ARTICLE HIGHLIGHTS
- Phage L522 showed a high stability under various temperatures, pH, and ultraviolet light.
- In the in vitro test, phage L522 controlled the growth of Xanthomonas oryzae pv. oryzae (Xoo) for approximately 45 h.
- In the in vivo trial, phage L522 had an equivalent efficacy against bacterial leaf blight in rice compared to a commercial pesticide.
1. INTRODUCTION
Bacterial leaf blight (BLB) caused by Xanthomonas oryzae pv. oryzae (Xoo) could lead to enormous yield losses of up to 50% in rice production areas [1]. Most Vietnamese high-quality rice varieties are susceptible to BLB resulting in 15–30% reductions in annual rice yield [2]. In southern Vietnam, BLB often occurs throughout the rainy season (from May to October). The soil-borne pathogens infiltrate natural openings or wounds in plants, leading to their rapid multiplication and widespread dissemination across the cultivation area through wind and heavy rain. The conventional approaches to control BLB, such as cultural practices and using chemical pesticides or biological control agents, remain ineffective, especially during the epiphytic stage [3]. Besides, rampant usage of chemical pesticides in agriculture has raised concerns about adverse effects on the ecosystem and human health in recent decades. In addition to decreasing the efficiency of BLB management, the development of antibiotic resistance in the Xoo population may be responsible for antibiotic resistance transmission into non-target microbiota [4,5]. In efforts to find more environmental-friendly alternatives to antibiotics, phage biocontrol has been considered a promising strategy for the control of phytopathogens.
Phage biocontrol against Xanthomonas sp. has been studied [6]. The host cells are specifically infected and lysed through the replication cycles of lytic bacteriophages. Therefore, phages are harmless to other non-target microbes in the normal microbiota and nontoxic to eukaryotic cells. Moreover, the observed reduction in virulence of phage-resistant Xoo may indirectly enhance the effectiveness of phage biocontrol [7]. Several studies indicated positive results in the inhibitory activity of isolated phages on Xoo in laboratory and greenhouse conditions [8,9]. In addition to genome properties [10], phage viability in various influence factors, such as temperature, pH, and ultraviolet (UV) radiation, should be investigated for phage production, storage, and practical application purposes. In a previous study of the genome properties with the NCBI accession number OP948730, phage L522 was considered a safe candidate for phage biocontrol [11]. The current study investigated the phage stability under environmental factors and the in vivo efficacy of phage L522 to control BLB disease in rice.
2. MATERIALS AND METHODS
2.1. Phage Stability Test
The differences in phage L522 viability under various physicochemical conditions (temperature, pH, UV-A, and UV-B) were assessed according to the method of Xuan et al. [12]. To determine the heat stability, phage preparation was diluted in SM buffer (100 mM NaCl, 10 mM MgSO4, 0.01% gelatin, 50 mM Tris-HCl, pH = 7.5) at approximately 107 PFU/mL, then incubated at different temperatures (4, 20, 30, 37, and 50°C) for 1 h. Samples taken at 10-min intervals were titrated using a plaque assay with bacterial strain Xoo LA1+. In the pH stability test, either 1 M HCl or 1 M NaOH was used to adjust the pH value of tryptone soya broth (TSB) to obtain solutions with a pH range of 3–11. The phage suspension (~ 107 PFU/mL) was added to an equal volume of the adjusted TSB and incubated at 30°C. After 24-h incubation, the phage titer was determined. The effect of UV radiation was also studied at 311 nm (UV-B, model Philips PL-S 9W/01/2P) and 365 nm (UV-A, model Phillips Actinic BL TL-D 15W). Phage stock was diluted in SM buffer to an initial titer of ~ 108 PFU/mL. A 2-mL dilution was placed on an Φ-6mm petri dish and exposed to UV radiation from a distance of 30 cm for 1 h. Sampling was carried out every 10 min and then titrated. The experiment was conducted in triplicate.
