1. INTRODUCTION
Lactic acid bacteria (LAB) are a group of bacteria that are Gram-positive and non-spore forming [1]. In recent years, LAB have attracted a lot of attention owing to their excellent probiotic functions, including the regulation of the intestinal barrier and promoting human health. Research indicates that the probiotic functions of LABS are related to their bacterial secretions [2]. Because microbes are capable of synthesizing functional secretory products and due to the presence of efficient technology available to purify the secreted products, microorganisms are preferred over plant and animal systems [3]. They can be produced in large quantities to meet the increasing demands of society. The feasibility of genetically modifying the microbial genome using strain improvement techniques has also increased the application and synthesis of microbial secretory products [4]. Exopolysaccharides (EPS) are one such secretory products that contribute to the beneficial properties of many probiotic bacteria [5,6]. EPS are long-chain polymers that are produced through metabolic pathways of microorganisms such as bacteria, fungi, and cyanobacteria [7]. Some LABs known for their ability to produce EPS are Lactobacillus, Lactococcus, Streptococcus, Leuconostoc, Pediococcus, and Enterococcus [1]. The beneficial roles of EPSs include immunomodulation [8], anti-biofilm [9], anti-cancer properties [10], blood glucose regulation [11], and cholesterol-lowering abilities [12]. Several studies have also linked probiotic EPS with adhesion, colonization, and host-bacteria interactions [13]. The functions of EPSs are dependent on their molecular weight and monosaccharide composition [14], which, in turn, are dependent on factors such as pH, temperature, salt concentration, and carbon and nitrogen contents [6,15]. Different LAB species produce a wide variety of EPS, and several researchers have worked on isolation, identification, purification, and characterization of EPS from different strains of bacteria [16].
The structure-function relationship of EPS is still a major research topic. The current study evaluates the influence of salt concentration, pH, carbon sources, and nitrogen sources on the production of EPS. Research on the chemical structures and molecular arrangements of EPSs are important to establish their structure-function relationship. Hence, the study also evaluates the chemical composition of the obtained EPS using Fourier-transform infrared spectroscopy (FTIR)\thermogravimetric analysis (TGA)-analysis, scanning electron microscopy-energy dispersive X-ray (SEM-EDX) and atomic force microscopy (AFM). The structural characterization of the isolated EPS was elucidated using FT-IR spectroscopy, and morphological analysis was performed using SEM and AFM.
2. MATERIALS AND METHODS
2.1. Isolation of the Bacteria and Extraction of EPS
The bacteria were isolated from dried anchovy fish, and 16S ribosomal RNA (16S rRNA) sequencing revealed the organism to be Bacillus subtilis (Accession number: MN960600) [17]. B. subtilis was cultured in MRS medium (De Man-Rogosa-Sharpe Lactobacillus MRS broth granulated catalog number GM369) media and incubated at 37°C for 16 h. The broth was subjected to centrifugation at 8,000 RPM for 8 min at 4°C, and the supernatant collected was treated with cold ethanol in three times the volume and stored at 4°C overnight for EPS precipitation. The overnight culture was subjected to centrifugation at 6000 rpm for 5 min at 4°C and the pellet was collected [18].
2.1.1. EPS purification
The culture was centrifuged at 12,000g for 20 min at 4°C. The supernatant was treated with different concentrations of trichloroacetic acid (TCA) (20–70%) and was held at 4°C to precipitate the proteins overnight. The pellet was discarded and the supernatant was treated with double the volume of chilled 95% (v/v) ethanol and stored at −20°C for 24 h in order to precipitate the polysaccharides. The sample was centrifuged at 10,000 g for 15 min at 4°C and the obtained EPS pellets were re-suspended in phosphate buffer. The carbohydrate content in the sample was estimated at 630 nm using the anthrone method using glucose as standard. The precipitated EPS was detected by SDS-PAGE and visualized using a silver staining procedure that was modified [19]. The samples were dialyzed using a dialysis membrane with 12-kDa cutoff. The dialyzed EPS was subsequently purified by Sephadex G75 chromatography [20].
2.2. Parameters Influencing Production of EPS
2.2.1. Effect of carbon source on EPS production
The bacteria were inoculated into different MRS minimal media containing 5% of dextrose, fructose, maltose, and xylose as the only carbon sources and were incubated for 24 h at 37°C. The overnight culture was subject to centrifugation at 6000 rpm for 5 min at 4°C, and the pellet was weighed after air drying. The supernatant was used for EPS precipitation using cold ethanol at 4°C overnight. Centrifugation at 10,000 rpm for 10 min at 4°C yielded EPS pellets that were collected and resuspended in 5 mL of sterile distilled water. Quantification of the obtained EPS was carried out by phenol-sulfuric acid assay [21].
2.2.2. Effect of nitrogen supplementation on EPS production
The bacteria were inoculated into MRS minimal media containing 5% each of meat extract, yeast extract, ammonium chloride (NH4Cl) or ammonium sulfate ([NH4]2, SO4) as the only nitrogen sources and was incubated at 37°C for 24 h. The overnight culture was subject to centrifugation at 6000 rpm for 5 min at 4°C, and the pellet was weighed after air drying. The supernatant was used for EPS precipitation using cold ethanol at 4°C overnight. Centrifugation at 10,000 rpm for 10 min at 4°C yielded EPS pellets that were collected and resuspended in 5 mL of sterile distilled water. Quantification of EPS was carried out by the phenol-sulfuric acid method [21].
