1. INTRODUCTION
The microflora of the earth is significantly influenced by microbes. They coexist in their biological environment in symbiotic relationships with other creatures [1]. These microbial interactions with other organisms take place at the molecular level through the release of a variety of molecules known as bioactive substances [2]. These bioactive molecules play a vital role due to their different chemical features, leading researchers to investigate their full potential [3]. The distinct properties of these bioactive chemicals, as well as their impact and effects in biotechnology domains like the food industry and medicine discovery and development, have been studied.
Over the last few years, researchers and scientists have investigated these bioactive metabolites for potential applications in the pharmaceutical and therapeutics industry. These bioactive compounds have tremendous potential and are thought to be more reliable than existing chemical substitutes in drugs [4]. Numerous isolated metabolites from fungi and bacteria have also demonstrated tremendous potential in treating lethal diseases such as diabetes, cancer, and Crohn’s disease [5-7]. Even though bioactive metabolites are widely recognized for their therapeutic properties, it is crucial to establish efficient technologies that will allow bioactive molecules to achieve the designated target.
The advancement of knowledge in this field is increasing day by day, and a significant number of microbial-derived drugs are being discovered daily. It is worth noting that this emerging field has evolved into a fruitful scientific endeavor in the investigation of bioactive compounds from microbial communities. This review covers the summary of taxonomic diversity and various technologies for the extraction of bioactive compounds producing microbes. Significant advances in genomics and metagenomics related to novel bioactive compounds from microbes, the clinical status of these compounds, and then, the biotechnological applications of various bioactive compounds in human health.
2. BIOACTIVE COMPOUNDS PRODUCING MICROBES
2.1. Fungi
Fungi are ubiquitous, occurring eukaryotic, heterotrophic organisms found worldwide in diverse habitats. Fungi have been used as remedies and in everyday life for thousands of years. Nearly 3000 years ago, fungi were used to treat intestinal ailments [8]. Fungi, especially endophytic fungi, are among the novel bioresources of natural bioactive compounds with their major biotechnological potential in the food industry, medicine, and agriculture. Numerous valuable bioactive compounds with a range of the bioactivities, such as antimicrobial, cytotoxic, and anticancer, have been successfully discovered from endophytic fungi [9]. Existing drugs of fungal origin include β-lactam antibiotics, cyclosporine A, ergot alkaloids, griseofulvin, lovastatin, and taxol; however, increasingly more novel natural products of the varied chemical structure be produced by fungi [10,11].
2.1.1. Macrofungi
Macrofungi, including mushrooms are an emerging bioresource of bioactive compounds. Many bioactive compounds have been reported from mushrooms. Ruksiriwanich et al. [12] reported allantoin, alkaloid, monosaccharide, mucopolysaccharide, and polyphenolic from Dictyophora indusiata. Zaki et al. [13] reviewed bioactive compounds from Leucocalocybe mongolica. About 100 chemical components have been isolated from L. mongolica. This mushroom is known to be a rich source of amino acids, lectins, laccase, polysaccharides, sterols, and volatile compounds. Further, the bioactive compounds from L. mongolica possess antitumor, antiproliferative, antidiabetic, and hypotensive activities. Krümmel et al. [14] reported linoleic, chlorogenic and vanillic acids from Pleurotus sajor-caju. WA, Daba [15] reviewed the bioactive potential of some edible mushrooms, including the species of Amanita, Grifola macrolepiota, Russula, and Vovariella.
2.1.2. Microfungi
Microfungi are considered to be a treasure trove of bioactive compounds. Wang and Xu [16] reviewed beauvericin, which is a cyclic hexadepsipeptide mycotoxin. It is known to possess cytotoxic, antiviral, insecticidal, and antimicrobial activities. It is also known to be a potential agent for pesticides and medicines. Its production has also been known in Beaveria bassiana and Fusarium spp. Kumar et al. [17] reviewed endophytic fungi as a source of bioactive compounds with antimicrobial activities. Manganyi et al. [18] screened bioactive compounds from endophytic fungi of Pelargonium sidoides. The chemical analysis of the extract from Alternaria spp. indicated that linoleic acid (9,12-octadecadienoic acid (Z,Z) and cyclodecasiloxane and concluded that both the compounds could be responsible for the antibacterial activity. Nuraini et al. [19] reported dihydropyran and 4H-Pyran4-one, 5-hydroxy-2-(hydroxymethyl-(CAS) Kojic acid from Aspergillus minisclerotigens and Aspergillus oryzae respectively. Jamal et al. [20] reported bioactive metabolites of isoelemicin, terpinen-4-ol, eucalyptol, oleic acid, and β-pinene from endophytic fungi isolated from Gynura procumbens. Kumar and Prasher [21] reported dodecane, ethyl 2-thiopheneacetate, tetradecane, hexadecane, octadecane, benzaldehyde, 4-(1-methylethyl)-, and griseofulvin from Fomitopsis meliae.
2.2. Bacteria
Bacteria are another important bioresources for the isolation and screening of bioactive compounds. Bioactive compounds such as alkaloids, flavonoids, peptides, polyketones, quinols, steroids, terpenoids, and phenols have been known to be produced by bacteria, especially endophytes [22-24]. These compounds have agricultural, industrial, and medical applications [25]. Christina et al. [26] reviewed a diverse range of bioactive compounds, especially from different endophytic bacteria. Pseudomonas viridiflava is known to produce ecomycins. Ecomycins possess bioactivities against various human and plant pathogenic fungi [27]. Pseudomycins are peptide antifungal compounds reported from Pseudomonas syringae [28]. Ghiasvand et al. [29] reported harmine, myricetin, and achillin, from Paenibacillus polymyxa.
2.3. Actinomycetes
Actinomycetes are an untapped source of potential bioactive compounds. The diverse range of actinomycetes have been isolated and used for the production of key drugs and biomedical agents [30]. Penicillin, cephalosporins, carbapenems, thienamycin, cephamycin, and nocardicin are some of the important β-lactam antibiotics reported from actinomycetes [31]. Balachandar et al. [32] reported the presence of 3, octadecene (E), behnic alcohol phenol, 2,4-bis(1,1-dimethyl ethyl) 1-nonadecene, 1-heneicosanol, milbemycin 3-eicosene (E), and 1-docosanol from vermicast isolated actinomycetes. Janardhan et al. [33] reported (Z)-1-([1-hydroxypenta-2,4-dien1-yl] oxy) anthracene-9,10-dione from Nocardiopsis alba.
2.4. Microalgae
Microalgae have been explored for their ability to produce bioactive compounds with promising applications as antibacterial, antiviral, antifungal, and antialgal agents [34]. Numerous secondary metabolites with antioxidant, antitumor, anticancer, and anti-inflammatory activities, including β-carotene, astaxanthin, lutein, zeaxanthin, violaxanthin, and fucoxanthin have been reported from microalgae [35]. Diatoms are rich sources of fucoxanthin [36]. Fucoxanthin has inhibitory effects on cancer cells by having proapoptotic activities [37,38]. The major algal species used to produce astaxanthin belongs to the genus Haematococcus. Some of the species of Chlorella, such as Chlorella zofingiensis, is also known producer of astaxanthin [39] [Tables 1 and 2].
Table 1: Diversity of bioactive producing microbes and their activities.
| Microbes | Activity | Activity against | References |
|---|---|---|---|
| Microfungi | |||
| Alternaria alternata | Bactericidal | Gram positive and Gram negative bacteria | Chatterjee et al. [222] |
| Alternaria alternata | Antioxidant | - | Chatterjee et al. [222] |
| Alternaria brassicae | Antimicrobial | Escherichia coli | Gauchan et al. [223] |
| Alternaria brassicae | Antimicrobial | Staphylococcus aureus | Gauchan et al. [223] |
| Alternaria brassicae | Antimicrobial | Bacillus subtilis | Gauchan et al. [223] |
| Alternaria brassicae | Cytotoxicity | Shrimp nauplii | Gauchan et al. [223] |
| Alternaria spp. | Antibacterial | Enterococcus gallinarum | Manganyi et al. [18] |
| Alternaria spp. | Antibacterial | Enterococcus faecium | Manganyi et al. [18] |
| Alternaria spp. | Antibacterial | Enterococcus gallinarum | Manganyi et al. [18] |
| Aspergillus minisclerotigens | Antioxidant | - | Nuraini et al. [19] |
| Aspergillus oryzae | Antioxidant | - | Nuraini et al. [19] |
| Cladosporium cladosporioides | Antimicrobial | Escherichia coli | Gauchan et al. [223] |
| Cladosporium cladosporioides | Antimicrobial | Staphylococcus aureus | Gauchan et al. [223] |
| Cladosporium cladosporioides | Antimicrobial | Bacillus subtilis | Gauchan et al. [223] |
| Cladosporium cladosporioides | Cytotoxicity | Shrimp nauplii | Gauchan et al. [223] |
| Cochliobolus sativus | Antileishmanial | - | Do Nascimento et al. [224] |
| Fomitopsis meliae | Antibacterial | Bacillus subtilis | Kumar and Prasher [21] |
| Fomitopsis meliae | Antibacterial | Pseudomonas aeruginosa | Kumar and Prasher [21] |
| Fomitopsis meliae | Antibacterial | Staphylococcus aureus | Kumar and Prasher [21] |
| Fomitopsis meliae | Antibacterial | Escherichia coli | Kumar and Prasher [21] |
| Penicillium oxalicum | Antioxidant | DPPH, nitric oxide, superoxide anion and hydroxyl free radicals | Verma et al. [225] |
| Penicillium oxalicum | Anti-proliferative | HuT-78 | Verma et al. [225] |
| Penicillium oxalicum | Anti-proliferative | MDA-MB-231 | Verma et al. [225] |
| Penicillium oxalicum | Anti-proliferative | MCF-7 | Verma et al. [225] |
| Macrofungi | |||
| Clavaria vermiculris | Antimicrobial | - | Ramesh and Pattar [226] |
