells [58]. In a study by Nuanaon et al., LEDs synthesized AgNP using fungal pigment had exhibited an antimicrobial activity against E. coli and S. aureus [59].
Table 1: Antimicrobial activity of fungal pigment and AgNP Fusarium solani.
| Test organisms | Diameters of zone of inhibition (in mm) | ||||
|---|---|---|---|---|---|
| Acetone extract of fungal pigment | Fungal pigment filtrate | AgNP | |||
| 40 µL pigment per well | 10 µL pigment per well | 40 µL pigment per well | 10 µL pigment per well | 40 µL pigment per well | |
| E. coli | 25 | 20 | 26 | 15 | 25 |
| Vibrio harveyi | 28 | 18 | 18 | 0 | 20 |
| Streptococcus sp. | 15 | 7 | 10 | 5 | 14 |
| Klebsiella sp. | 18 | 5 | 18 | 5 | 20 |
| Bacillus sp. | 18 | 5 | 18 | 5 | 21 |
The fungal pigment filtrate, the acetone extract of fungal pigment, and fungal AgNP depicted high antimicrobial properties against E. coli, Klebsiella spp., Vibrio harveyi, Streptococcus spp., and Bacillus spp. [Table 1]. The maximum zone of 28 mm was observed in the acetone extract against Vibrio harveyi. The maximum zone of 26 mm among the filtrate was shown against E.coli. This indicates that the pigment showed better antibacterial properties against the tested Gram-negative bacteria. The maximum ZOI was shown against E. coli. Negative control and vehicle control DMSO did not exhibit any zones of clearance. These results indicate that this fungal pigment could be potentially developed as an antibiotic drug. In a study by Saravanan and Radhakishnan, the pigment produced by the fungal isolate MF5 showed a broad spectrum of antibacterial activity [60]. In East Asia, fungal pigments produced by Aspergillus niger and Monascus purpureus were employed as natural colorants and food additives. Growth and pigment production of the fungus Talaromyces verruculosus had been optimized to obtain the highest yield and for its potential application as a replacement for synthetic dyes that are also hazardous [61].
The Western Ghats region of Southern India is home to a diverse group of soil and endophytic fungi which can be tapped more for antimicrobial properties [62]. Of late, there has been a lot of exploration on the antibacterial and antibiofilm properties of nanoparticles [63,64]. The recent years saw the priority being shifted toward the green technologies of synthesis of nanoparticles, without the use of harmful synthetic chemicals [65]. Fungal endophytes that are potential sources of industrially important enzymes [24,66,67] and those with antibacterial and antiangiogenic properties have been reported earlier too [68].
3.6.2. Brine shrimp cytotoxicity test
It was observed that the mortality rate was gradually increasing along with increase in the concentration of the pigment. As depicted in Figure 7, the LC50 value obtained from brine shrimp lethality bioassay was found to be 78 μg/mL which depicts negligible toxicity. Earlier studies defined the toxicity based on LC50 values as: highly toxic if LC50 < 1.0 μg/mL, toxic if LC50 = 1.0-10.0 μg/mL, moderately toxic if LC50 = 10.0–30.0 μg/mL, mildly toxic if 30 < LC50 < 100 μg/mL, and non-toxic if LC50 > 100 μg/mL [69].