2.2. Bacterial Challenge Test
To evaluate the inhibitory activity of phage L522 against Xoo, the bacterial challenge test was conducted. The culture of Xoo strain LA1+ was incubated at 30°C with shaking at 150 rpm in TSB medium. Until the absorbance at 600 nm reached approximately 0.1 (~ 6 × 107 CFU/mL), a volume of the phage suspension was added at a multiplicity of infection (MOI) of 0.01, 0.1, or 1.0. The sample without phage was used as the control. Mixtures were incubated as described above. Sampling was carried out periodically to determine the OD600, which indicated the changes in bacterial growth by phage lysis. The experiment was performed in triplicate.
2.3. Preparation of Phage Suspension for the In Vivo Trial
The culture of Xoo strain LA1+ was incubated at 30°C with shaking at 200 rpm in TSB medium. Until the absorbance at 600 nm reached approximately 0.1, a volume of the phage stock was added at an MOI of 0.1. After 8 h shaking as above condition, the mixture became clear and was then distributed into 2-mL centrifuge tubes and centrifuged at 9,727 × g for 5 min at 4°C. The supernatant was passed through 0.22-μm filter membranes to obtain the final phage suspension.
2.4. In Vivo Trial of Phage in Rice
The in vivo efficacy of phage L522 to control BLB was assessed at Southern Research Center for Plant Protection in Long An province, Vietnam. Jasmin 85 rice variety provided by Cuu Long Delta Rice Research Institute was used. After pre-germinated at room temperature for 48 h, 5 seeds were sown in each 17.5 × 12 cm plastic pot containing 1 kg of soil. Then rice seedlings were pruned to 3 plants per pot. After 45 days of sowing, the experiment was conducted in an arrangement of completely randomized design at environmental temperature with three replications per treatment. Rice plants were infected with the Xoo L024 from microbial-type culture collection in the laboratory, by wound-inducing and spraying bacterial suspension at a concentration of approximately 108 CFU/mL. At day 1 and day 5 after the bacterial infection, four treatments were sprayed phages at different titers such as ~106, 107, 108, 109 PFU/mL, respectively. Another treatment was sprayed a popular commercial pesticide in Vietnam (Starner 20WP) according to the manufacturer’s instruction. The control was infected plants sprayed with sterile water. The plants were observed daily. On day 7 after the second treatment with phage, disease rate (%) and disease incidence (%) were measured as following equations:
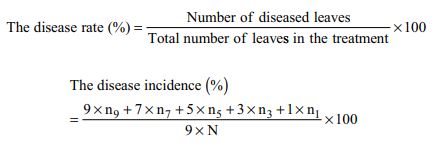
n1, n3, n5, n7: Corresponding to the number of leaves whose diseased area was <1%, 5%, 25%, 50%; n9: The number of leaves whose diseased area was >50%. N: total number of leaves in the treatment.
Curative ratio (%) was calculated as following equation:

DI stands for Disease Incidence. The treated plant in this equation indicated plants treated with phage or pesticide in each test. The above assessment was based on the national technical regulation on surveillance methods of rice pests [13]. Statistical analysis was performed using one-way analysis of variance by IBM SPSS Statistics 20 Software, and the significant differences between means were determined by Duncan’s multiple range test at P ≤ 0.05.
3. RESULTS AND DISCUSSION
3.1. Effect of Temperature, pH, and UV Radiation on the Viability of Phage
As shown in Figure 1a, the temperature stability of phage L522 was stable at all tested temperatures during 1-h incubation. Even at 50°C, the phage concentration was similar to the initial value (P > 0.05). The same results were found with several published Xanthomonas phages [6]. In addition, the titer of the phage remained unchanged over a wide pH range of 4 to 11 as presented in Figure 1b. It is clarified that phage L522 is more resistant to acidic environment than phage ΦXOF4, the titer of which was affected by pH below 6 after 1-h exposure [14]. The phage titer only sharply reduced at pH 3. While temperature and environment acidification are the main factors limiting phage activity [15], the high tolerance of phage L522 contributed information to devise suitable application strategies in rice farming.