| Clavaria vermiculris | Antioxidant | - | Ramesh and Pattar [226] |
| Dictyophora indusiata | MMP-2 inhibition | ||
| Dictyophora indusiata | Anti-inflammatory | Nitric oxide (NO), interleukin (IL)-β, IL-6, and tumour necrosis factor (TNF)-α secretion | Ruksiriwanich et al. [12] |
| Hypsizigus tessulatus | Antimicrobial | - | Chowdhury et al. [227] |
| Hypsizigus tessulatus | Antioxidant | - | Chowdhury et al. [227] |
| Lactarius deliciosus | Antibacterial | Bacillus cereus | Barros et al. [228] |
| Lactarius deliciosus | Antibacterial | Bacillus subtilis | Barros et al. [228] |
| Lactarius deliciosus | Antifungal | Candida albicans | Barros et al. [228] |
| Lactarius deliciosus | Antifungal | Cryptococcus neoformans | Barros et al. [228] |
| Lentinula edodes | Antimicrobial | - | Chowdhury et al. [227] |
| Lentinula edodes | Antioxidant | - | Chowdhury et al. [227] |
| Lycoperdon perlatum | Antimicrobial | - | Ramesh and Pattar [226] |
| Lycoperdon perlatum | Antioxidant | - | Ramesh and Pattar [226] |
| Macrolepiota procera | Antioxidant | - | Erbiai et al. [229] |
| Marasmius oreades | Antimicrobial | - | Ramesh and Pattar [226] |
| Marasmius oreades | Antioxidant | - | Ramesh and Pattar [226] |
| Pleurotus eryngii | Antioxidant | - | Koutrotsios et al. [230] |
| Pleurotus ostreatus | Antioxidant | - | Zawadzka et al. [231] |
| Pleurotus ostreatus | Antimicrobial | - | Chowdhury et al. [227] |
| Pleurotus ostreatus | Antioxidant | - | Chowdhury et al. [227] |
| Pleurotus pulmonarius | Antimicrobial | - | Ramesh and Pattar [226] |
| Pleurotus pulmonarius | Antioxidant | - | Ramesh and Pattar [226] |
| Sarcodon imbricatus | Antibacterial | Bacillus cereus | Barros et al. [228] |
| Sarcodon imbricatus | Antibacterial | Bacillus subtilis | Barros et al. [228] |
| Sarcodon imbricatus | Antifungal | Candida albicans | Barros et al. [228] |
| Sarcodon imbricatus | Antifungal | Cryptococcus neoformans | Barros et al. [228] |
| Tricholoma portentosum | Antibacterial | Bacillus cereus | Barros et al. [228] |
| Tricholoma portentosum | Antibacterial | Bacillus subtilis | Barros et al. [228] |
| Tricholoma portentosum | Antifungal | Candida albicans | Barros et al. [228] |
| Tricholoma portentosum | Antifungal | Cryptococcus neoformans | Barros et al. [228] |
| Bacteria | |||
| Bacillus amyloliquefaciens | Antibacterial | Bacillus cereus | Bhoonobtong et al. [232] |
| Bacillus amyloliquefaciens | Antibacterial | Escherichia coli | Bhoonobtong et al. [232] |
| Bacillus aryabhattai | Antibacterial | Staphylococcus aureus | Beiranvand et al. [233] |
| Bacillus australimaris | Antifungal | Candida albicans | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Antibacterial | Bacillus subtilis | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Antibacterial | Staphylococcus aureus | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Antibacterial | Escherichia coli | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Antibacterial | Pseudomonas aeruginosa | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Anticancer | - | Ghiasvand et al. [29] |
| Planomicrobium spp. | Antibacterial | Bacillus cereus | Beiranvand et al. [233] |
| Actinobacteria | |||
| Kytococcus schroeteri | Anticancer | - | Ghiasvand et al. [29] |
| Microbacterium maritypicum | Anticancer | - | Ghiasvand et al. [29] |
| Microbacterium maritypicum | Antioxidant | - | Ghiasvand et al. [29] |
| Nocardiopsis alba | Antioxidant | - | Janardhan et al. [33] |
| Streptomyces spp. | Antioxidant | - | Naine et al. [234] |
| Amycolatopsis tolypomycina | Antibacterial | Bacillus cereus | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Bacillus subtilis | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Citrobacter freundii | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Escherichia coli | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Klebsiella pneumoniae | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Proteus mirabilis | Beiranvand et al. [233] |
| Streptomyces spp. | Antibacterial | Pseudomonas aeruginosa | Naine et al. [234] |
| Amycolatopsis tolypomycina | Antibacterial | Shigella flexneri | Beiranvand et al. [233] |
| Amycolatopsis tolypomycina | Antibacterial | Staphylococcus aureus | Beiranvand et al. [233] |
| Microalgae | |||
| Chlorococcum minutum | Antimicrobial | Bacillus subtilis | Elshobary et al. [235] |
| Chlorococcum minutum | Antimicrobial | Klebsiella pnemoniae | Elshobary et al. [235] |
| Chlorococcum minutum | Antimicrobial | Proteus vulgaris | Elshobary et al. [235] |
| Oscillatoria spp. | Antibacterial | Pseudomonas aeruginosa | Bhuyar et al. [236] |
| Chlorococcum minutum | Antimicrobial | Salmonella typhi | Elshobary et al. [235] |
| Chlorococcum minutum | Antimicrobial | Staphylococcus aureus | Elshobary et al. [235] |
| Oscillatoria spp. | Antibacterial | Staphylococcus aureus | Bhuyar et al. [236] |
Table 2: Structures of bioactive compounds from microbes.
| Bioactive compound producing microbes | Bioactive compound | Structure | References |
|---|---|---|---|
| Alternaria spp. | Linoleic acid (9,12-octadecadienoic acid (Z,Z) |  | Manganyi et al. [18] |
| Alternaria spp. | Cyclodecasiloxane | 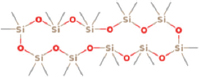 | Manganyi et al. [18] |
| Armillaria mellea | Vanillic acid |  | Erbiai et al. [229] |
| Armillaria mellea | Cinnamic acid |  | Erbiai et al. [229] |
| Aspergillus minisclerotigens | Dihydropyran | 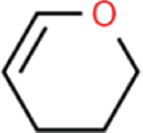 | Nuraini et al. [19] |
| Aspergillus oryzae | 4H-Pyran4-one,5-hydroxy-2-(hydroxymethyl-(CAS) Kojic acid | 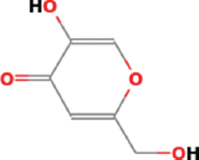 | Nuraini et al. [19] |
| Cochliobolus sativus | Cochlioquinone A | 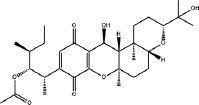 | Do Nascimento et al. [224] |
| Cochliobolus sativus | Isocochlioquinone A | - | Do Nascimento et al. [224] |
| Cochliobolus sativus | Anhydrocochlioquinone A | - | Do Nascimento et al. [224] |
| Fomitopsis meliae | Dodecane | 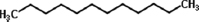 | Kumar and Prasher [21] |
| Fomitopsis meliae | Ethyl 2-thiopheneacetate |  | Kumar and Prasher [21] |
| Fomitopsis meliae | Tetradecane |  | Kumar and Prasher [21] |
| Fomitopsis meliae | Hexadecane | 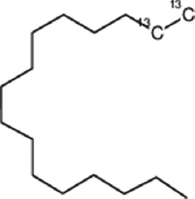 | Kumar and Prasher [21] |
| Fomitopsis meliae | Octadecane | 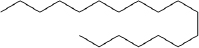 | Kumar and Prasher [21] |
| Fomitopsis meliae | Benzaldehyde | 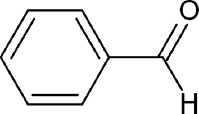 | Kumar and Prasher [21] |
| Fomitopsis meliae | 4-(1-methylethyl)- | 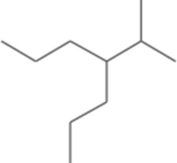 | Kumar and Prasher [21] |
| Fomitopsis meliae | Griseofulvin | 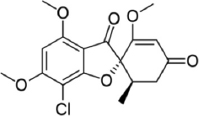 | Kumar and Prasher [21] |
| Kytococcus schroeteri | Berberine | 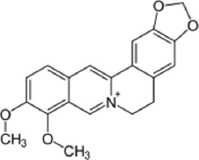 | Ghiasvand et al. [29] |
| Kytococcus schroeteri | Camptothecin | 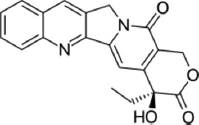 | Ghiasvand et al. [29] |
| Macrolepiota procera | Protocatechuic acid |  | Erbiai et al. [229] |
| Microbacterium maritypicum | Harmine |  | Ghiasvand et al. [29] |
| Microbacterium maritypicum | Myricetin | 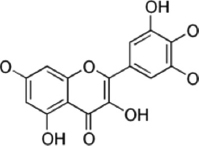 | Ghiasvand et al. [29] |
| Microbacterium maritypicum | Achillin |  | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Sanguinarine |  | Ghiasvand et al. [29] |
| Paenibacillus polymyxa | Daunorubicin | 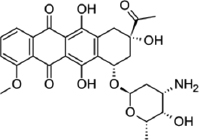 | Ghiasvand et al. [29] |
| Pleurotus sajor-caju | Linoleic acid |  | Krümmel et al. [14] |
| Pleurotus sajor-caju | Chlorogenic acid | 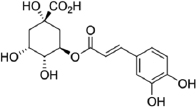 | Krümmel et al. [14] |
| Pleurotus sajor-caju | Vanillic acid |  | Krümmel et al. [14] |
3. TAXONOMIC DIVERSITY OF BIOACTIVE COMPOUNDS PRODUCING MICROBES
Microbes are amazing bioresources for the production of bioactive compounds. Bioactive compounds are greatly used as antibiotics. These compounds may be effective against many HIV-1, conditions of multiple bacterial infections or neural tube defects [40-42]. Some among them have also been found to show activity against carcinomas [43]. Exploiting the diversity of microbial communities and their huge potential in finding new bioactive compounds is of immense importance. Ouchari et al. [44] investigated the antimicrobial potential of actinomycetes and their taxonomic diversity. In the study, the rep-PCR revealed a high taxonomic diversity of isolates. Dendro grams from the BOXA1R-PCR fingerprints showed a grouping of 36 isolates in 16 clusters, containing from two to four isolates while some of them could not be grouped. The study of Liao et al. [45] explored the microbial taxonomy and functionality of two diverse mangrove ecosystems and their potential abilities to produce bioactive compounds. The study observed 83 bacterial phyla, 11 archaeal phyla, and 9 fungal phyla, in all the rhizospheric samples. Further, 675, 656, 452, 379, 267, 205, 132, 90, and 766 biosynthesis gene clusters were inferred to synthesize terpene, non-ribosomal peptide synthetase, bacteriocins, NRPS-like, type I polyketide synthases, aryl polyene, type III polyketide synthases, beta lactone, and other products, respectively. In another study by Teimoori-Boghsani et al. [46], taxonomic diversity and unique profiles of secondary metabolites from endophytic fungi of native Salvia abrotanoides plants have been reported. Molecular approaches classified endophytic fungi into 15 genera. Coniolariella hispanica, Paraphoma radicina, Penicillium canescens, and Penicillium murcianum have been reported to be the major producers of cryptotanshinone, the major bioactive compound of S. abrotanoides. Niego et al. [47] reviewed the taxonomy, and diversity Oudemansielloid/Xeruloid taxa Hymenopellis, Mucidula, Oudemansiella, and Xerula, which constitute an important bioresource of bioactive compounds. Several studies have shown antimicrobial, anti-oxidative, anti-cancer, anti-inflammatory, and other bioactivities of their extracts.