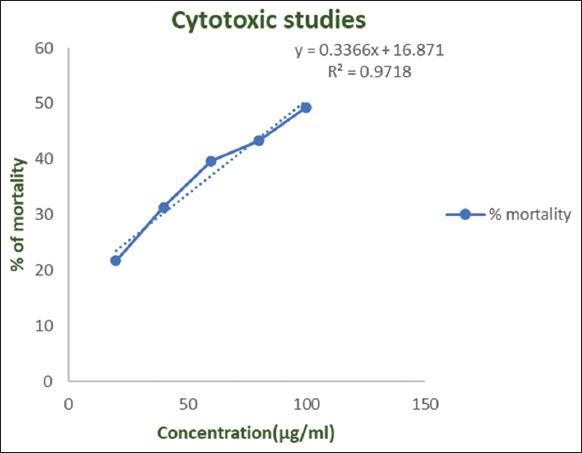 | Figure 7: Brine shrimp lethality bioassay depicting the cytotoxic activity of Fusarium solani fungal pigment extract. [Click here to view] |
4. CONCLUSION
The present study aimed at the optimization of fungal pigment production from Fusarium solani. On biochemical analysis, 18 compounds were identified in the fungal pigment extract. Green synthesis of AgNP from Fusarium solani was also standardized. Cytotoxic properties of fungal pigment and AgNP were studied and it was found that the antimicrobial activity of fungal AgNP was significantly higher than that of fungal pigment. Both the fungal AgNP and pigment extract showed good antioxidant activity, along with negligible lethal effects in brine shrimp cytotoxicity assay. These results indicate the potential of fungal pigment and AgNP from Fusarium solani to be utilized as an antibacterial and/or antioxidant agent that has almost negligible toxic effects. Green synthesis of these AgNP highlights the significance of environment friendly ways to produce bioactive compounds with numerous applications especially as natural dyes that can be a potential replacement for synthetic dyes.
5. ACKNOWLEDGMENTS
All authors greatly acknowledge the Department of Life Sciences, CHRIST (Deemed to be University), Bangalore, for providing all the necessary infrastructure and resources to conduct this work. SJ would like to express her gratitude towards KSTePS (Karnataka Science and Technology Promotion Society) – DST (Department of Science and Technology) Ph.D. fellowship (award letter number LIF-05:2021-22/1017) and BPP would like to acknowledge KSTA Karnataka Science and Technology Academy (KSTA) – Padma Vibhushan Prof. U. R. Rao award 2021 (PG level) for providing the financial assistance and stipend.
6. AUTHORS’ CONTRIBUTIONS
BPP and SB carried out sample collection and optimization. SJ, AK, MU, and SS did the bioactivity studies. All authors involved in the manuscript preparation and editing. SS conceptualized and supervised the research design and experimental planning.
All authors have made significant contributions toward the conceptualization, designing, analysis, and data interpretation of this manuscript along with reviewing and editing.
7. CONFLICTS OF INTEREST
All authors would like to report that there are no financial or any other forms of conflicts of interest in this research work.
8. ETHICAL APPROVALS
This research work does not involve experiments performed on animals or humans.
9. DATA AVAILABILITY
All the data related to this study have been included in this research article.
10. PUBLISHER’S NOTE
This journal declares that it remains neutral in relation with the jurisdictional claims of the affiliation of the publication.
REFERENCES
1. Lagashetti AC, DufosséL, Singh SK, Singh PN. Fungal pigments and their prospects in different industries. Microorganisms 2019;7:604. [https://doi.org/10.3390/microorganisms7120604]
2. Mani VM, Soundari AP, Karthiyaini D, Preeth K. Bioprospecting endophytic fungi and their metabolites from medicinal tree Aegle marmelos in Western Ghats, India. Mycobiology 2015;43:303-10. [https://doi.org/10.5941/MYCO.2015.43.3.303]
3. Rao MP, Xiao M, Li WJ. Fungal and bacterial pigments:Secondary metabolites with wide applications. Front Microbiol 2017;8:1113. [https://doi.org/10.3389/fmicb.2017.01113]
4. Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, DufosséL. Multifaceted applications of microbial pigments:Current knowledge, challenges and future directions for public health implications. Microorganisms 2019;7:186. [https://doi.org/10.3390/microorganisms7070186]