2.2.3. Effect of pH on EPS production
The bacteria were inoculated into MRS broth with pH ranging from 4 to 10 and incubated at 37°C for 24 h. The overnight culture was subject to centrifugation at 6000 rpm for 5 min at 4°C, and the pellet was weighed after air drying. The supernatant was used for EPS precipitation using cold ethanol at 4°C overnight. Centrifugation at 10,000 rpm for 10 min at 4°C yielded EPS pellets that were collected and resuspended in 5 mL of sterile distilled water. Quantification of EPS was carried out by phenol-sulfuric acid assay [22].
2.2.4. EPS production and the effect of sodium chloride (NaCl)
The bacteria were inoculated into MRS broth containing NaCl at concentrations ranging from 2% to 5% and incubated at 37°C for 48 h. EPS was precipitated as previously described and quantified using the phenol-sulfuric acid assay [23].
2.3. Characteristics of the Isolated EPS
2.3.1. FTIR analysis
FTIR was used to detect the major structural groups of the purified EPS (FTIR: Shimadzu IR Spirit-L, Detector: IRSpirit-L model: LiTa03 detector). EPS was precipitated as previously described and pelleted by centrifugation at 10,000RPM for 10 min at 4°C. The pellets were further dried in a hot air oven at 90°C for a duration of 5 h. The dried EPS samples were analyzed for its chemical composition and physical state [16].
2.3.2. TGA
TGA/DSC: Perkin Elmer STA 6000 equipment (Christ University, Bangalore, India) was used to determine the carbonization temperature. The samples were characterized under a nitrogen atmosphere in the range of 30−800°C [8].
2.3.3. SEM-EDX
The structure and morphology of non-purified EPS were analyzed using SEM-EDX: Jeol 6390 LA/OXFORD XMX N (CUSAT, Kochi, India) at an accelerating voltage of 0.5–30 kV. EDAX detector area was maintained at 30 mm2 [24].
2.3.4. AFM
The slides were exposed to a mixture of 15 mL of hydrochloric acid and 5 mL of nitric acid for 30 min. This was followed by a subsequent treatment with a mixture of sulfuric acid and hydrogen peroxide for a duration of 30 min. The slides were rinsed with double distilled water. About 30 μL of the freshly prepared EPS sample was dropped and casted on the surface of a freshly cleaved mica disc sample carrier and was allowed to settle on the mica disc at room temperature. The samples were washed thrice with 100 μL of 0.22 μm filtered milliQ water for every 5 min and dried in a vacuum for 30 min at 37°C. Later, the AFM imaging was carried out using the peak force tapping principle in Bruker bioscope resolve via Peak Force Quantitative Nanomechanical Mapping mode. Imaging was done at a 0.9 Hz scanning rate with 256 samples per line using SCANASYST probe (Bruker) with a 5nm tip radius. Images were processed and analyzed using Nanoscope Analysis 1.8 software [25].
2.3.5. Emulsification assays
EPS fractions were dissolved in water to obtain a concentration of 0.4 mg/mL. The emulsifying potential of EPS was determined by mixing the EPS with hydrocarbons such as olive oil, coconut oil, hexane, xylene, benzene, and paraffin oil (light) in a ratio 3:2. All the samples were mixed vigorously for 3 min and kept undisturbed for 3 days [7]. The volume of the emulsified layer was noted at 1 h, 24 h, 48 h and 72 h to calculate the emulsification index (EI) using the formula:
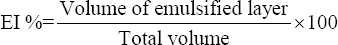 |
2.3.6. Anti-oxidant assays
(i) Total antioxidant capacity (TAC) of EPS
TAC was determined using an ascorbic acid standard. TAC reagents namely 0.6 M sulphuric acid, 28 mM sodium phosphate and 1% ammonium molybdate were added to the diluted EPS samples. The samples were incubated at 95°C for 10 min and brought to room temperature. Absorbance was measured at 695 nm [26].
(ii) Free radical scavenging assay
A working standard was prepared using ascorbic acid (1 mg/mL) following which 0.5 mL of the sample was diluted in 1 ml of distilled water and 0.625 mL of 1% potassium ferricyanide. The samples were incubated at 50°C for 5 min. After the addition of 10% TCA the sample was centrifuged at 3000 rpm for 10 min. 300 μL of 0.1% FeCl3 was added to 1.8 mL of the supernatant diluted using 1.8 mL of distilled water. Further, the absorbance was recorded at 700 nm. An increase in absorbance indicates a higher reducing power [26].
2.3.7. Heavy metal binding ability of EPS
Heavy metal assimilation of EPS was determined [27] by adding 0.1 mg/mL of EPS to heavy metal solutions of Fe (0.6 ppm), Cu (0.5 ppm), and Zn (0.4 ppm) and incubated overnight in dialysis bags with a 12 KDa cut-off. The EPS samples were collected from the dialysis tubes after 24 h and acid-digested using concentrated nitric acid on a hot plate. The heat-digested samples were quantified for heavy metal assimilation by the EPS samples using Atomic Absorption Spectrophotometer (SHIMADZU, AA-6880) with measurement range 185-900nm, detector photomultiplier tube, optical double beam, and flame type air-C2H2.