4. TECHNOLOGIES FOR EXTRACTION AND PRODUCTION OF BIOACTIVE COMPOUNDS
In modest amounts, natural compounds are usually synthesized in conjunction with or in conjugation with some other type [48]. As a result, the recurrent separation of extremely complex extracts into individual bioactive compounds is required for the purification and concentration of these conjugated products, which is a labor-intensive process that is unaffordable for industries. Many cutting-edge techniques for extracting and producing bioactive compounds from natural sources have been developed [49].
There are several steps that are involved in the extraction and production of natural compounds. The first step is to choose raw materials based on their nutritional or medicinal properties. Standard protocols are used to assess the toxicity of the materials chosen. The materials’ chemical composition and potential bioactivities are then determined using elemental analysis. The bioactive compounds are isolated from the crude extracts and are further evaluated under in vivo and in vitro conditions for their potential activities. In the last stage, the bioactive compounds are commercialized as medicinal products and found to be effective in the treatment of diseases [50]. Conventional and non-conventional methods are used for the extraction of bioactive compounds.
4.1. Solvent Extraction
Solvent extraction is one of the most popular and traditional techniques for extracting metabolites from microorganisms. To improve extraction efficiency, raw materials are frequently blended into powder form. For the extraction, non-polar and polar solvents such as ether, ethanol, chloroform, benzene, water, ethyl acetate, and hexane, also their mixtures in various ratios, were used [51]. Because of its ease of use and low price, this technique has been widely adopted. Nevertheless, certain organic solvents, which are commonly employed in enormous quantities during processing and extraction, are highly hazardous and/or combustible. As a result, users must adhere to proper handling rules to ensure environmental compliance and users’ safety.
It is worth noting that organic solvents can cause bioactive compounds to degrade thermally [52]. Furthermore, the extraction procedure takes a lot of time and requires a lot of effort. Other advanced methods, such as soxhlet, ultrasound, and microwave extraction, have been developed to address these issues. Researchers have developed more advanced techniques like microwave-assisted extraction (MAE), pulsed-electric field extraction (PEF), pressurized liquid extraction (PLE), ultrasound-assisted extraction (UAE), and supercritical fluid extraction to overcome the shortcomings of conventional extraction methods [53]. It has been reveled that when, the extraction time was halved, and the thermal decomposition of compounds was prevented.
4.2. Soxhlet Extraction
It is a standard extraction method used to compare the results of other liquid-solid extraction methods [54]. The Soxhlet apparatus is a type of condenser used in this technique, which was developed in 1879 [55]. The traditional Soxhlet extractor is made up of a thimble-holder and a distillation flask. The solvent vaporizes and reaches the matrix when it attains boiling point, solubilizing suitable compounds. The solvent then strikes the condenser’s cooling pipes and condenses back into the original flask with the extracted compounds. This procedure is repeated until the entire extraction has been completed [55,56].
This method of extraction has a number of advantages. To begin with, the continuous renewal of the solvent in touch with the matrix creates an imbalance between the compounds in the test and the absence of them in the solvent, endorsing compound extraction. Second, the system’s temperature is maintained all throughout the procedure. The final crude extract from Soxhlet extraction need not necessitate filtration or centrifugation because they are nicely isolated from the initial biomass. Finally, because the basic equipment is relatively inexpensive and simple, it enables the treatment of numerous samples in parallel at a minimal price and with simple operational processes [57].
Nevertheless, there are some drawbacks to Soxhlet extraction, such as the vast concentrations of organic solvents needed, the long time it takes to finish the last cycles [57], the high temperatures used to heat up the solvents, which can deteriorate the compounds [55], and the fact that this process cannot be speeded up by introducing agitation [58].
Nonetheless, Soxhlet extraction has evolved over time to make up for some of these drawbacks, such as automating the process and trying to minimize extraction times. More recently, Soxhlet extraction has been paired with innovative technologies such as supercritical fluid-Soxhlet extraction, automated Soxhlet extraction, and high-pressure Soxhlet extraction, or by using auxiliary energies such as microwaves or ultrasounds, which results in higher efficiency [57,59].
4.3. Distillation
Distillation is among the oldest extraction methods still being used today. Its primary use is to separate liquid mixtures by using the boiling points of every component in the sample, followed by condensation steps [60]. Although distillation methods are still widely used, they have a number of disadvantages. The requirement for large amounts of energy to be consumed over long durations, including the use of high temperatures can deteriorate the ingredients of concern. Furthermore, the huge quantities of solvent are requisite, as well as the lengthy extraction times [57,61].
4.4. Infusions
Infusions are very brief macerations in which the plant is immersed in boiling or cold water for a short period [58]. Maceration entails breaking down the sample into smaller pieces to enhance the surface area available for mixing with the solvents. Both diffusion and the removal of the concentrated solution from the sample’s surface are made easier by the agitation involved in the maceration process. This method has been used to obtain bioactive compounds and essential oils for a long time [62]. Since infusions are very susceptible to fungus and bacterial growth due to the vast volume of water they consist of, they have a really limited lifespan and must be used right away. As a result, infusions are very seldom used in the industrial sector [63].
4.5. Green Extraction Techniques
Large volumes of organic solvents are often used in conventional extraction techniques, endangering the environment from chemical exposure. The idea of “green chemistry” was created to lessen the risks associated with chemicals, limit their use, and limit environmental exposure. By enabling to use nature without harming it, green chemistry also supports environmental sustainability. This idea has been used in synthesis, catalysis, separation, and monitoring, among other chemical processes. Waste, energy, and hazard are the three most important aspects of green chemistry, according to Anastas and Warner’s twelve principles [64]. The goal of these green extraction processes, according to Jacotet-Navarro et al. [65], is to obtain a quicker extraction rate, more efficient energy use, enhanced heat and mass transfer, smaller equipment, and fewer processing steps [65].
4.6. Supercritical Fluid Extraction
Supercritical extraction is characterized by pressure and temperature changes that transform the gas into a supercritical fluid with indistinguishable gas and liquid phases [66]. The extraction procedure is divided into several stages. Initially, the plant matrix absorbs the supercritical solvent, bulging the cellular structure and dilation the inter-cellular channels. As a result, the resistance to mass transfer decreases. Furthermore, the matter is being transferred from the internal matrix to the surface at the same time. Following that, these molecules are transported from the surface to the supercritical solvent before being excluded from the solvent [67].
The exclusion of harmful residues in the final product, high selectivity, short durations, high stability of the product acquired, low solvent consumption, and the fact that the residual biomass can be treated with other strategies to proceed with the extraction are all advantages of this method. This method can also be used to eliminate unwanted compounds such as pesticides, pollutants, and toxins [68]. Supercritical fluid extraction is an efficient and environmentally beneficial process. The most used solvent for supercritical fluid extraction is carbon dioxide (CO2); however, methane, ethylene, fluorocarbons, nitrogen, and xenon are also utilized [69]. This technique has been used to collect biologically active substances from marine invertebrates and microalgae (such as crawfish, starfish, crustaceans, shrimp, crab, urchin, squid), macroalgae and cyanobacteria [70]. Dunaliella salina a microalga belonging to the class Chlorophyta contains fatty acids and β-carotene. In order to extract these molecules, supercritical CO2 (SC-CO2) was used at different operative conditions [71].
4.7. MAE
In 1986, Ganzler et al. published the first description of MAE [72]. Microwaves are electromagnetic fields that oscillate perpendicularly between 300 MHz and 300 GHz. The solute is dissolved by the solvent as it diffuses into the solid matrix, but the concentration is constrained by the solid’s physical characteristics [73].
This technique has several advantages, including rapid temperature rise, high efficiency, improved process monitoring, short extraction time, and low energy consumption and cost [74]. The breakdown of some compounds as a result of the heat produced by irradiation is one drawback of MAE. The MAE’s efficiency is determined by factors such as the power of microwave irradiation, the nature of the extractant, the temperature, and the extraction time, as well as the matrices’ characteristics and the solvent-food relationship. Due to the local heating that contributes to matrix rupture, the extraction efficiency is usually directly proportional to the microwave power. However, microwave power has a limit, which can result in a decrease in extraction efficiency [56]. Compound extraction, on the other side, is influenced by the solvent used. A combination of organic solvents and water has been found to increase extraction effectiveness. Contrarily, compared to MAE which exclusively employs organic solvents, the inclusion of water in organic solvents causes the extractant to penetrate the matrix molecules more deeply, enhancing microwave heating and improving overall efficiency and extraction time [75].
The toxicity of the solvent is also an essential aspect to consider when selecting an appropriate extractant for MAE [76]. According to some theories, the selectivity and efficiency of MAE are affected by the dielectric constant of the solvent mixture [77]. The agitator effect influences the extraction process, which mitigates the adverse effects of the S/F ratio on extraction recovery [78].
4.8. UAE
UAE involves the mechanism of diffusion across cells and cell breakage caused by mass transfer. To extract the chemical components, UAE uses a sound wave at 20 kHz-100 MHz to compress and expand the cells [79]. By rupturing plant cell walls, ultrasounds can expedite mass and heat transfer and improve the release of the target substances from a range of natural sources [80].
Compared to other extraction techniques, ultrasound is relatively simple to use; it is flexible, versatile, and requires a low investment. Polysaccharides, peptides, essential oils, dyes, proteins, pigments, and bioactive compounds have all been extracted using ultrasound [81]. This phenomenon can occur in either an indirect or direct manner [82].
Among the benefits of this technology are reduced solvent consumption, time and temperature, low investment for equipment, and ease of implementation, allowing it to be used industrially in local companies [83]. Heating can degrade the additives present in the sample, which is one of the main disadvantages of UAE [84]. Compared to traditional methods, UAE uses less solvent and is more efficient and cost-effective at extracting polyphenols and other compounds.
4.9. PLE
PLE, commonly called pressurized fluid extraction, and accelerated solvent extraction, was first introduced by Ganzler et al. [71], enhanced solvent extraction or Pressurized Hot Water Extraction [85]. PLE operates at high temperatures (50–200°C) and high pressures (1450–2175 psi) to maintain the solvent liquid above the typical boiling point [86]. The polarity of the solvent is reduced at high temperatures, but the solubility and mass transfer rate are increased due to an increase in the dielectric constant. A very little solvent is needed because the liquid is pushed into the extracting cells under high pressure, and the extraction yield is higher as a result. Additionally, automated methods shorten extraction times and do not need solvents [87]. Using pressurized liquid extraction, pheophytins, and the carotenoid diatoxanthin were removed from Euglena cantabrica [88].
4.10. PEF
PEF is a non-thermal technique that extracts bioactive compounds using a short electric field pulse. During PEF, the electric field distorts or destroys the cell membrane, transferring electric potential to the cells [89]. Its mode of action is based on causing cell membrane permeability in a short period and with minimal energy consumption. This is performed by using well-known techniques for preservation, enzyme, and microbial inactivation, which involve administering brief pulses (μs to ms) of moderate electric voltage (usually 0.5–20 kV/cm) to a substrate of interest positioned between two electrodes [90]. Because of these properties, various studies have been conducted to improve the extraction performance of bioactive compounds such as anthocyanins, polyphenols, and vegetable oil from plant tissues and byproducts, as well as the soluble intracellular matter of microorganisms [91]. On the other hand, in plant systems and cell cultures, low to light PEF treatment intensities are frequently regarded as an efficient pretreatment technique for raising secondary metabolites extraction efficiency [92]. Several procedures, such as pressing, extraction, drying, and diffusion, have used PEF. This method accelerates mass transfer while speeding up extraction by disrupting the membrane of the raw materials. The distortion or damage of the cell membrane is important for increasing permeability and proving to be beneficial over traditional extraction methods [50].