5. Caro Y, Venkatachalam M, Lebeau J, Fouillaud M, DufosséL. 2017. Pigments and Colorants from Filamentous Fungi in Fungal Metabolites. Cham, Switzerland:Springer International Publishing;499-568. [https://doi.org/10.1007/978-3-319-25001-4_26]
6. Kristensen SB, Fini MN, Pedersen TB, Sørensen JL, Muff J. Membrane based separation and purification of fusarubins from Fusarium solani. Sep Purif Technol 2021;278:119576. [https://doi.org/10.1016/j.seppur.2021.119576]
7. Thomas TA, Tirumale S. Production of a polyketide pigment by Fusarium chlamydosporum. J Pure Appl Microbiol 2022;16:1318-29. [https://doi.org/10.22207/JPAM.16.2.60]
8. Calvo AM, Wilson RA, Bok JW, Keller NP. Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev 2002;66:447-59. [https://doi.org/10.1128/MMBR.66.3.447-459.2002]
9. Menezes BS, Solidade LS, Conceição AA, Santos MN Jr., Leal PL, de Brito ES, et al. Pigment production by Fusarium solani BRM054066 and determination of antioxidant and anti-inflammatory properties. AMB Express 2020;10:117. [https://doi.org/10.1186/s13568-020-01054-y]
10. Ammar MS, Gerber NN, McDaniel LE. New antibiotic pigments related to fusarubin from Fusarium solani (Mart.) Sacc. I. Fermentation, isolation, and antimicrobial activities. J Antibiot (Tokyo) 1979;32:679-84. [https://doi.org/10.7164/antibiotics.32.679]
11. Gerber NN, Ammar MS. New antibiotic pigments related to fusarubin from Fusarium solani (Mart.) Sacc. II. Structure elucidations. J Antibiot (Tokyo) 1979;32:685-8. [https://doi.org/10.7164/antibiotics.32.685]
12. Shah A, Rather MA, Hassan QP, Aga MA, Mushtaq S, Shah AM, et al. Discovery of anti-microbial and anti-tubercular molecules from Fusarium solani:An endophyte of Glycyrrhiza glabra. J Appl Microbiol 2017;122:1168-76. [https://doi.org/10.1111/jam.13410]
13. Claydon N, Grove JF, Pople M. Insecticidal secondary metabolic products from the Entomogenous fungus Fusarium solani. J Invertebr Pathol 1977;30:216-23. [https://doi.org/10.1016/0022-2011(77)90222-1]
14. Tatum JH, Baker RA. Naphthoquinones produced by Fusarium solani isolated from citrus. Phytochemistry 1983;22:543-7. [https://doi.org/10.1016/0031-9422(83)83042-8]
15. Hateet RR, Muhsin TM, Humadi KJ. Antibacterial activities secondary metabolites from endophytic fungus Fusarium solani. J Basrah Res Sci 2014;40:94-101.
16. Mane RS, Paarakh PM, Vedamurthy AB. Brief review on fungal endophytes. Int J Secondary Metab 2018;5:288-303. [https://doi.org/10.21448/ijsm.482798]
17. Mani VM, Priya MS, Dhaylini S, Preethi K. Antioxidant and antimicrobial evaluation of bioactive pigment from Fusarium sp isolated from stressed environment. Int J Curr Microbiol Appl Sci 2015;4:1147-58.
18. Wang W, Liao Y, Chen R, Hou Y, Ke W, Zhang B, et al. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar Drugs 2018;16:61. [https://doi.org/10.3390/md16020061]
19. Kalra R, Conlan XA, Goel M. Fungi as a potential source of pigments:Harnessing filamentous fungi. Front Chem 2020;8:369. [https://doi.org/10.3389/fchem.2020.00369]
20. Seyedin A, Yazdian F, Hatamian-Zarmi A, Rasekh B, Mir-Derikvand M. Natural pigment production by Monascus purpureus:Bioreactor yield improvement through statistical analysis. Appl Food Biotechnol 2015;2:23-30.
21. Patil SA, Sivanandhan G, Thakare DB. Effect of physical and chemical parameters on the production of red exopigment from Penicillium purpurogenum isolated from spoilt onion and study of its antimicrobial activity. Int J Curr Microbiol Appl Sci 2015;4:599-609.