3. RESULTS AND DISCUSSION
3.1. EPS Purification
EPS recovery was found to be maximum in the 50% TCA fraction, and it was quantified to be 628 mg/L. The 50% TCA fraction represented the maximum EPS concentration as depicted in Figure 1. Hence this fraction was taken for further purification by dialysis. After 48 h of dialysis, the band number reduced to 2 major bands indicating partial purity in target EPS. The 48 h dialysate of TCA, 50% fraction, taken for column filtration by Sephadex G75 yielded 5 different fractions. Table 1 shows that fraction 4 after column chromatography yielded the highest EPS concentration of 1928 mg/L with the single band in the SDS page indicating homogeneity.
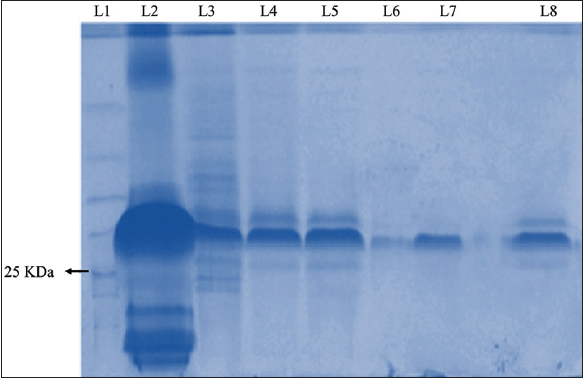 | Figure 1: Sodium dodecyl-sulfate polyacrylamide gel electrophoresis gel image of trichloroacetic acid (TCA) 50% fraction after dialysis and column filtration. Lane summary: Lane 1: Ladder, Lane 2: Fraction TCA 50%, Lane 3: Fraction TCA 50% (48 h dialysate), Lane 4: Fraction 1 (0.5 mL/min), Lane 5: Fraction 2 (0.5 mL/min), Lane 6: Fraction 3 (0.5 mL/min), Lane 7: Fraction 4 (0.5 mL/min), Lane 8: Fraction 5 (0.5 mL/min). [Click here to view] |
Table 1: Quantification of 50% fraction of trichloroacetic acid after dialysis and column filtration.
| Sample | Fractions | OD at 630nm | EPS conc.μg/0.5ml | EPS conc.μg/ml | EPS conc.mg/ltr |
|---|---|---|---|---|---|
| Fraction TCA 50% after 48 hours dialysis and column filtration | 1 (0.5ml/min) | 0.235 | 470 | 941 | 941 |
| 2 (0.5ml/min) | 0.267 | 552 | 1103 | 1103 | |
| 3 (0.5ml/min) | 0.088 | 104 | 208 | 208 | |
| 4 (0.5ml/min) | 0.432 | 964 | 1928 | 1928 | |
| 5 (0.5ml/min) | 0.215 | 422 | 843 | 843 |
3.2. EPS Production and the Effect of Carbon Sources
Different carbon sources were tested for their ability to promote EPS production by B. subtilis. Figure 2a displays the influence of various sugars on EPS synthesis. Maximum EPS production of 20 mg/mL was detected when dextrose was used as the carbon source, followed by 17 mg/mL with fructose, and the lowest production of EPS was found when maltose (10 mg/mL) and xylose (12 mg/mL) were used. The increase in bacterial cell weight could not be correlated with enhanced EPS production. The sugars are used by EPS producing bacteria as carbon sources as they aid in the synthesis of EPS [28]. The sugars preferred by microorganisms’ change from species to species. Bacillus thermoantarcticus has shown mannose as its preferred carbon source [29]. When Streptococcus thermophilus ASCC 1275 and Bacillus amyloliquifaciens were examined for sugar-associated changes in their EPS production, it was found that both bacteria were able to utilize three sugars, namely sucrose, glucose, and lactose, but the sugar that resulted in maximum EPS yield was sucrose [30,31].
 | Figure 2: The effects of (a) carbon sources (b) nitrogen sources (c) NaCl and (d) pH on the production of exopolysaccharides. [Click here to view] |
3.3. EPS Production and the Effect of Nitrogen Sources
Various nitrogen sources were tested for their ability to promote EPS production by B. subtilis. Figure 2b shows the influence of nitrogen sources on EPS production. Maximum EPS production of 0.7 mg/ml was observed when yeast extract and ammonium chloride were separately used as the nitrogen sources. This was followed by meat extract (0.6 mg/mL), and the lowest yield was seen when ammonium sulfate was used (0.4 mg/mL). The increase in bacterial cell weight could not be correlated with enhanced EPS production. Bacillus amyloliquefaciens BPRGS showed maximum EPS production in the presence of yeast extract [31], followed by the rest of the nitrogen sources. In Chryseobacterium indologenes MUT.2 highest production of EPS was achieved when glutamic acid was used as the nitrogen source [32]. As with carbon sources, different bacterial species prefer different nitrogen sources.