5. CLINICAL STATUS OF BIOACTIVE COMPOUNDS OF MICROBIAL ORIGIN
A bioactive compound tetrodotoxin isolated from microalgae Alexandrium tamarense had been undergoing phase III clinical trials. This compound has been developed as Halneuron® for the treatment of chemotherapy-induced neuropathic pain on cancer patients. Utilizing the satisfactory portions of tetrodotoxin has been displayed to have gainful impacts against intense, provocative, and neuropathic pain in animal models. The clinical trial has shown the benefits of tetrodotoxin on patients older than 18 years with severe cancer-related pain. Wex Pharmaceuticals Inc. has tested Halneuron® on more than 500 patients and reported that this medication is able to give pain relief for a longer duration with low side effects [93]. Kumamoto University had done a study on sacran a polysaccharide extracted from Aphanothece sacrum Cyanobacteria. The anti-inflammatory effect on 25 patients of atopic dermatitis was studied. The results of this study showed that all 25 patients treated with sacran had an improvement in the atopic dermatitis average symptoms. After 4 weeks of treatment, there was also significant improvement in the problem of sleep disorder and itching [94].
Plinabulin (NPI-2358), a compound derived from a marine fungus, is being tested in phase II clinical trials as a powerful and targeted vascular disruptor. This fungus compound was shown to have effectiveness over multi-drug resistant human tumor cell lines, according to preclinical investigations. This compound also has shown enhanced efficiency of current chemotherapy and radiotherapy in animal models [95]. Another promising anticancer drug is salinosporamide A, which is undertaking phase I studies under the direction of Nereus Pharmaceuticals (San Diego, CA). It is a brand-new proteasome inhibitor that was discovered in the Salinispora tropica bacterium. Proteasome inhibition properties were effective at inhibiting a variety of solid tumor models and hematologic malignancies while causing minimal damage in normal cells [96]. A marine bacterium-derived drug called Tasidotin, Synthadotin (ILX-651) is also undergoing Phase II clinical trials with Genzyme Corporation (Cambridge, MA). Under the direction of Aska Pharmaceuticals, Soblidotin (TZT 1027), another bacterial bioactive substance, is undergoing Phase III clinical preliminary testing (Tokyo, Japan). These two substances are both potential cancer-fighting substances [97].
Recent reports have verified the synthesis of the well-known marine anticancer drug bryostatin 1 from Candidatus Endobugula sertula [98]. The National Cancer Institute (NIH, U.S.) is also conducting Phase I clinical trials with bryostatin 1 [99]. Sorbicillactone-A, another anti-leukemic drug made by Penicillium chrysogenum, has begun to be researched for human trials. This alkaloid substance was said to have antiviral and neuroprotective effects [100].
6. GENOMICS AND METAGENOMICS-BASED BIOPROSPECTING OF MICROBES FOR NOVEL BIOACTIVE COMPOUNDS
Microbes have long been considered a possible source of bioactive molecules that may be turned into medications to fight cancer and diseases. In the past, novel microbe-produced chemicals were found through traditional bioprospecting, which involved isolating putative producers and testing their extracts in various bioassays [101]. There are currently greater chances for the identification of novel bioactive chemicals due to recent developments in numerous methodologies, including metagenomics, genomics, combinatorial biosynthesis, screening techniques, expression systems, proteomics, and bioinformatics [102]. The integration of these modern techniques with classic techniques serves as an important tool in the identification of novel bioactive compounds [102].
Formerly, conventional microscopy was used to identify the microbes in a sample. However, it is now much simpler to identify and characterize the biodiversity and function within a microbial population due to improvements in DNA/RNA-based methodologies and sequence technology. Both partial community analysis methods and entire community analysis methods have been used to describe these methodologies [103]. PCR-based techniques and other ways are frequently used in partial community analysis. While focusing solely on one or a small number of genes, whole-community analysis methodologies provide a more comprehensive understanding of the genetic diversity inside a community [103]. These methods genuinely make an effort to examine every piece of genetic data included in the whole DNA that has been isolated from an ambient sample or a pure culture of bacteria. These methods include whole-microbial genome sequencing, metagenomics, DNA-DNA hybridization kinetics, G+C DNA content, and developing omics technologies.
A new method was created to cover the whole microbial variety from various biotopes in light of the lack of growing techniques for the majority of microorganisms. The foundation of this cultivation-independent strategy is the creation of elaborate libraries using ambient DNA. The metagenome, also referred to as the collective genome of all extant microorganisms, is made up of isolated microbial DNA from a certain habitat [104,105].
The term “metagenomics” refers to the genetic analysis of microbes using the direct extraction method and the cloning of DNA from a variety of different species from environmental samples [106]. Gene clusters, genes that code for enzymes, and the creation of bioactive molecules may be the focus of metagenomics [107]. Extreme environments, naturally or intentionally enriched environments for the target gene, and extremely diversified habitats are some of the key categories of environments taken into account when utilizing this strategy [108]. The vast amount of metagenomic data that the sequencing platforms have collected is then analysed, which calls for the use of the appropriate data-analysis tools. Tools for managing huge datasets have been created using bioinformatics software.
7. BIOTECHNOLOGICAL APPLICATIONS OF BIOACTIVE COMPOUNDS IN HUMAN HEALTH
7.1. Antiviral Activity
Microalgae are quite possibly the most encouraging hotspots for new useful food items, because of their capacity to incorporate polyunsaturated unsaturated lipids, colors, and regular cancer prevention agents [109]. In a study, four strains of microalgae prospected were refined, and their antiviral effect was assessed in vitro against MAYV. The cell reasonability tests were done on VERO cells (Verda Reno Cells) to evaluate the lethality of the concentrates by CC50 designation and the deactivation limit of the MAYV as assessed by TCID50. The outcomes demonstrated that all the microalgae strains introduced adversary of the Mayaro movement and the overall strength was greater than ribavirin, the current antiviral. In addition, the basic portrayal by TLC and NMR 1H inquiry demonstrated in the concentrated configuration of terpenoid atoms and the greater part occurrence of unsaturated aliphatic particles [110].
Another study analyzed the polyunsaturated fatty acids. Carotenoids and cancer prevention agent movement of Phaeodactylum tricornutum, Nannochloropsis oculata, and Porphyridium cruentum activity got from SC-CO2 and subcritical n-butane extraction techniques SC-CO2 strategy was particular in removing immersed unsaturated fats saturated fatty acids for all microalgae species contemplated [110,111]. To add up to carotenoids and cancer prevention agent action utilizing DPPH scavenging test, a critical connection coefficient (R2 = 0.80) was found among the concentrates autonomous of the extraction strategy tried. This shows that carotenoid mixtures may be a significant supporter of the cell reinforcement limits of these microalgae. Polysaccharide-rich fractions isolated from D. salina and Haematococcus pluvialis extracts exhibit higher antiviral activity against herpes simplex virus type 1 [112]. Oscillatoria agardhii, Nostoc ellipsosporum reported lectins (Agglutinin OAA, Cyanovirin-N) which showed anti-influenza A-B viruses, anti-HIV1 and HIV2 properties [113,114].
Every year, marine fungi produce between 150 and 200 novel compounds, such as sesquiterpenes, alkaloids, polyketides, and aromatic compounds [115]. Recent studies have shown the enormous potential of marine fungi as a viable source for the creation of novel antivirals against a variety of significant viruses, such as the influenza virus, the human immunodeficiency virus, and herpes simplex viruses [116]. Till date, Pleurotus citrinopileatus exhibits the highest anti-HIV-RT activity with IC50 (0.93). Pholiota adipose and Schizophyllum commune have lentins bioactive compounds with inhibitory activity toward HIV-1RT with low IC50 values [117]. Trichoderma spp. of fungi, when tested against the human Enterovirus 71, SCSIO41004 (which contains 5-acetyl-2-methoxy-1,4,6-trihydroxy-anthraquinone) significantly inhibited viral growth [118]. Isolated polyketides from the fungus Diaporthe spp. SCSIO 41011 exhibited strong antiviral activity against three different strains of the influenza A virus [119]. Isoprenylated cyclohexanols present in Truncatella angustata have shown inhibition activity against HIV and H1N1 virus [120].
7.2. Anticancer Activity
Triterpenes from Ganoderma lucidum are known to initiate apoptosis of DU-145 cells in prostate malignant growth cells. Triterpenes had restrained the development of HT-29 cells by capturing cell cycle at the G0/G1 stage and furthermore accepted the modified cell passing Type II. Triterpenes from G. lucidum (GLT) restrain the development of prostate disease cells, stifle the relocation and intrusion and actuate apoptosis through the restraint of MMP articulation [121]. Triterpenes had likewise been displayed to hinder the development of growths in a xenograft model of colon disease [122]. Triterpenoids acquired from polyporus mushrooms, for example, ganolucidic corrosive E, ganoderenic corrosive D, iucidumol A, ganodermanontriol, 15-hydroxy ganoderic acis 5, 7-oxo-ganoderic corrosive Z, and ganoderic corrosive DM showed diminished cell development in three human carcinoma cell lines CaCo2, HEPA C12 and HeLa cells [123].
In another review, seven parts of triterpenoids were assessed for the anticancer exercises on malignant growth cell lines. Ganoderic corrosive D had shown high cytotoxic movement against Hep G2, Hela, and Caco-2 cell lines [124]. In a comparative report, lanostane triterpenoids were cleansed from Inonotus obliquus, for example, inotodiol, 3b,22dihydroxylanosta-7,6 (11), 24-triene, 3bhydroxylanosta-8,24-dien-21-al, 22R-epoxylanost-8ene-3b,24S-diol, lanosterol, trametenolic corrosive, inonotsulides A, B, and C, inonotsuoxides An and B, inonotsutriols A, B, and C, lanosta-8,23E-diene-3b,22R,25-triol and lanosta-7:9(11), 23E-triene-3b,22R,25-triol, spiroinonotsuoxodiol, inonotsudiol An and inonotsuoxodiol A, and inonotsutriols D and E had shown enemy of growth impact [125]. Triterpenes of the fruiting varieties of Fomitopsis pinicola and Fumaria officinalis were compared to the cell lines HeLa, A549, hepatocellular liver carcinoma (HepG2), and MCF7. These triterpenes show a significant influence on disease cells by inhibiting the expression of VEGF, IL4, and IFN gamma growth factors [126].