22. Poorniammal R, Prabhu S, Sakthi AR. Evaluation of in vitro antioxidant activity of fungal pigments. Pharma Innov J 2019;8:326-30.
23. Malik K, Tokas J, Anand RC. Characterization and cytotoxicity assay of pigment producing microbes. Int J Curr Microbiol Appl Sci 2016;5:370-6. [https://doi.org/10.20546/ijcmas.2016.506.042]
24. Jayaram S, Biswas S, Philip I, Umesh M, Sarojini S. Differential laccase production among diverse fungal endophytes in aquatic plants of Hulimavu Lake in Bangalore, India. J Pure Appl Microbiol 2023;2023:17. [https://doi.org/10.22207/JPAM.17.1.19]
25. Ashfaq M, Ali Q, Haleem A, Ullah A, Umar A, Ullah I, et al. Orange-brown Pigment Production from an Endophytic Fungus Aspergillus sp. N11 and its Potential Pharmaceutical Applications. United States:Research Square;2022. [https://doi.org/10.21203/rs.3.rs-1126132/v1]
26. Qiu M, Xie R, Shi Y, Chen H, Wen Y, Gao Y, et al. Isolation and identification of endophytic fungus SX01, a red pigment producer from Ginkgo biloba L. World J Microbiol Biotechnol 2010;26:993-8. [https://doi.org/10.1007/s11274-009-0261-6]
27. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5:Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731-9. [https://doi.org/10.1093/molbev/msr121]
28. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X:Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 2018;35:1547-9. [https://doi.org/10.1093/molbev/msy096]
29. Sibero MT, Sahara R, Syafiqoh N, Tarman K. Antibacterial activity of red pigment isolated from coastal endophytic fungi against multi drug resistant bacteria. Biotropia 2017;24:161-72. [https://doi.org/10.11598/btb.2017.24.2.725]
30. Geweely NS. Investigation of the optimum condition and antimicrobial activities of pigments from four potent pigment-producing fungal species. J Lifesci 2011;5:697-711.
31. Sandhu SS, Shukla H, Shukla S. Biosynthesis of silver nanoparticles by endophytic fungi:Its mechanism, characterization techniques and antimicrobial potential. Afr J Biotechnol 2017;16:683-98. [https://doi.org/10.5897/AJB2017.15873]
32. Sharma A, Sagar A, Rana J, Rani R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst Lett 2022;10:2. [https://doi.org/10.1186/s40486-022-00144-9]
33. Saravanan M, Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf B Biointerfaces 2010;77:214-8. [https://doi.org/10.1016/j.colsurfb.2010.01.026]
34. Omran BA, Nassar HN, Fatthallah NA, Hamdy A, El-Shatoury EH, El-Gendy NS. Characterization and antimicrobial activity of silver nanoparticles mycosynthesized by Aspergillus brasiliensis. J Appl Microbiol 2018;125:370-82. [https://doi.org/10.1111/jam.13776]
35. Anbazhagan S, Azeez S, Morukattu G, Rajan R, Venkatesan K, Thangavelu KP. Synthesis, characterization and biological applications of mycosynthesized silver nanoparticles. 3 Biotech 2017;7:333. [https://doi.org/10.1007/s13205-017-0961-9]
36. Ayyolath A, Kallingal A, Kundil VT, Variyar EJ. Studies on the bioactive properties of Penicillium mallochi ARA-1 pigment isolated from coffee plantation. Biocatal Agric Biotechnol 2020;30:101841. [https://doi.org/10.1016/j.bcab.2020.101841]
37. Hemeda NA, Hegazy GE, Abdelgalil SA, Soliman NA, Abdel-Meguid DI, El-Assar SA. Maximization of red pigment production from Streptomyces sp. LS1 structure elucidation and application as antimicrobial/antifouling against human pathogens and marine microbes. J Genet Eng Biotechnol 2022;20:168. [https://doi.org/10.1186/s43141-022-00452-y]
38. Suwannarach N, Kumla J, Nishizaki Y, Sugimoto N, Meerak J, Matsui K, et al. Optimization and characterization of red pigment production from an endophytic fungus, Nigrospora aurantiaca CMU-ZY2045, and its potential source of natural dye for use in textile dyeing. Appl Microbiol Biotechnol 2019;103:6973-87. [https://doi.org/10.1007/s00253-019-09926-5]
39. Shetty AV, Dave N, Murugesan G, Pai S, Pugazhendhi A, Varadavenkatesan T, et al. Production and extraction of red pigment by solid - state fermentation of broken rice using Monascus sanguineus NFCCI 2453. Biocatal Agric Biotechnol 2021;33:101964. [https://doi.org/10.1016/j.bcab.2021.101964]
40. Kantifedaki A, Kachrimanidou V, Mallouchos A, Papanikolaou S, Koutinas AA. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J Clean Prod 2018;185:882-90. [https://doi.org/10.1016/j.jclepro.2018.03.032]
41. Babitha S, Soccol CR, Pandey A. Jackfruit seed -A novel substrate for the production of Monascus pigments through solid-state fermentation. Food Technol Biotechnol 2006;44:465-71.