3.4. EPS Production and the Effect of NaCl
B. subtilis was grown at different concentrations of NaCl. Figure 2c displays the influence of NaCl on EPS production. The highest EPS production (9 mg/mL) was observed at 2% NaCl supplementation groups, followed by 3% NaCl which yielded 7 mg/mL and the lowest production of EPS was found to be at 4% (4 mg/mL) and 5% NaCl (6 mg/mL). There was no significant correlation between the production of EPS and the cell weight of the culture. The EPS production saw a sharp decline with an increase in salinity. It has been previously shown that NaCl at high concentrations can reduce EPS production in LAB [13,33]. Decreased production of EPS due to increased NaCl concentrations has been demonstrated previously in Lactobacillus helveticus ATCC 1580710 and Pediococcus parvulus 2.6.11 [13].
3.5. EPS Production and the Effect of pH
B. subtilis was grown within a pH range of 4–10. Figure 2d displays the effect of pH on EPS production. Maximum EPS production of 0.8 mg/mL was seen at pH 4, followed by 0.73 mg/mL at pH6 and the lowest EPS production was found to be at pH 10 (0.28 mg/mL) and pH 7 (0.48 mg/mL). There was no correlation between EPS production and the cell weight of the culture. We observed that at pH 4 EPS production was high even when the cell count was the lowest. In most of the situations, there was a decline in EPS production with the increase in pH except at pH 8. Bacteria use different mechanisms, such as the production of EPS to overcome stressful situations [34]. The increased production of EPS at pH 4 may be as a protective effect against the acidic environment. This was also witnessed when the strain Lactobacillus salivarius UCO_979C-1 showed that when exposed to an acidic environment of pH 2 there was an increased EPS production [15].
3.6. Characteristics of the Isolated EPS
3.6.1. FTIR of EPS produced by the isolated bacteria
FTIR is a method that relies on the fact that the vibration of bonds occurs at their characteristic frequencies. This helps in detecting and characterizing covalent bonding and detecting functional groups. Figure 3 depicts the FTIR spectrum of the isolated EPS. EPS isolated were studied between the spectrum of 400 cm-1 and 4000 cm-1 in which numerous peaks from 3195.74 cm-1 to 561.18 cm-1 were identified. An absorption band seen in the region of 3195.74 cm−1 is indicative of a hydroxyl group and is associated with polyhydroxilic compounds [35-37]. Stretching in the 2911.58 cm−1 region is characteristic of methyl and methylene groups because of the C-H stretching vibrations [38,39]. The region of 1640 cm−1 shows a band that is characteristic of the stretching vibrations of C=O group [16]. Vibrations around 981 cm-1 indicate the vibrations of the C-O-C glycoside link [40]. These results show that the extracted EPS was a polysaccharide as the infrared spectra displayed shows distinctive characteristics of polysaccharides.
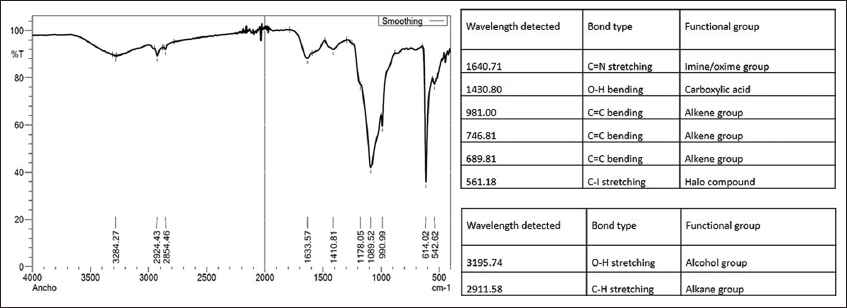 | Figure 3: Fourier-transform infrared spectroscopy spectrum of purified exopolysaccharides of Bacillus subtilis in the range of 500–4000 cm−1. [Click here to view] |
3.6.2. Thermogravimetric analysis
Figure 4 shows a 3 Phase degradation where the 1st phase showcases the loss of moisture from a region of 50–150°C with a weight loss of 8%. Similar observations of loss of weight were made in EPS from L. plantarum KF5 [41]. The EPS remained unstable and continued to lose weight with the increase in temperature (200–440°C). The second phase showed a major loss of mass (26%) due to unfolding and EPS depolymerization. The final stage of degradation was observed from 750°C before, which the EPS were thermally stable at 500–640°C. The thermodynamic results state that EPS can be used as a high thermal tolerance product in various industries.
 | Figure 4: Thermogravimetric analysis analysis of the exopolysaccharides. [Click here to view] |
3.6.3. SEM-EDX
SEM is a useful means to examine the surface morphology of molecules and can elucidate their physical properties [42]. The information generated about a sample is more when SEM is used in combination with energy-dispersive X-ray (EDX), as EDX generates information about the elemental composition of the sample [43]. The scanning electron micrographs under different magnifications and EDX analysis of isolated exopolysaccharide is presented in Figure 5. EPS of B. subtilis has a coarse surface and a three-dimensional structure with irregular lumps. The SEM analysis of EPS from a B. subtilis isolated from a marine source called EPSR4 revealed densely packed polysaccharide particles with a relatively high degree of porosity [44]. While SEM micrographs of B. subtilis strains were limited, SEMs from other species were available for comparison. The EPS from Streptococcus thermophilus strain CC30 was porous in nature with a web-like structure [45]. EPS from Lactobacillus fermentum CFR 2195 was highly compact and exhibited a flaky appearance [16]. An exopolysaccharide from Lactobacillus plantarum displayed a stable 3-dimensional porous structure [8]. The EPS of B. infantis shows a loose structure with relatively thinner and fragmented filaments and that of Bifidobacterium longum subsp. infantis CCUG 52486 displayed a porous web-like structure [42]. EPS-E8 secreted by Pediococcus pentosaceus has a rough surface, irregular reticular-like shape, and a near-spherical structure [24]. These results indicate that the structures of EPS vary from species to species, and the chemical composition of the polysaccharides is responsible for the visible physical changes. EDX analysis of the EPS showed carbon with oxygen, silicon, chlorine, magnesium, sodium, phosphorous, sulfur, chlorine, potassium, and calcium. Reports on the structure and elemental composition of the EPS synthesized by other B. subtilis were not found during our literature search.