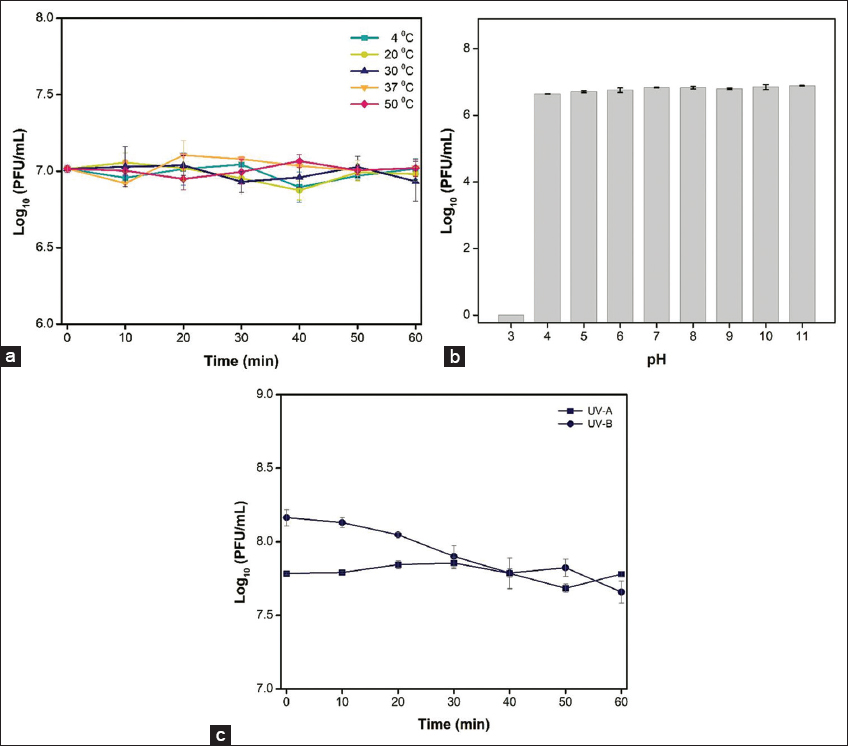 | Figure 1: Stability of the phage L522 under various conditions including temperatures (a), pHs (b), and ultraviolet (UV)-A and UV-B exposure (c). Error bars indicate 95% confidence intervals for the averaged values (n = 3). [Click here to view] |
Figure 1c presents that the phage viability was gradually less affected by UV-A and UV-B light during 1-h exposure. Viable phage counts slightly declined and reached by ~ 0.5 log 10 units at the end of the experiment under UV-B. However, no significant changes in phage titer were recorded under UV-A radiation. Natural sunlight UV level (UV-A and UV-B) is one of the stability issues when applied in both greenhouse and field conditions. Efforts are being made to prolong the viability of phages on the rice leaf surface, including preventing daylight exposure or improving phage formulation [16].
3.2. Inactivation of Xoo in Broth by Phage
The inhibition of phage L522 on Xoo growth in TSB medium was evaluated at three MOI (0.01, 0.1, 1.0). From the initial concentration (OD600 ~ 0.1), the growth of host culture was inhibited post-infection (p.i.) by phage and the mixture became clear at 6 h p.i. Figure 2 shows that the host OD600 began to decrease at different times depending on the MOI. In detail, while the OD600 of the mixture with MOI of 0.1 and 1.0 started to decline at 2 h p.i, the OD600 of host culture with an MOI of 0.01 remained at about 0.1 until 4 h p.i. It is proved that the higher phage concentration resulted in a faster inhibitory effect at the beginning. Growth inhibition was maintained for approximately 45 h by phage infection, whereas the control culture without phage grew sharply and entered the stationary phase at around 30 h (OD600 ~ 2). After 45 h p.i, there were gradual increases of OD600 in all three phage treatments disregarding MOI that reflected the growth of phage-resistant bacteria. The OD600 returned to ~ 0.1 after 55 h. There was no significant difference in the inhibition time among different MOIs. The results of the in vitro test revealed that L522 phage is reasonable to be studied further in the in vivo trial.