42. Velmurugan P, Hur H, Balachandar V, Kamala-Kannan S, Lee KJ, Lee SM, et al. Monascus pigment production by solid-state fermentation with corn cob substrate. J Biosci Bioeng 2011;112:590-4. [https://doi.org/10.1016/j.jbiosc.2011.08.009]
43. Lagashetti AC, Singh SK, DufosséL, Srivastava P, Singh PN. Antioxidant, antibacterial and dyeing potential of crude pigment extract of Gonatophragmium triuniae and its chemical characterization. Molecules 2022;27:393. [https://doi.org/10.3390/molecules27020393]
44. Visalakchi S, Muthumary J. Antimicrobial activity of the new endophytic Monodictys castaneae SVJM139 pigment and its optimization. Afr J Microbiol Res 2009;3:550-6.
45. Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp:A convenient general bioassay for active plant constituents. Planta Med 1982;45:31-4. [https://doi.org/10.1055/s-2007-971236]
46. Afroz T, Hasan IM, Kafi AK, Azad AK, Rahman MM, Akter A, et al. Phytochemical screening and pharmacological studies of methanol extract of Polyalthia suberosa RoxB. Pharmacologyonline 2019;1:104-16.
47. Mahdieh M, Zolanvari A, Azimee AS. Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci Iran 2012;19:926-9. [https://doi.org/10.1016/j.scient.2012.01.010]
48. Clarance P, Luvankar B, Sales J, Khusro A, Agastian P, Tack JC, et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J Biol Sci 2020;27:706-12. [https://doi.org/10.1016/j.sjbs.2019.12.026]
49. Ingle A, Rai M, Gade A, Bawaskar M. Fusarium solani:A novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 2009;11:2079. [https://doi.org/10.1007/s11051-008-9573-y]
50. Taha ZK, Hawar SN, Sulaiman GM. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol Lett 2019;41:899-914. [https://doi.org/10.1007/s10529-019-02699-x]
51. Li G, He D, Qian Y, Guan B, Gao S, Cui Y, et al. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci 2012;13:466-76. [https://doi.org/10.3390/ijms13010466]
52. Fayaz AM, Balaji K, Kalaichelvan PT, Venkatesan R. Fungal based synthesis of silver nanoparticles--an effect of temperature on the size of particles. Colloids Surf B Biointerfaces 2009;74:123-6. [https://doi.org/10.1016/j.colsurfb.2009.07.002]
53. Dashora A, Rathore K, Raj S, Sharma K. Synthesis of silver nanoparticles employing Polyalthia longifolia leaf extract and their in vitro antifungal activity against phytopathogen. Biochem Biophys Rep 2022;31:101320. [https://doi.org/10.1016/j.bbrep.2022.101320]
54. Taylor JR, Dewar J. Developments in sorghum food technologies. Adv Food Nutr Res 2001;43:217-64. [https://doi.org/10.1016/S1043-4526(01)43006-3]
55. Srianta I, Zubaidah E, Estiasih T, Yamada M, Harijono H. Comparison of Monascus purpureus growth, pigment production and composition on different cereal substrates with solid state fermentation. Biocatal Agric Biotechnol 2016;7:181-6. [https://doi.org/10.1016/j.bcab.2016.05.011]