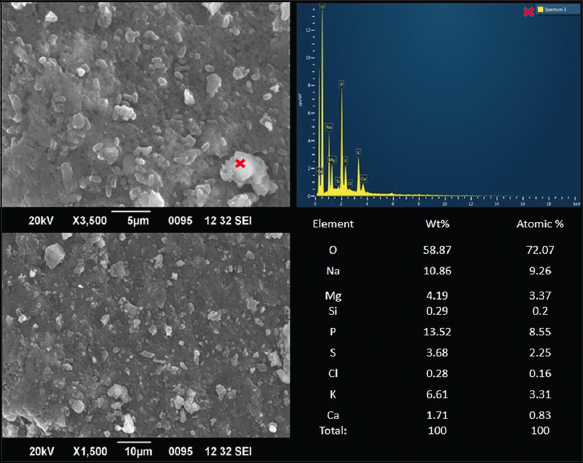 | Figure 5: Scanning electron microscopy images and energy dispersive X-ray spectrum of purified exopolysaccharides of anchovies. Wt%: Percentage by weight; atomic %: Percentage by atomic weight; O: Oxygen; Na: Sodium, Mg: Magnesium, Si: Silicon P: Phosphorous S: Sulphur Cl: chlorine K: Potassium Ca: Calcium. [Click here to view] |
3.6.4. AFM
AFM is a useful imaging tool that helps to characterize the morphological features of biological samples, such as expolysacchrides by providing three-dimensional views and structural details [46]. AFM analysis has helped to understand the nanoscale morphology of EPS better by providing high-resolution images of the EPS surface [47]. In the present study, AFM images of EPS showed a highly cross-linked polymerized structure with an average count of 35 particles per field and an average density of 1.4 μg/μm2 [Figure 6]. Its mean area was 14877.319 nm2 (minimum 9536.74 nm2, maximum 7389.662 nm2). The average diameter of the molecules was observed to be at 134.75nm (minimum 110.193 nm, maximum 245.412 nm). AFM analysis of the EPS revealed spike-like lumps with an average height of 3.537 nm (minimum 1.962 nm and maximum 22.798 nm). The estimated maximum height of 22.78 nm is higher than the length of a single EPS chain (0.1–1 nm), indicating that EPS molecular chains intend to create polymeric clusters [48]. The presence of a rough surface morphology and topology is indicative of clustering and cross-linking of the polysaccharide chains. EPS of varied shapes and structures have been reported from other sources. AFM of ESPR4 from B. subtilis strain AG4 showed a great degree of crystallinity with significant porosity [44]. AFM images of EPS-E8 highlighted spherical clusters indicating molecular aggregation of polysaccharide chains [24]. AFM images of the CC30 EPS showed molecules tightly packed, suggesting that the CC30 EPS has a strong affinity for water molecules [45]. Images of the strain YW11 EPS presented tangled networks with spherical lumps [49].
 | Figure 6: Atomic force microscopic analysis of exopolysaccharides representing the topology in 2D (a) and 3D (b). [Click here to view] |
3.6.5. Emulsification assays
The emulsification property of biopolymers was determined by their ability to retain emulsions for some time [50]. EI of the EPS with hydrocarbons such as olive oil, coconut oil, hexane, xylene, benzene, and paraffin oil (light) are shown in Figure 7. EPS was able to stabilize emulsions with different hydrocarbons. The value was the highest for coconut oil, followed by olive oil, with a 72-h EI of 44 and 21%, respectively. Reports on the emulsification activity of the EPS produced by B. subtilis species were not found. However, similar observations were seen in the case of biopolymers formed by other bacterial species. EPS produced by Bacillus megaterium RB-05 showed maximum emulsification with coconut oil [51]. Emulsions prepared with EPS of Bifidobacterium longum subsp. Infantis and Bifidobacterium infantis also showed a high EI percentage in the presence of coconut oil along with orange oil and sunflower seed oil [42]. EPS from Leuconostoc mesenteroides strain SN-8 showed the best emulsification activity with peanut oil [7]. Since the extracted EPS showed good activity with coconut oil, it can be purified to be used as a potential bio-emulsifier with considerable activity when used along with coconut oil and olive oil. However, its use as a food additive needs to be ascertained.