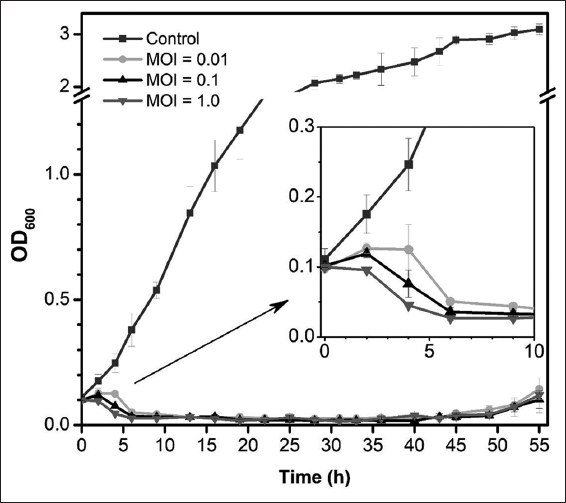 | Figure 2: Changes in optical density at 600 nm during inactivation of Xoo LA1+ by L522 in tryptone soya broth medium at an multiplicity of infection of 0.01, 0.1, or 1.0. The sample without phage was used as the control. Error bars indicate 95% confidence intervals for the averaged values (n = 3). [Click here to view] |
3.3. In Vivo Efficacy of Phage in Rice
The concentration of phage suspension prepared for this experiment was approximately 109 PFU/mL. This phage preparation was then diluted to desired concentrations. In the planta pot experiment, treatments at four phage titers (106, 107, 108, 109 PFU/mL) and a commercial pesticide (Starner 20WP), were applied twice at day 1 and day 5 after the bacterial inoculation. On day 7 after the second treatment, the tests showed significant differences [Figure 3 and Table 1]. Compared to the control, rice leaves treated with phage L522 and the pesticide were obviously less damaged. Table 1 shows that the lowest disease rate and disease incidence of BLB occurred when applying the phage concentration of 109 PFU/mL. With the highest phage titer, the disease rate decreased by approximately 9%, whereas that of the pesticide treatment did not significantly differ from the control plants. Moreover, the efficacy of these two treatments (the phage concentration of 109 PFU/mL and the pesticide) in reducing the disease incidence was equivalent. The disease severity and extent declined to one-half of the control. These results may induce the similarity in the curative ratio of phage preparation at 109 PFU/mL (44.7%) and the pesticide application (45.7%). Further, the in vivo efficacy to control BLB decreased at lower phage concentrations (106, 107, 108 PFU/mL). As shown in Table 1, the phage titer at 108 PFU/mL is considered as a second option of treatment following 109 PFU/mL, when its curative ratio was obviously higher than other phage treatments (106 and 107 PFU/mL). The active chemical of Starner 20WP is oxolinic acid, one of the antibiotics most commonly used against plant pathogen bacteria worldwide [17]. However, the gradual decrease in oxolinic acid efficacy due to resistant strains was recorded during 2009 – 2014 in Israel [18]. The antibiotic resistance could spread to the surrounding environment via plasmid-mediated quinolone resistance genes [19]. As comparable efficacy to Starner 20WP, the phage L522 application is suggested as a more environmental-friendly alternative to control BLB.
Table 1: In vivo efficacy to control bacterial leaf blight in rice by Starner 20WP treatment and phage treatments at various concentrations (106, 107, 108, 109 PFU/mL) at day 12 after Xoo L024 infection.
| Phage concentration | Disease rate (%) | Disease incidence (%) | Curative ratio (%) |
|---|---|---|---|
| 106 | 28.0a | 9.44b | 18.2c |
| 107 | 20.8bc | 8.59b | 25.4c |
| 108 | 23.7b | 7.54c | 34.5b |
| 109 | 20.5c | 6.38d | 44.7a |
| Starner 20WP | 27.1a | 6.25d | 45.7a |
| Control | 29.3a | 11.5a | na |
| cv (%) | 6.48 | 6.57 | 12.2 |
The same letters on the same columns indicate numbers are not significant according to Duncan’s multiple range test at 0.05 level.