Polysaccharides cleansed from G. lucidum had shown antitumor action against different malignant growth cell lines. These mixtures hinder the development of Hep2 cells by the guideline of hepatic miRNAs and safe related miRNA. It has been seen that polysaccharides in blend with 5-fluorouracil showed synergistic cytotoxicity, apoptosis, and cell cycle capture against human colon malignant growth cells [127]. The polysaccharide from the maitake mushroom (Grifola frondosa), Part D, has demonstrated anticancer activity. When human breast cancer cells (MCF7) were exposed to maitake (D part) extract at various fixations, a significant decrease in the viability of the malignant growth cell line was observed. Due to the overexpression of BAK-1 and cytochrome C records, the apoptotic activity in a portion of the subordinate way significantly increased [128].
In a comparable report, the D-part of polysaccharides has likewise exhibited a direct effect on human and canine growth disease cell lines. This portion impacts the cell expansion through a tweak of quality articulation, cell passing, and metastasis in MCF7 human bosom disease cells. It was additionally exhibited that this compound has been able to straightforwardly follow up on the mammary growth cells and to module cell processes that are associated with the turn of events and movement of disease in people [129]. Polysaccharide compound se-gp11 was purged from Se-advanced G. frondosa which is made out of mannose, glucose, and galactose. This compound repressed the development of Hepa growth by expanding interleukin-2 and serum rot factor alpha which increment the heaviness of the thymus and spleen [130]. Polysaccharide GP11, which was removed from G. frondosa, inhibited the growth of Heps cells as well as exerted indirect cytotoxicity against HepG-2 cells. This substance stimulated the production of several distinct factors, including interleukin-1β, nitric oxide (NO), and tumor necrosis factor (TNF-α). It was hypothesized that the TLR-4-intervened up-regulation of the production of NO and TNF-α was responsible for the anticancer effect of GP11 polysaccharide by strengthening the host insusceptible framework [131].
GFG-3a is an original glycoprotein refined from G. frondosa had shown cell apoptosis and capture of the cell cycle at S gradually works in human gastric malignant growth SGC-7901 cells [132]. Polysaccharide peptide compound krestin refined from tinea versicolor goes about as an adjuvant in the counteraction of bosom malignant growth. This compound had likewise been announced for antitumor impacts in growth-bearing transgenic mice and had critical hindrances of bosom disease development [133]. A polysaccharide removes (Khz) decontaminated from the intertwined mycelia of Polyporus umbellatus and G. lucidum had shown hindrance to the development of A549 cellular breakdown in the lung cells. A high sub-atomic weight novel polysaccharide PL-N1) was secluded from Phellinus linteus mycelium remove. This polysaccharide contains arabinose, xylose, glucose, and galactose and has shown antitumor exercises and critical hindrance against the development of HepG2 malignant growth cell lines [134]. Two polysaccharides filtered from Grifola umbellata GUMP-1-1 and GUMP-1-2 had shown huge restraint of cancer development in hepatoma H22 relocated mice [135]. Water-dissolvable intracellular polysaccharides were removed from refined mycelia of Phellinus igniarius, and their decontaminated ethanol part had shown anticancer exercises against Su480 and HepG2 cell lines [136].
Numerous mixes that have been restricted from mushrooms contain anticancer workouts. Grifolin, a substance extracted from the crisp fruiting bodies of Albatrellus intersection, had been shown to inhibit the growth of various disease-related cell lines in vitro by up-guiding DAPK1 [137]. The anticancer and cancer prevention agent action of low atomic weight subfractions disconnected from auxiliary metabolites created by the wood corrupting growth Cerrena unicolor was additionally revealed against human colon disease cells [138]. Anticancer movement of this compound additionally covered nasopharyngeal and osteosarcoma disease by the enlistment of apoptosis and concealment of the ERK1/2 pathway [139]. This compound can hinder DNMT1 articulation which helps in the working of mitochondrial oxidative phosphorylation edifices in nasopharyngeal carcinoma [140]. Isosullin acquired from the oil ether concentrate of Saillus flavus had shown apoptosis and G0/G1 capture of the cell cycle in KS62 cell lines [141]. This compound had likewise answered to fundamentally diminish the cell practicality, G1 capture, and apoptosis in the SMMC-772 liver cell lines. This compound had the potential for cellular breakdown in the lung therapy because of the acceptance of apoptosis in H446 malignant growth cell lines [142,143].
Ergone and polyporusterone B disconnected from P. umbellatus had shown anticancer movement against HepG2, Hep-2, and Hela disease cells. However, ergone had shown particular cytotoxic action against these malignant growth cell lines [144]. Ergosterol peroxide and trametenolic corrosive disconnected from Chaga mushroom had shown cytotoxicity in human prostatic carcinoma cell PC3 and bosom carcinoma MDA-MB-231 cell lines [145]. The ergosterol peroxide got from I. obliquus showed against malignant growth impacts in colorectal disease by down-guideline of the β-catenin pathway. This compound had shown apoptosis in CRC cell lines, and furthermore repressed colitis-related colon disease in AOM/DSS-treated mice [146].
Hispolon, a phenolic compound removed from I. obliquus could initiate apoptosis of bosom and bladder-malignant growth cells [147]. A vigorously glycosylated protein, proteoglycan, which was cleansed from P. linteus had shown antitumor impact on human disease cells by the hindrance of expansion of human colon adenocarcinoma (HT-29), human HepG2, human cellular breakdown in the lungs (NCIH 460), and human bosom adenocarcinoma (MCF-7) cells [148]. The polysaccharide compound of T. versicolor at a centralization of 20 mg/L had shown critical restraint of hepatoma malignant growth cell line (QGY) by influencing articulation of cell cycle-related qualities (p53, Bcl-2, and Fas,) and actuated apoptosis [149]. Trametenolic corrosive, a bioactive compound filtered from Trametes lactinea (Berk.) Pat, had likewise known to show cytotoxic exercises against gastric malignant growth cell lines [150]. GA3P (d-galactan sulfate) isolated from Gymnodinium microalgae had reported growth inhibition of different cell lines (HCC2998, KM-12, HT-29, WiDr, HCT-15, and HCT-116) [151]. A glycolipid mono galactosyl diacylglycerol had reported activity toward the HT-29 human colon adenocarcinoma tumors. Violaxanthin isolated from Chlorella ellipsoidea had proapoptotic and anti-proliferative activity against the HCT-116 colon cancer cell line [152].
7.3. Antifungal Activity
A melodramatic shift approaching a more sustainable, environmentally stable, and natural way of living has been observed in recent years. Besides, many demerits and ill-effects related to existing antimicrobial agents, it is no wonder that a considerable number of humans, particularly those belonging to developing nations, are using naturally accessible bioactive alternatives for their health-care systems [153]. A substantial number of natural drugs can be obtained either from microbes or by their interaction with hosts [154]. Among the many antimicrobial agents’ antifungal peptides have also been extracted from numerous sources. It was in 1948 when scientists isolated antifungal compounds from Bacillus subtilis [155], the studies on the biosynthesis and their mode of action of antifungal substances began to limelight [156]. Antifungal compounds have been extracted from three kinds of microbes: Actinomycetes, bacteria, and fungi. Bacteria constitute the largest source among these three and Bacillus amyloliquefaciens, Bacillus cereus and B. subtilis are more widely utilized in research. B. cereus have the tendency to produce bacereutin, cispentacin, azoxybacilin, and mycocerein, which show strong activity against the proliferation of Candida albicans, Saccharomyces spp., Aspergillus species, and many other fungi [157]. Chernin et al. [158] reported the antifungal compound, namely pyrrolnitrin from Enterobacter agglomerans which shows antimicrobial action against a number of pathogenic fungi such as Aspergillus niger, Candida spp., dermatophytes, and other phytopathogenic fungi. Antifungal compounds extracted from a strain of B. amyloliquefaciens sybc H47 had shown a significant effect on a number of pathogenic fungi-like Aspergillus niger, Candida albicans Fusarium oxysporum and Penicillium citrinum [159]. In addition, many fungi, such as Aspergillus have also been known to synthesize antifungal compounds like echinocandins which are resistant to mycosis [160]. In recent years, the remarkable antifungal activity of marine actinomycetes has invited the attention of many researchers globally [161].
Various studies have been done on Streptomyces species which in known to have marked antifungal activity in addition to antiviral, antibacterial, and antiparasitic properties [162,163]. The crude extract of seven phyllospheric bacteria was tested against P. oryzae which demonstrated excellent antifungal activity [164]. Enormous tactics have been utilized to deal with numerous deadly fungal infections. Amphotericin B which is produced by Streptomyces nodusus, a Gram-positive bacteria, is a first-line drug used against the commented problem and it works by disrupting the membrane system. Another great approach is the use of combinatorial therapy, which can lessen the risk of the antifungal battle against monotherapy [165]. Recently, the extract from macroalgae viz. Gracilariopsis persica has demonstrated excellent antifungal activity against four pathogenic fungi [166]. Recently, bioactive compounds from Lactobacillus harbinensis K V9.3.1Np were identified using nuclear magnetic resonance and mass spectrometry. This was accompanied by checking the activity against Penicillium expansum and Yarrowia lipolytica, which revealed a polyamine and benzoic acid as active compounds from L. harbinensis K.V9.3.1Np [167].
7.4. Antibacterial Activity
Soil is the best medium for the growth of microorganisms that produce antibiotics that can further be incredibly used in the treatment of bacterial diseases in humans. The demand for such kinds of antibiotics has been growing day by day [168,169]. Bacterial isolates from B. cereus and Klebsiella pneumoniae were discovered to have antibacterial action toward Escherchia coli, Salmonella typhi and Staphylococcus aureus and thus these could be used for the production of the broad range of antibiotics [170]. Lactic acid bacteria are known to produce antibacterial compounds such as bacteriocin, hydrogen peroxide, diacetyl, and organic acids, which are efficient against harmful bacteria. Bacteriocins are generally peptides that are antagonists for bacteria; however, their number is quite less in comparison to the developed antimicrobial peptides [171,172]. Lactobacillus pentosus ST712BZ produces bacteriocin, which is effective against the proliferation of E. coli, E. faecalis, K. pneumoniae, Lactobacillus casei, Lactobacillus curvatus and Pseudomonas aeruginosa [173,174]. Bacteriocin properties of Serraticin A from Serratia proteomaculans have been reported [175]. The compound works by interfering with the synthesis of DNA [176]. Three methoxyphenol phytometabolites, namely eugenol, capsaicin, and vanillin exhibited antibacterial and antioxidant activities against Brochothrix thermosphacta, Shewanella putrefaciens, Lactobacillus and E. coli, P. aeruginosa, and S. aureus [177]. The isolates from Streptomyces spp. exhibited strong antibacterial activity against E. coli, S. aureus, P. aeruginosa, and B. cereus [178]. Nisin and gramicidin are the famous antimicrobial peptides extracted B. subtilis, Bacillus brevis, and Lactococcus lactis [179]. Antibacterial peptides constitute a large share of AMPs and have an extensive inhibitory effect on some common pathogenic bacteria, like Listeria monocytogenes, S. aureus Acinetobacter baumannii, and E. coli [180,181]. In addition, a total of 64 fungal families were reported to show antibacterial activity against 32 species of bacteria from over the world [182]. Antibacterial activity of endophyte fungi strains against the growth of pathogenic bacteria like S. aureus and E. coli has been studied [183].