 | Figure 7: Emulsifying percentage of exopolysaccharides with different hydrocarbons. [Click here to view] |
3.6.6. Antioxidant assays
Tests that quantify the antioxidant effect in biological fluids are helpful in assessing the potentiality to resist oxidative damage [51]. TAC gives a measure of the free radical scavenging capacity of a test solution [52]. The ferric reducing ability of plasma is a type of indirect spectrophotometric TAC that works by determining the metal complex-reducing ability of a sample [53]. The intensity of color produced by the end product is proportional to the antioxidant capacity [52]. The in vitro antioxidant activity of EPS was determined and compared with that of ascorbic acid in this study. The TAC of the EPS was found to be 47.95% which was lesser than that of ascorbic acid, indicating moderate antioxidant capacity. At the same time, the reducing power of the EPS was found to be 37.41667%. The antioxidant capacities of EPS-E8 of Pediococcus pentosaceus E8 were considerably less than ascorbic acid [24]. The EPS from Streptococcus thermophilus CC30 was also reported to have moderate antioxidant activity [45]. Factors such as the methods of extraction and isolation, molecular weight and monosaccharide composition may affect the antioxidant activity of EPS. Some reports show that EPS with low molecular weight shows stronger antioxidant activities [8].
3.6.7. Heavy metal binding ability of EPS
Bacterial EPS have the ability to bind to heavy metals with different affinity and specificity [54]. The presence of ionizable functional groups such as hydroxyl and carboxyl may be responsible for the ability of EPS to bind to heavy metals [27]. This experiment examined the ability of the EPS to adsorb metals such as Fe, Cu and Zn. The highest adsorption was seen toward copper, followed by iron, as shown in Figure 8. The least activity was seen with zinc which is in accordance with the study on Pseudomonas stutzeri AS22 [27]. EPS from a non-pathogenic Pseudomonas veronii 2E strain adsorbed 82.8% of 1 mM Cu(II) in 96 h and was also able to desorb 73.2% of the adsorbed metal [55]. Comparable results were seen when EPS produced from nitrogen-limited glycerol/ethanol-rich waste- water were used to examine the adsorption and subsequent desorption of copper with 99.9% adsorption and 86% desorption abilities [56]. These properties are useful in the field of bioremediation, especially in recovering and recycling metals from industrial wastes.
 | Figure 8: Heavy metal assimilation by exopolysaccharides. [Click here to view] |
4. CONCLUSION
The use of bacterial EPS is environmentally advantageous as they are hydrophilic, biodegradable, and non-toxic when compared to synthetic polymers [20,57]. These polysaccharides can be successfully used in the removing heavy metals from industrial wastes, oil recovery, and other remediation techniques [58]. B. subtilis produces an efficient quantity of EPS. The purified EPS can be a potential emulsifier. Future studies will focus on further analysis of its structure, biological roles, and possible use as a potential probiotic.
5. ACKNOWLEDGMENTS
The authors acknowledge the Department of Life Sciences and Centre for Research, CHRIST University, India, for their support (MRP-1936).
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Werning ML, Hernández-Alcántara AM, Ruiz MJ, Soto LP, Dueñas MT, López P, et al. Biological functions of exopolysaccharides from lactic acid bacteria and their potential benefits for humans and farmed animals. Foods 2022;11:1284. [CrossRef]
2. Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán CG, Salminen S. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J Food Prot 2006;69:2011-5. [CrossRef]
3. Green ER, Mecsas J. Bacterial secretion systems:An overview. Microbiol Spectr 2016;4:10.1128/microbiolspec.VMBF-0012-2015. [CrossRef]
4. Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health:A review. J Food Drug Anal 2018;26:927-39. [CrossRef]
5. Oerlemans MM, Akkerman R, Ferrari M, Walvoort MT, de Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J Funct Foods 2021;76:104289. [CrossRef]
6. Sheng S, Fu Y, Pan N, Zhang H, Xiu L, Liang Y, et al. Novel exopolysaccharide derived from probiotic Lactobacillus pantheris TCP102 strain with immune-enhancing and anticancer activities. Front Microbiol 2022;13:1015270. [CrossRef]
7. Li Y, Liu Y, Cao C, Zhu X, Wang C, Wu R, et al. Extraction and biological activity of exopolysaccharide produced by Leuconostoc mesenteroides SN-8. Int J Biol Macromol 2020;157:36-44. [CrossRef]
8. Wang J, Zhao X, Yang Y, Zhao A, Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol 2015;74:119-26. [CrossRef]
9. Shaaban M, Abd El-Rahman OA, Al-Qaidi B, Ashour HM. Antimicrobial and antibiofilm activities of probiotic Lactobacilli on Antibiotic-Resistant Proteus mirabilis. Microorganisms 2020;8:960. [CrossRef]
10. ?li?ewska K, Markowiak-Kope?P, ?li?ewska W. The role of probiotics in cancer prevention. Cancers (Basel) 2020;13:20. [CrossRef]
11. Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus:A meta-analysis of randomized clinical trials. Sci Rep 2020;10:11787. [CrossRef]
12. Rahbar Saadat Y, Yari Khosroushahi A, Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr Polym 2019;217:79-89. [CrossRef]
13. Seesuriyachan P, Kuntiya A, Hanmoungjai P, Techapun C, Chaiyaso T, Leksawasdi N. Optimization of exopolysaccharide overproduction by Lactobacillus confusus in solid state fermentation under high salinity stress. Biosci Biotechnol Biochem 2012;76:912-7. [CrossRef]
14. Liu Z, Zhang Z, Qiu L, Zhang F, Xu X, Wei H, et al. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J Dairy Sci 2017;100:6895-905. [CrossRef]
15. Sanhueza E, Paredes-Osses E, González CL, García A. Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the pH acid acclimated variant. Electron J Biotechnol 2015;18:343-6. [CrossRef]
16. Yadav V, Prappulla SG, Jha A, Poonia A. A novel exopolysaccharide from probiotic Lactobacillus fermentum CFR 2195:Production, purification and characterization. Biotechnol Bioinf Bioeng 2011;1:415-21.