56. Khan N, Afroz F, Begum MN, Rony SR, Sharmin S, Moni F, et al. Endophytic Fusarium solani:A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol Rep 2018;5:970-6. [https://doi.org/10.1016/j.toxrep.2018.08.016]
57. Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomedicine 2020;15:2555-62. [https://doi.org/10.2147/IJN.S246764]
58. Meikle TG, Dyett BP, Strachan JB, White J, Drummond CJ, Conn CE. Preparation, characterization, and antimicrobial activity of cubosome encapsulated metal nanocrystals. ACS Appl Mater Interfaces 2020;12:6944-54. [https://doi.org/10.1021/acsami.9b21783]
59. Nuanaon N, Bhatnagar S, Motoike T, Aoyagi H. Light-emitting-diode-assisted, fungal-pigment-mediated biosynthesis of silver nanoparticles and their antibacterial activity. Polymers (Basel) 2022;14:3140. [https://doi.org/10.3390/polym14153140]
60. Saravanan D, Radhakrishnan M. Antimicrobial activity of pigments produced by fungi from Western Ghats. J Chem Pharm Res 2016;8:634-8.
61. Chadni Z, Rahaman MH, Jerin I, Hoque KM, Reza MA. Extraction and optimisation of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology 2017;8:48-57. [https://doi.org/10.1080/21501203.2017.1302013]
62. Sivakumar B, Rao NR, Poornamath BP, Jayaram S, Sarojini S. Multifarious pigment producing fungi of Western Ghats and their potential. Plant Sci Today 2022;9:733-47. [https://doi.org/10.14719/pst.1759]
63. Santhosh SK, Sarojini S, Umesh M. Anti-biofilm activities of nanocomposites:Current scopes and limitations. In:Pal K, editor. Bio-manufactured Nanomaterials. Cham:Springer;2021. [https://doi.org/10.1007/978-3-030-67223-2_5]
64. Sarojini S, Jayaram S. An impact of antibacterial efficacy of metal oxide nanoparticles:A promise for future. In:Pal K, editor. Bio-manufactured Nanomaterials. Cham:Springer;2021. [https://doi.org/10.1007/978-3-030-67223-2_18]
65. Sreekumar MB, Annadurai N, Jayaram S, Sarojini S. Industrial applications of hybrid nanocatalysts and their green synthesis. Top Catal 2022;65:1910-22. [https://doi.org/10.1007/s11244-022-01712-4]
66. Jayaram S, Sarojini S. Bioprospecting of fungal endophytes in Hulimavu Lake for their repertoire of bioactive compounds. Electrochem Soc 2022;107:10471-81. [https://doi.org/10.1149/10701.10471ecst]
67. Joe S, Sarojini S. An efficient method of production of colloidal chitin for enumeration of Chitinase producing bacteria. Mapana J Sci 2017;16:37-45. [https://doi.org/10.12723/mjs.43.4]
68. Vellingiri MM, Ashwin JK, Soundari AJ, Sathiskumar S, Priyadharshini U, Paramasivam D, et al. Mycofabrication of AgONPs derived from Aspergillus terreus FC36AY1 and its potent antimicrobial, antioxidant, and anti-angiogenesis activities. Mol Biol Rep 2021;48:7933-46. [https://doi.org/10.1007/s11033-021-06824-w]
69. Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK, et al. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north Western Tanzania. Tanzan J Health Res 2010;12:63-7. [https://doi.org/10.4314/thrb.v12i1.56287]