7.5. Antimalarial Activity
Malaria is a disease which is widespread in subtropical and tropical regions, including parts of America, Asia, and Africa. An estimated 241 million malaria cases and 6,27,000 malaria deaths have been reported worldwide in 2020. Plasmodium falciparum, one of the five species of infectious malaria parasites is the most dangerous one because it causes dreadful infection even death. The available drugs against P. falciparum are increasingly losing efficacy because of the growing emergence of resistance, so there is an ongoing need to develop new, effective, and affordable antimalarial agents [184]. Ferreira et al. [185] studied 285 fungal isolates and their antimicrobial and antimalarial activities were examined. These endophytic fungal isolates were grown in solid-state fermentation and their crude extracts were recovered in dichloromethane. Chromatographic fractionation and NMR were used to analyze the bioactive extracts, which showed five fungi producing antimicrobial and antimalarial compounds. Extracts of endophyte Diaporthemiriciae produced epoxycytochalasin H which displayed high antimalarial activity against chloroquine-sensitive and chloroquine-resistant strains of P. falciparum. The compound epoxycytochalasin H with high anti-malarial activity against the chloroquine-resistant strains of P. falciparum has IC50 approximately 3.5-fold lower than that with chloroquine. Bioactive compounds from Streptomyces, a Gram-positive bacterium, have been used as a most popular source of antibiotics [186]. Trioxacarcins A, B, C, and D isolated from marine Streptomyces have been tested against P. falciparum. Of these, Trioxacarcins A and D had a high antiplasmodial activity with IC50 value1.6 ± 0.1 and 2.3 ± 0.2 ng/mL, respectively comparable to artemisinin with IC50 value 0.7 ± 0.1 ng/mL [187] Trioxacarcin B with IC50 value 102 ± 4.9 ng/mL has anti plasmodial activity about 100 times less than that of trioxacarcins A and Dwhereastrioxacarcin C with IC50 value is >5000 ng/mL has been found to be inactive. Coronamycin from endophytic Streptomyces spp. growing inside an epiphytic vine, Monstera spp., showed an antiplasmodial activity against P. falciparum with IC 50 of 9.0 ng/mL [188]. A series of unique wide-spectrum antibiotics called munumbicins A, B, C, and D of which Munumbicin D showed activity against P. falciparum(with IC50 of 4.5±0.07 ng/mL) [189].
Similarly, Kakadumycin A, Munumbicins E-4, and E-5 also showed antimalarial activity [190,191]. In vivo testing of Gancidin- W isolated from Streptomyces spp. SUK 10 on P. berghei NK 65 infected mice showed a remarkable 80% inhibition of malarial parasite at 6.25 and 3.125 µg/kg body weight [192]. McCarthy et al. [193] screened the role of marine microbes as potential antimalarial agents. They identified 17 dominant extracts produced by the actinomycetes, fungi and Gram-negative bacteria out of 2365 tested samples due to their inhibitory action against the multidrug chloroquine-resistant P. falciparum and their cytotoxic nature toward the mammalian cells. Parapini et al. [194] screened a group of 14 different inhibitors of Rac1 as potent antimalarial agents. Their study showed E-Hop-016 as the potent inhibitor of P. falciparum in vitro but did not impede the parasitic invasion of erythrocytes. An outcome of the results depicted the role of E-Hop-016 in affecting the intraerythrocytic growth of the parasite.
7.6. Antidiabetic Activity
Diabetes mellitus is a collection of non-communicable metabolic disorders characterized by a persistently high blood glucose level resulting from decreased insulin production, insulin action, or even both. Diabetes mellitus, if left untreated, can cause serious health problems, which include cardiovascular disease, blindness, chronic kidney disease, neuropathy, stroke, and even death [195-197]. American diabetes association, 1997, classified Diabetes mellitus as Type 1 Diabetes mellitus, which results from the destruction of beta cells of the pancreas due to an autoimmune disorder accounting for 3–10% of cases, Type 2 Diabetes mellitus which occurs due to the body’s ineffective use of insulin and accounts for 85–90% cases and Gestational Diabetes (2–5% cases) which occurs during pregnancy [198]. Several researchers have reported that gut microbiota such as Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Faecalibacterium, Clostridium cluster IV and subcluster XIVa and Akkermansia have shown promising results against T2DM [199-201]. Jayant and Vijayakumar [202], reported in vitro anti oxidant and anti-diabetic potential of ten fungal endophytes isolated from Ficus religiosa viz., Aspergillus aculeatus, Penicillium spp., Aspergillus sydowii, Curvularia lunata, Cephaliophora irregularis, Diaporthe spp., Aspergillus quadrilineatus, A. flavus, A. versicolor and Aspergillus spp.
The antidiabetic and antioxidant potential of the fungal extracts were determined by inhibition of DPPH and enzyme α-amylase. Interestingly, all the extracts displayed moderate DPPH and α-amylase inhibitory activity with Curvularia lunata being the lead. In another study made by Ushasri et al. [203], it was reported that ethanol and acetone extracts of endophyte Syncephalastrum racemosum extracted from the sea weed Gracilaria corticata exhibited maximal α-amylase inhibitory action. Besides these, several wild edible mushrooms possess various nutritional and pharmacologically active components such as fibers, alkaloids, lectins, proteins, polysaccharides, and polyphenols which show antitumor, anti-inflammatory, immunomodulatory, antioxidant, antihypercholesterolemic, antihypertensive, hypoglycemic, antimicrobial and various other properties [204-207]. Anti-diabetic action of six medicinal and edible mushrooms such as Agaricus blazei, Coprinus comatus, Cordyceps militaris, I. obliquus, Morchella conica and P. linteus has been reported [208]. In addition, hypogycemic potential of several mushroom species viz., Lentinus swartzii, Tremella fuciformis (glucuronoxylomannan [209], Wolfiporia extensa (dehydrotumulosic acid, dehydrotrametenolic acid, and pachymic acid) [210], G. lucidum (G. lucidum polysaccharides) [211], Ganoderma applanatum (Exo-polymer (GAE) [212] and Collybia confluens (Exo-polymer (CCE) [212], Auricularia auricula-judae (Polysaccharide (FA) [213], Agaricus campestris, Agaricus subrufescens (Beta-glucans and Oligosaccharides [AO]) [214], I. obliquus, Hericium erinaceus, Agrocybe aegerita, C. comatus (Vanadium) [215], Cordyceps sinensis (Polysaccharide CSP-1) [216], G. frondosa (Alpha-glucan, MT-alpha-glucan) have been reported [217-221].
8. CONCLUSION
A greater knowledge of the significance of bioactive compounds and the identification of novel specialized metabolites would provide the world with hope for the future. The extraction of bioactive metabolites via traditional methods was time-consuming and labor-intensive. UAEs, microwave extraction, pressurized liquid extraction, and supercritical fluid extraction all have improved extraction yield and efficiency. Unquestionably, genomics has made a significant contribution to the revitalization of microbial product screening. Furthermore, microbes are an environmentally friendly and renewable drug discovery option. As a result, compounds derived from microorganisms may be the answer to the ongoing battle against antibiotic-resistant bacteria and a variety of terrible diseases. The discovery of novel biologics may be aided by the advancement and development of easily accessible NGS technologies, bioinformatics tools, and other emerging approaches. The growing demand for bioactive compounds of microbial origin in the pharmaceutical industry necessitates the development of more efficient, productive, and environmentally friendly extraction techniques. As a result, we are optimistic that the near future will persist to provide a plentiful bounty of novel bioactive molecules derived from microbial sources.
9. AUTHORS CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
10. FUNDING
The authors are grateful to the Department of Environment, Science and Technology (DEST), Shimla funded project “Development of Microbial Consortium as Bio-inoculants for Drought and Low Temperature Growing Crops for Organic Farming in Himachal Pradesh” for providing the facilities and financial support, to undertake the investigations.
11. CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Webster NS. Cooperation, communication, and co-evolution:Grand challenges in microbial symbiosis research. Front Microbiol 2014;5:164. [CrossRef]
2. Braga RM, Dourado MN, Araújo WL. Microbial interactions:Ecology in a molecular perspective. Braz J Microbiol 2016;47 suppl 1:86-98. [CrossRef]
3. Kalia VC. The dawn of the era of bioactive compounds. In:Saini AK, editors. Metabolic Engineering for Bioactive Compounds:Strategies and Processes. Singapore:Springer Singapore;2017. 3-10. [CrossRef]
4. Cragg GM, Newman DJ. Natural products:A continuing source of novel drug leads. Biochim Biophys Acta 2013;1830:3670-95. [CrossRef]
5. Aftab U, Zechel DL, Sajid I. Antitumor compounds from Streptomyces sp. KML-2, isolated from Khewra salt mines, Pakistan. Biol Res 2015;48:58. [CrossRef]
6. Rani A, Saini KC, Bast F, Mehariya S, Bhatia SK, Lavecchia R, et al. Microorganisms:A potential source of bioactive molecules for antioxidant applications. Molecules 2021;26:1142. [CrossRef]
7. Mazzoli R, Riedel K, Pessione E. Editorial:Bioactive compounds from microbes. Front Microbiol 2017;8:392. [CrossRef]
8. Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 2003;67:491-502. [CrossRef]
9. Zhao J, Shan T, Mou Y, Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem 2011;11:159-68. [CrossRef]
10. Grabley S, Sattler I. Natural products for lead identification:Nature is a valuable resource for providing tools. In:Hillisch A, Hilgenfeld R, editors. Modern Methods of Drug Discovery. Basel:Birkhäuser Basel;2003. 87-107. [CrossRef]
11. Stadler M, Keller NP. Paradigm shifts in fungal secondary metabolite research. Mycol Res 2008;112:127-30. [CrossRef]
12. Ruksiriwanich W, Khantham C, Linsaenkart P, Chaitep T, Rachtanapun P, Jantanasakulwong K, et al. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int J Food Sci Tech 2022;57:110-22. [CrossRef]
13. Zaki AH, Zahid MT, Haiying B. Bioactive compounds of the culinary-medicinal mushroom Leucocalocybe mongolica (Agaricomycetes):Pharmacological and therapeutic applications-a review. Int J Med Mushrooms 2022;24:19-33. [CrossRef]
14. Krümmel A, Rodrigues LG, Vitali L, Ferreira SR. Bioactive compounds from Pleurotus sajor-caju mushroom recovered by sustainable high-pressure methods. LWT 2022;160:113316. [CrossRef]
15. Elkhateeb WA, Daba GM. Bioactive potential of some fascinating edible mushrooms Macrolepiota, Russula, Amanita, Vovariella and Grifola as a treasure of multipurpose therapeutic natural product. J Mycol Mycol Sci 2022;5:000157.