17. Bhandary T, Riyaz AL, Alagesan Paari K. Probiotic properties of Bacillus subtilis isolated from dried anchovies (Stolephorus indicus) and evaluating its antimicrobial, antibiofilm and growth-enhancing potential in Danio rerio. J Anim Health Prod 2021;9:205. [CrossRef]
18. Kodali VP, Das S, Sen R. An exopolysaccharide from a probiotic:Biosynthesis dynamics, composition and emulsifying activity. Food Res Int 2009;42:695-9. [CrossRef]
19. Fomsgaard A, Freudenberg MA, Galanos C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J Clin Microbiol 1990;28:2627-31. [CrossRef]
20. Sharma K, Sharma N, Handa S, Pathania S. Purification and characterization of novel exopolysaccharides produced from Lactobacillus paraplantarum KM1 isolated from human milk and its cytotoxicity. J Genet Eng Biotechnol 2020;18:56. [CrossRef]
21. Polak-Berecka M, Wa?ko A, Szwajgier D, Chomaz A. Bifidogenic and antioxidant activity of exopolysaccharides produced by Lactobacillus rhamnosus E/N cultivated on different carbon sources. Pol J Microbiol 2013;62:181-8. [CrossRef]
22. Abid Y, Joulak I, Ben Amara C, Casillo A, Attia H, Gharsallaoui A, et al. Study of interactions between anionic exopolysaccharides produced by newly isolated probiotic bacteria and sodium caseinate. Colloids Surf B Biointerfaces 2018;167:516-23. [CrossRef]
23. Ayyash M, Stathopoulos C, Abu-Jdayil B, Esposito G, Baig M, Turner MS, et al. Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79:Characterization, bioactivities and rheological properties influenced by salt and pH. LWT 2020;131:109741. [CrossRef]
24. Jiang G, He J, Gan L, Li X, Xu Z, Yang L, et al. Exopolysaccharide Produced by Pediococcus pentosaceus E8:Structure, bio-activities, and its potential application. Front Microbiol 2022;13:923522. [CrossRef]
25. Vinothkanna A, Sathiyanarayanan G, Balaji P, Mathivanan K, Pugazhendhi A, Ma Y, et al. Structural characterization, functional and biological activities of an exopolysaccharide produced by probiotic Bacillus licheniformis AG-06 from Indian polyherbal fermented traditional medicine. Int J Biol Macromol 2021;174:144-52. [CrossRef]
26. Shankar T, Palpperumal S, Kathiresan D, Sankaralingam S, Balachandran C, Baskar K, et al. Biomedical and therapeutic potential of exopolysaccharides by Lactobacillus paracasei isolated from sauerkraut:Screening and characterization. Saudi J Biol Sci 2021;28:2943-50. [CrossRef]
27. Maalej H, Hmidet N, Boisset C, Buon L, Heyraud A, Nasri M. Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J Appl Microbiol 2015;118:356-67. [CrossRef]
28. Yilmaz M, Celik GY, Aslim B, Onbasili D. Influence of carbon sources on the production and characterization of the exopolysaccharide (EPS) by Bacillus sphaericus 7055 Strain. J Polym Environ 2012;20:152-6. [CrossRef]
29. Manca MC, Lama L, Improta R, Esposito E, Gambacorta A, Nicolaus B. Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 1996;62:3265-9. [CrossRef]
30. Padmanabhan A, Tong Y, Wu Q, Zhang J, Shah NP. Transcriptomic insights into the growth phase- and sugar-associated changes in the exopolysaccharide production of a high EPS-producing Streptococcus thermophilus ASCC 1275. Front Microbiol 2018;9:1919. [CrossRef]
31. Rao BP, Kasirajan S, Sekaran R, Mandal A. Characterization of Exopolysaccharide from Bacillus amyloliquefaciens BPRGS for its Bioflocculant Activity. Int J Sci Eng Res 2013;4:1696-704.