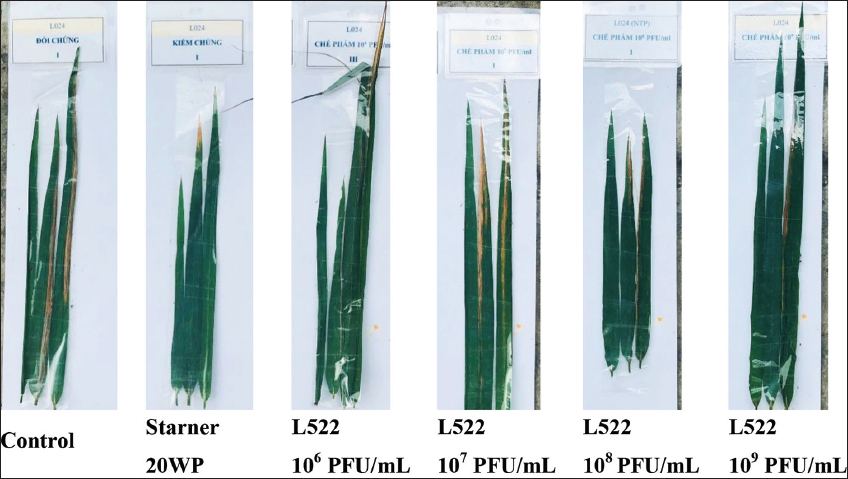 | Figure 3: Differences in the lesion length of rice leaves by treatment with Starner 20WP and phage at various concentrations (106, 107, 108, 109 PFU/mL) at day 12 after Xoo L024 infection. In the control, bacterial infected plants were sprayed with sterile water. [Click here to view] |
4. CONCLUSION
Phage biocontrol is a potential solution in the fight against plant diseases, especially BLB in rice. Our research contributed a profile to the existing Xoo phage collection for practical applications under different soil and climatic conditions. The phage stability, in vitro, and in vivo control efficacy of phage L522 were evaluated. Besides the safety reported in the previous paper, the results of this study suggest that phage L522 has an equivalent efficacy in control BLB in rice to the commercial pesticide Starner 20WP. Therefore, phage L522 is a promising candidate for BLB management in rice farming.
5. ACKNOWLEDGMENTS
This research was funded by the Department of Science and Technology of HCMC, Vietnam, under contract number 110/2020/HD-QPTKHCN.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Liu W, Liu J, Triplett L, Leach JE, Wang GL. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol 2014;52:213-41. [https://doi.org/10.1146/annurev-phyto-102313-045926]
2. Nguyen HT, Vu QH, Van Mai T, Nguyen TT, Vu LD, Nguyen TT, et al. Marker-assisted selection of Xa21 conferring resistance to bacterial leaf blight in indica rice cultivar LT2. Rice Sci 2018;25:52-6. [https://doi.org/10.1016/j.rsci.2017.08.004]
3. Kumar A, Kumar R, Sengupta D, Das SN, Pandey MK, Bohra A, et al. Deployment of genetic and genomic tools toward gaining a better understanding of rice-Xanthomonas oryzae pv. oryzae interactions for development of durable bacterial blight resistant rice. Front Plant Sci 2020;11:1152. [https://doi.org/10.3389/fpls.2020.01152]
4. Sciallano C, Auguy F, Boulard G, Szurek B, Cunnac S. The complete genome resource of Xanthomonas oryzae pv. oryzae CIX2779 includes the first sequence of a plasmid for an African representative of this rice pathogen. Mol Plant Microbe Interact 2022;36:73-7. [https://doi.org/10.1094/MPMI-09-22-0191-A]
5. Xu Y, Luo QQ, Zhou MG. Identification and characterization of integron-mediated antibiotic resistance in the phytopathogen Xanthomonas oryzae pv. oryzae. PLoS One 2013;8:e55962. [https://doi.org/10.1371/journal.pone.0055962]
6. Nakayinga R, Makumi A, Tumuhaise V, Tinzaara W. Xanthomonas bacteriophages:A review of their biology and biocontrol applications in agriculture. BMC Microbiol 2021;21:291. [https://doi.org/10.1186/s12866-021-02351-7]
7. Liu M, Tian Y, Zaki HE, Ahmed T, Yao R, Yan C, et al. Phage resistance reduced the pathogenicity of Xanthomonas oryzae pv. oryzae on rice. Viruses 2022;14:1770. [https://doi.org/10.3390/v14081770]