16. Wang Q, Xu L. Beauvericin, a bioactive compound produced by fungi:A short review. Molecules 2012;17:2367-77. [CrossRef]
17. Kumar S, Aharwal RP, Shukla H, Rajak RC, Sandhu SS. Endophytic fungi:As a source of antimicrobials bioactive compounds. World J Pharm Pharm Sci 2014;3:1179-97.
18. Manganyi MC, Tchatchouang CD, Regnier T, Bezuidenhout CC, Ateba CN. Bioactive compound produced by endophytic fungi isolated from Pelargonium sidoides against selected bacteria of clinical importance. Mycobiology 2019;47:335-9. [CrossRef]
19. Nuraini FR, Setyaningsih R, Susilowati A. Antioxidant activity of bioactive compound produced by endophytic fungi isolated from endemic plant of South Kalimantan Mangifera casturi Kosterm. AIP Conf Proc 2019;2120:080013. [CrossRef]
20. Jamal HA, Husaini A, Sing NN, Roslan HA, Zulkharnain A, Akinkunmi WA. Characterization of bioactive compounds produced by endophytic fungi isolated from Gynura procumbens (Sambung Nyawa). Braz J Microbiol 2022;53:1857-70. [CrossRef]
21. Kumar V, Prasher IB. Antimicrobial potential of endophytic fungi isolated from Dillenia indica L. and identification of bioactive molecules produced by Fomitopsis meliae (Undrew.) Murril. Nat Prod Res 2022;36:6064-8. [CrossRef]
22. Li J, Zhao GZ, Chen HH, Wang HB, Qin S, Zhu WY, et al. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett Appl Microbiol 2008;47:574-80. [CrossRef]
23. Kusari S, Verma VC, Lamshoeft M, Spiteller M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol 2012;28:1287-94. [CrossRef]
24. Molina G, Pimentel MR, Bertucci TC, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem Eng Trans 2012;27:289-94.
25. Singh M, Kumar A, Singh R, Pandey KD. Endophytic bacteria:A new source of bioactive compounds. 3 Biotech 2017;7:315. [CrossRef]
26. Christina A, Christapher V, Bhore SJ. Endophytic bacteria as a source of novel antibiotics:An overview. Pharmacogn Rev 2013;7:11-6. [CrossRef]
27. Miller CM, Miller RV, Garton-Kenny D, Redgrave B, Sears J, Condron MM, et al. Ecomycins, unique antimycotics from Pseudomonas viridiflava. J Appl Microbiol 1998;84:937-44. [CrossRef]
28. Harrison L, Teplow DB, Rinaldi M, Strobel G. Pseudomycins, a family of novel peptides from Pseudomonas syringae possessing broad-spectrum antifungal activity. J Gen Microbiol 1991;137:2857-65. [CrossRef]
29. Ghiasvand M, Makhdoumi A, Matin MM, Vaezi J. Exploring the bioactive compounds from endophytic bacteria of a medicinal plant:Ephedra foliata (Ephedrales:Ephedraceae). Adv Tradit Med 2020;20:61-70. [CrossRef]
30. Matsumoto A, Takahashi Y. Endophytic actinomycetes:Promising source of novel bioactive compounds. J Antibiot (Tokyo) 2017;70:514-9. [CrossRef]
31. De Simeis D, Serra S. Actinomycetes:A never-ending source of bioactive compounds-an overview on antibiotics production. Antibiotics (Basel) 2021;10:483. [CrossRef]
32. Balachandar R, Karmegam N, Saravanan M, Subbaiya R, Gurumoorthy P. Synthesis of bioactive compounds from vermicast isolated actinomycetes species and its antimicrobial activity against human pathogenic bacteria. Microb Pathog 2018;121:155-65. [CrossRef]
33. Janardhan A, Kumar AP, Viswanath B, Saigopal DV, Narasimha G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int 2014;2014:217030. [CrossRef]
34. Sanmukh S, Bruno B, Ramakrishnan U, Khairnar K, Swaminathan S, Paunikar W. Bioactive compounds derived from microalgae showing antimicrobial activities. J Aquac Res Dev 2014;5:224. [CrossRef]
35. Bule MH, Ahmed I, Maqbool F, Bilal M, Iqbal HM. Microalgae as a source of high-value bioactive compounds. Front Biosci (Schol Ed) 2018;10:197-216. [CrossRef]
36. Yi Z, Xu M, Magnusdottir M, Zhang Y, Brynjolfsson S, Fu W. Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar Drugs 2015;13:6138-51. [CrossRef]
37. Okuzumi J, Nishino H, Murakoshi M, Iwashima A, Tanaka Y, Yamane T, et al. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-myc expression and cell cycle progression in human malignant tumor cells. Cancer Lett 1990;55:75-81. [CrossRef]
38. Ishikawa C, Tafuku S, Kadekaru T, Sawada S, Tomita M, Okudaira T, et al. Anti-adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int J Cancer 2008;123:2702-12. [CrossRef]
39. Fu W, Nelson DR, Yi Z, Xu M, Khraiwesh B, Jijakli K, et al. Bioactive compounds from microalgae:Current development and prospects. Stud Nat Prod Chem 2017;54:199-225. [CrossRef]
40. Cragg GM, Newman DJ. Medicinals for the millennia:The historical record. Ann N Y Acad Sci 2001;953:3-25. [CrossRef]
41. Finglas PM, Wright AJ, Wolfe CA, Hart DJ, Wright DM, Dainty JR. Is there more to folates than neural-tube defects?Proc Nutr Soc 2003;62:591-8. [CrossRef]
42. Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1-26. [CrossRef]
43. Bhatnagar I, Kim SK. Immense essence of excellence:Marine microbial bioactive compounds. Mar Drugs 2010;8:2673-701. [CrossRef]
44. Ouchari L, Boukeskasse A, Bouizgarne B, Ouhdouch Y. Antimicrobial potential of actinomycetes isolated from the unexplored hot Merzouga desert and their taxonomic diversity. Biol Open 2019;8:bio035410. [CrossRef]
45. Liao S, Wang Y, Liu H, Fan G, Sahu SK, Jin T, et al. Deciphering the microbial taxonomy and functionality of two diverse mangrove ecosystems and their potential abilities to produce bioactive compounds. mSystems 2020;5:e00851-19. [CrossRef]
46. Teimoori-Boghsani Y, Ganjeali A, Cernava T, Müller H, Asili J, Berg G. Endophytic fungi of native Salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front Microbiol 2020;10:3013. [CrossRef]
47. Niego AG, RaspéO, Thongklang N, Charoensup R, Lumyong S, Stadler M, et al. Taxonomy, diversity and cultivation of the oudemansielloid/xeruloid taxa Hymenopellis, Mucidula, Oudemansiella, and Xerula with respect to their bioactivities:A review. J Fungi (Basel) 2021;7:51. [CrossRef]
48. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals:Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel) 2017;6:42. [CrossRef]
49. Sweetlove LJ, Nielsen J, Fernie AR. Engineering central metabolism-a grand challenge for plant biologists. Plant J 2017;90:749-63. [CrossRef]
50. Gill BS, Qiu FN. Technologies for extraction and production of bioactive compounds. In:Verma ML, Chandel AK, editors. Biotechnological Production of Bioactive Compounds. Ch. 1. Amsterdam:Elsevier;2020. 1-36. [CrossRef]
51. Negi A, Gill BS. Success stories of enolate form of drugs. PharmaTutor 2013;1:45-53.
52. Teo CL, Idris A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour Technol 2014;171:477-81. [CrossRef]
53. Gill BS, Kumar S. Differential algorithms-assisted molecular modeling-based identification of mechanistic binding of ganoderic acids. Med Chem Res 2015;24:3483-93. [CrossRef]
54. De Castro MD, Priego-Capote F. Soxhlet extraction:Past and present panacea. J Chromatogr A 2010;1217:2383-9. [CrossRef]
55. Ashraf R, Ghufran S, Akram S, Mushtaq M, Sultana B. Cold pressed coriander (Coriandrum sativum L.) seed oil. In:Cold Pressed Oils. Amsterdam:Elsevier;2020. 345-56. [CrossRef]
56. Carreira-Casais A, Lourenço-Lopes C, Otero P, Rodriguez MC, Pereira AG, Echave J, et al. Application of Green Extraction Techniques for Natural Additives Production. London, UK:Intechopen;2021. 1-21. [CrossRef]
57. Garcia-Vaquero M, Rajauria G, Tiwari B. Conventional extraction techniques:Solvent extraction. In:Sustainable Seaweed Technologies. Amsterdam:Elsevier;2020. 171-89. [CrossRef]
58. Manousi N, Sarakatsianos I, Samanidou VF. Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In:Grumezescu AM, Holban AM, editors. Engineering Tools in the Beverage Industry. United Kingdom:Woodhead Publishing;2019. 283-314. [CrossRef]
59. López-Bascón MA, de Castro MD. Soxhlet extraction. In:Poole CF, editor. Liquid-phase Extraction. Amsterdam:Elsevier;2020. 327-54. [CrossRef]
60. Górak A, Sorensen E. Distillation:Fundamentals and Principles. Unites States:Academic Press;2014. [CrossRef]
61. Prado JM, Vardanega R, Debien IC, Meireles MA, Gerschenson LN, Sowbhagya HB, et al. Conventional extraction. In:Food Waste Recovery. Amsterdam:Elsevier;2021. 109-27. [CrossRef]
62. Azmir J, Zaidul IS, Rahman MM, Sharif KM, Mohamed A, Sahena F, et al. Techniques for extraction of bioactive compounds from plant materials:A review. J Food Eng 2013;117:426-36. [CrossRef]
63. Handa SS, Khanuja SP, Longo G, Rakesh DD, editors. An overview of extraction technology for medicinal and aromatic plants. In:Extraction Technologies for Medicinal and Aromatic Plants. United Nations Industrial Development Organization:Vienna;2008. 1-58.
64. Anastas PT, Warner JC. Principles of green chemistry. In:Green chemistry:Theory and Practice. United Kingdom:Oxford University Press;1998. 29.