32. Khani M, Bahrami A, Chegeni A, Ghafari MD, Mansouran Zadeh A. Optimization of Carbon and Nitrogen Sources for Extracellular Polymeric Substances Production by Chryseobacterium indologenes MUT.2. Iran J Biotechnol 2016;14:13-8. [CrossRef]
33. Cui YW, Gong XY, Shi YP, (Drew) Wang Z. Salinity effect on production of PHA and EPS by Haloferax mediterranei. RSC Adv 2017;7:53587-95. [CrossRef]
34. Sutherland IW. Structure-function relationships in microbial exopolysaccharides. Biotechnol Adv 1994;12:393-448. [CrossRef]
35. Cao W, Li XQ, Liu L, Yang T-H, Li C, Fan HT, et al. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr Polym 2006;66:149-59. [CrossRef]
36. Liu C, Lin Q, Gao Y, Ye L, Xing Y, Xi T. Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr Polym 2007;67:313-8. [CrossRef]
37. Paulo EM, Boffo EF, Branco A, Valente AM, Melo IS, Ferreira AG, et al. Production, extraction and characterization of exopolysaccharides produced by the native Leuconostoc pseudomesenteroides R2 strain. An Acad Bras Cienc 2012;84:495-508. [CrossRef]
38. Botelho PS, Maciel MI, Bueno LA, Marques Mde F, Marques DN, Sarmento Silva TM. Characterisation of a new exopolysaccharide obtained from of fermented kefir grains in soymilk. Carbohydr Polym 2014;107:1-6. [CrossRef]
39. Van Dyk JS, Low Ah Kee N, Frost CL, Pletschke BI. Extracellular polysaccharide production in Bacillus licheniformis Svd1 and its immunomodulatory effect. BioResources 2012;7:4976-93. [CrossRef]
40. Bramhachari PV, Kishor PB, Ramadevi R, Kumar R, Rao BR, Dubey SK. Isolation and characterization of mucous exopolysaccharide (EPS) produced by Vibrio furnissii strain VB0S3. J Microbiol Biotechnol 2007;17:44-51.
41. Wang H, Gao XD, Zhou GC, Cai L, Yao WB. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem 2008;106:888-95. [CrossRef]
42. Prasanna PH, Bell A, Grandison AS, Charalampopoulos D. Emulsifying, rheological and physicochemical properties of exopolysaccharide produced by Bifidobacterium longum subsp. Infantis CCUG 52486 and bifidobacterium infantis NCIMB 702205. Carbohydr Polym 2012;90:533-40. [CrossRef]
43. Nanakoudis A. EDX Analysis with SEM:How Does it Work?Adv Mater;2019. Available from:https://www.thermofisher.com/blog/materials/edx-analysis-with-sem-how-does-it-work/. [Last accessed on 2023 Feb 23].
44. Abdel-Wahab BA, Abd El-Kareem HF, Alzamami A, Fahmy CA, Elesawy BH, Mostafa Mahmoud M, et al. Novel exopolysaccharide from marine Bacillus subtilis with broad potential biological activities:insights into antioxidant, anti-inflammatory, cytotoxicity, and anti-alzheimer activity. Metabolites 2022;12:715. [CrossRef]
45. Kanamarlapudi SL, Muddada S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed Res Int 2017;2017:4201809. [CrossRef]
46. Dorobantu LS, Gray MR. Application of atomic force microscopy in bacterial research. Scanning 2010;32:74-96. [CrossRef]
47. Svetli?i?V, Zuti?V, Pletikapi?G, Radi?TM. Marine polysaccharide networks and diatoms at the nanometric scale. Int J Mol Sci 2013;14:20064-78. [CrossRef]
48. Hu D, Su F, Yang G, Wang J, Zhang Y. Purification, structural characterization, and anti-inflammatory effects of a novel polysaccharide isolated from Orostachys fimbriata. Molecules 2021;26:7116. [CrossRef]
49. Wang J, Zhao X, Tian Z, Yang Y, Yang Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from tibet kefir. Carbohydr Polym 2015;125:16-25. [CrossRef]
50. Chowdhury SR, Manna S, Saha P, Basak RK, Sen R, Roy D, et al. Composition analysis and material characterization of an emulsifying extracellular polysaccharide (EPS) produced by Bacillus megaterium RB-05:A hydrodynamic sediment-attached isolate of freshwater origin. J Appl Microbiol 2011;111:1381-93. [CrossRef]
51. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power“:The FRAP assay. Anal Biochem 1996;239:70-6. [CrossRef]
52. Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum:An update. BMC Vet Res 2016;12:166. [CrossRef]
53. Merola ET, Catherman AD, Yehl JB, Strein TG. Determination of total antioxidant capacity of commercial beverage samples by capillary electrophoresis via inline reaction with 2,6-dichlorophenolindophenol. J Agric Food Chem 2009;57:6518-23. [CrossRef]
54. Gutnick DL, Bach H. Engineering bacterial biopolymers for the biosorption of heavy metals;new products and novel formulations. Appl Microbiol Biotechnol 2000;54:451-60. [CrossRef]
55. Busnelli MP, Lazzarini Behrmann IC, Ferreira ML, Candal RJ, Ramirez SA, Vullo DL. Metal-Pseudomonas veronii 2E interactions as strategies for innovative process developments in environmental biotechnology. Front Microbiol 2021;12:622600. [CrossRef]
56. Ajao V, Nam K, Chatzopoulos P, Spruijt E, Bruning H, Rijnaarts H, et al. Regeneration and reuse of microbial extracellular polymers immobilised on a bed column for heavy metal recovery. Water Res 2020;171:115472. [CrossRef]
57. Zhou W, Shen B, Meng F, Liu S, Zhang Y. Coagulation enhancement of exopolysaccharide secreted by an Antarctic sea-ice bacterium on dye wastewater. Sep Purif Technol 2010;76:215-21. [CrossRef]
58. BalíkováK, VojtkováH, DuborskáE, Kim H, MatúšP, Urík M. Role of exopolysaccharides of Pseudomonas in heavy metal removal and other remediation strategies. Polymers (Basel) 2022;14:4253. [CrossRef]