8. Jain L, Kumar V, Jain SK, Kaushal P, Ghosh PK. Isolation of bacteriophages infecting Xanthomonas oryzae pv. oryzae and genomic characterization of novel phage vB_XooS_NR08 for biocontrol of bacterial leaf blight of rice. Front Microbiol 2023;1:1084025. [https://doi.org/10.3389/fmicb.2023.1084025]
9. Dong Z, Xing S, Liu J, Tang X, Ruan L, Sun M, et al. Isolation and characterization of a novel phage Xoo-sp2 that infects Xanthomonas oryzae pv. oryzae.JGen Virol 2018;99:1453-62. [https://doi.org/10.1099/jgv.0.001133]
10. Nguyen AN, Pham NQ, To HN, Vo N, Tu QV, Nguyen MT, et al. Potential of some phages against Pseudomonas solanacearum causing bacterial wilt in tomato. JAppl Biol Biotechnol 2023;11:232-8. [https://doi.org/10.7324/JABB.2023.145780]
11. My PD, Vinh TQ, Ngoc TH, Anh PN, Duyen LT, Thien NM, et al. Complete genome sequence of a novel lytic phage of Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Arch Virol 2023;168:157. [https://doi.org/10.1007/s00705-023-05788-5]
12. Xuan T, Hoang HA, Tam L. Stability and activity of TG25P phage in control of Aeromonas hydrophila in striped catfish pond water. Sci Technol Dev J 2018;21:64-70. [https://doi.org/10.32508/stdj.v21i2.429]
13. The national technical regulation on surveillance method of rice pests. QCVN 01-166:2014/BNNPTNT, 2014. Available from:https://www.ppd.gov.vn/uploads/news/2014_06/QC%20dich%20hai%20lua%20166.pdf [Last accessed on 2023 Jun 15].
14. Ranjani P, Gowthami Y, Gnanamanickam SS, Palani P. Bacteriophages:A new weapon for the control of bacterial blight disease in rice caused by Xanthomonas oryzae. Microbiol Biotechnol Lett 2018;46:346-59. [https://doi.org/10.4014/mbl.1807.07009]
15. Jonczyk-Matysiak E, Lodej N, Kula D, Owczarek B, Orwat F, Miedzybrodzki R, et al. Factors determining phage stability/activity:Challenges in practical phage application. Exp Rev Anti Infect Ther 2019;17:583-606. [https://doi.org/10.1080/14787210.2019.1646126]
16. Holtappels D, Fortuna K, Lavigne R, Wagemans J. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr Opin Biotechnol 2021;68:60-71. [https://doi.org/10.1016/j.copbio.2020.08.016]
17. Verhaegen M, Bergot T, Liebana E, Stancanelli G, Streissl F, Mingeot-Leclercq MP, et al. On the use of antibiotics to control plant pathogenic bacteria:A genetic and genomic perspective. Front Microbiol 2023;14:1221478. [https://doi.org/10.3389/fmicb.2023.1221478]
18. Shtienberg D, Manulis-Sasson S, Zilberstaine M, Oppenheim D, Shwartz H. The incessant battle against fire blight in pears:30 years of challenges and successes in managing the disease in Israel. Plant Dis 2015;99:1048-58. [https://doi.org/10.1094/PDIS-01-15-0101-FE]
19. Li J, Wang T, Shao B, Shen J, Wang S, Wu Y. Plasmid-mediated quinolone resistance genes and antibiotic residues in wastewater and soil adjacent to swine feedlots:Potential transfer to agricultural lands. Environ Health Perspect 2012;120:1144-9. [https://doi.org/10.1289/ehp.1104776]