65. Jacotet-Navarro M, Rombaut N, Deslis S, Fabiano-Tixier AS, Pierre FX, Bily A, et al. Towards a “dry“bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chem 2016;18:3106-15. [CrossRef]
66. Soquetta MB, de Marsillac Terra L, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J Food 2018;16:400-12. [CrossRef]
67. Brunner G. Supercritical gases as solvents:Phase equilibria. In:Gas Extraction:An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes. Heidelberg:Steinkopff;1994. 59-146. [CrossRef]
68. Echave J, Pereira AG, Carpena M, Prieto MÁ, Simal-Gandara J. Capsicum seeds as a source of bioactive compounds:Biological properties, extraction systems, and industrial application. In:Capsicum. London:IntechOpen;2020. [CrossRef]
69. Daintree L, Kordikowski A, York P. Separation processes for organic molecules using SCF Technologies. Adv Drug Deliv Rev 2008;60:351-72. [CrossRef]
70. Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 2006;17:300-12. [CrossRef]
71. Molino A, Larocca V, Di Sanzo G, Martino M, Casella P, Marino T, et al. Extraction of bioactive compounds using supercritical carbon dioxide. Molecules 2019;24:782. [CrossRef]
72. Ganzler K, SalgóA, ValkóK. Microwave extraction. A novel sample preparation method for chromatography. J Chromatogr 1986;371:299-306. [CrossRef]
73. Angiolillo L, Del Nobile MA, Conte A. The extraction of bioactive compounds from food residues using microwaves. Curr Opin Food Sci 2015;5:93-8. [CrossRef]
74. Sarfarazi M, Jafari SM, Rajabzadeh G, Galanakis CM. Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.). Ind Crops Prod 2020;145:111978. [CrossRef]
75. Alfaro MJ, Bélanger JM, Padilla FC, ParéJR. Influence of solvent, matrix dielectric properties, and applied power on the liquid-phase microwave-assisted processes (MAP™)1 extraction of ginger (Zingiber officinale). Food Res Int 2003;36:499-504. [CrossRef]
76. Casazza AA, Aliakbarian B, Mantegna S, Cravotto G, Perego P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J Food Eng 2010;100:50-5. [CrossRef]
77. Moreira MM, Barroso MF, Boeykens A, Withouck H, Morais S, Delerue-Matos C. Valorization of apple tree wood residues by polyphenols extraction:Comparison between conventional and microwave-assisted extraction. Ind Crops Prod 2017;104:210-20. [CrossRef]
78. Chan CH, Yusoff R, Ngoh GC, Kung FW. Microwave-assisted extractions of active ingredients from plants. J Chromatogr A 2011;1218:6213-25. [CrossRef]
79. Mason TJ, Chemat F, Vinatoru M. The extraction of natural products using ultrasound or microwaves. Curr Org Chem 2011;15:237-47. [CrossRef]
80. Roselló-Soto E, Koubaa M, Moubarik A, Lopes RP, Saraiva JA, Boussetta N, et al. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process:Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci Technol 2015;45:296-310. [CrossRef]
81. Briones-Labarca V, Plaza-Morales M, Giovagnoli-Vicuña C, Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds:Effects of extraction conditions and methods. LWT Food Sci Technol 2015;60:525-34. [CrossRef]
82. Kek S, Chin NL, Yusof YA. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod Process 2013;91:495-506. [CrossRef]
83. Boonkird S, Phisalaphong C, Phisalaphong M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab-and pilot-plant scale. Ultrason Sonochem 2008;15:1075-9. [CrossRef]
84. Chemat F, Zill-e-Huma, Khan MK. Applications of ultrasound in food technology:Processing, preservation and extraction. Ultrason Sonochem 2011;18:813-35. [CrossRef]
85. Nieto A, Borrull F, Pocurull E, MarcéRM. Pressurized liquid extraction:A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal Chem 2010;29:752-64. [CrossRef]
86. Dunford NT, Irmak S, Jonnala R. Pressurised solvent extraction of policosanol from wheat straw, germ and bran. Food Chem 2010;119:1246-9. [CrossRef]
87. Plaza M, Santoyo S, Jaime L, Reina GG, Herrero M, Señoráns FJ, et al. Screening for bioactive compounds from algae. J Pharm Biomed Anal 2010;51:450-5. [CrossRef]
88. Muñóz-Almagro N, Gilbert-López B, Pozuelo-Rollon MC, García-Fernandez Y, Almeida C, Villamiel M, et al. Exploring the microalga Euglena cantabrica by pressurized liquid extraction to obtain bioactive compounds. Marine Drugs 2020;18:308. [CrossRef]
89. Bryant G, Wolfe J. Electromechanical stresses produced in the plasma membranes of suspended cells by applied electric fields. J Membr Biol 1987;96:129-39. [CrossRef]
90. Kumari B, Tiwari BK, Hossain MB, Brunton NP, Rai DK. Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food Bioprocess Technol 2018;11:223-41. [CrossRef]
91. Zeng XA, Zhang Z. Pulsed electric field assisted extraction of bioactive compounds. In:Jia J, Liu D, Ma H, editor. Advances in Food Processing Technology. Singapore:Springer Singapore;2019. 125-35. [CrossRef]
92. Balasa A, Janositz A, Knorr D. Electric field stress on plant systems. In:Encyclopedia of Biotechnology in Agriculture and Food. Vol. 2. Milton Park:Taylor and Francis Group;2011. 208-11. [CrossRef]
93. Saide A, Martínez KA, Ianora A, Lauritano C. Unlocking the health potential of microalgae as sustainable sources of bioactive compounds. Int J Mol Sci 2021;22:4383. [CrossRef]
94. Tabarzad M, Atabaki V, Hosseinabadi T. Anti-inflammatory activity of bioactive compounds from microalgae and Cyanobacteria by focusing on the mechanisms of action. Mol Biol Rep 2020;47:6193-205. [CrossRef]
95. Rangel M, Falkenberg M. An overview of the marine natural products in clinical trials and on the market. J Coast Life Med 2015;3:421-8. [CrossRef]
96. Bhatnagar I, Kim SK. Marine antitumor drugs:Status, shortfalls and strategies. Mar Drugs 2010;8:2702-20. [CrossRef]
97. Nikapitiya C. Bioactive secondary metabolites from marine microbes for drug discovery. Adv Food Nutr Res 2012;65:363-87. [CrossRef]
98. Miller IJ, Vanee N, Fong SS, Lim-Fong GE, Kwan JC. Lack of overt genome reduction in the bryostatin-producing bryozoan symbiont “Candidatus Endobugula sertula“. Appl Environ Microbiol 2016;82:6573-83. [CrossRef]
99. Lam AP, Sparano JA, Vinciguerra V, Ocean AJ, Christos P, Hochster H, et al. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am J Clin Oncol 2010;33:121-4. [CrossRef]
100. Murti Y, Agrawal T. Marine derived pharmaceuticals-development of natural health products from marine biodiversity. Int J ChemTech Res 2010;2:2198-217.
101. Sekurova ON, Schneider O, Zotchev SB. Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb Biotechnol 2019;12:828-44. [CrossRef]
102. Rocha-Martin J, Harrington C, Dobson AD, O'Gara F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar Drugs 2014;12:3516-59. [CrossRef]
103. Rastogi G, Sani RK. Molecular techniques to assess microbial community structure, function, and dynamics in the environment. In:Ahmad I, Ahmad F, Pichtel J, editor. Microbes and Microbial Technology:Agricultural and Environmental Applications. New York:Springer;2011. 29-57. [CrossRef]
104. Handelsman J, Rondon MR, Brady SF, Clardy J, Goodman RM. Molecular biological access to the chemistry of unknown soil microbes:A new frontier for natural products. Chem Biol 1998;5:R245-9. [CrossRef]
105. NovákováJ, FarkašovskýM. Bioprospecting microbial metagenome for natural products. Biologia 2013;68:1079-86. [CrossRef]
106. Handelsman J. Metagenomics:Application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 2004;68:669-85. [CrossRef]
107. Wong DW. Applications of metagenomics for industrial bioproducts. In:Metagenomics:Theory, Methods and Applications. Norfolk, UK:Caister Academic Press;2010. 141-58.
108. Barone R, De Santi C, Esposito FP, Tedesco P, Galati F, Visone M, et al. Marine metagenomics, a valuable tool for enzymes and bioactive compounds discovery. Front Mar Sci 2014;1:38. [CrossRef]
109. Barkia I, Saari N, Manning SR. Microalgae for high-value products towards human health and nutrition. Mar Drugs 2019;17:304. [CrossRef]
110. Feller R. Microalgae Biomass as a Source of Natural Compounds:Chemical Characterization and New Approaches for Lipid Extraction and Culture Harvesting. Florianópolis, Brazil:Federal University of Santa Catarina;2017. 146.
111. Pandeirada CO, Maricato É, Ferreira SS, Correia VG, Pinheiro BA, Evtuguin DV, et al. Structural analysis and potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr Polym 2019;222:114962. [CrossRef]
112. De Jesus Raposo MF, de Morais AM, de Morais RM. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci 2014;101:56-63. [CrossRef]
113. Santoyo S, Jaime L, Plaza M, Herrero M, Rodriguez-Meizoso I, Ibañez E, et al. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J Appl Phycol 2012;24:731-41. [CrossRef]
114. Carbone DA, Pellone P, Lubritto C, Ciniglia C. Evaluation of microalgae antiviral activity and their bioactive compounds. Antibiotics (Basel) 2021;10:746. [CrossRef]
115. Moghadamtousi SZ, Nikzad S, Kadir HA, Abubakar S, Zandi K. Potential antiviral agents from marine fungi:An overview. Mar Drugs 2015;13:4520-38. [CrossRef]
116. Nosik DN, Nosik NN, Teplyakova T, Lobach OA, Kiseleva IA, Kondrashina NG, et al. Antiviral activity of extracts of basidiomycetes and humic compounds substances against Human Immunodeficiency Virus (Retroviridae:Orthoretrovirinae:Lentivirus:Human immunodeficiency virus 1) and Herpes Simplex Virus (Herpesviridae:Simplexvirus:Human alphaherpesvirus 1). Vopr Virusol 2020;65:276-83. [CrossRef]
117. Singh RS, Walia AK, Kennedy JF. Mushroom lectins in biomedical research and development. Int J Biol Macromol 2020;151:1340-50. [CrossRef]
118. Pang X, Lin X, Tian Y, Liang R, Wang J, Yang B, et al. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat Prod Res 2018;32:105-11. [CrossRef]
119. Luo X, Yang J, Chen F, Lin X, Chen C, Zhou X, et al. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front Chem 2018;6:282. [CrossRef]
120. Zhao Y, Liu D, Proksch P, Zhou D, Lin W. Truncateols O-V, further isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with antiviral activities. Phytochemistry 2018;155:61-8. [CrossRef]
121. Qu L, Li S, Zhuo Y, Chen J, Qin X, Guo G. Anticancer effect of triterpenes from Ganoderma lucidum in human prostate cancer cells. Oncol Lett 2017;14:7467-72.
122. Thyagarajan A, Jedinak A, Nguyen H, Terry C, Baldridge LA, Jiang J, et al. Triterpenes from Ganoderma lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK). Nutr Cancer 2010;62:630-40. [CrossRef]
123. Ruan W, Wei Y, Popovich DG. Distinct responses of cytotoxic Ganoderma lucidum triterpenoids in human carcinoma cells. Phytother Res 2015;29:1744-52. [CrossRef]
124. Ruan W, Lim AH, Huang LG, Popovich DG. Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth. Nat Prod Res 2014;28:2264-72. [CrossRef]
125. Handa N, Yamada T, Tanaka R. Four new lanostane-type triterpenoids from Inonotus obliquus. Phytochem Lett 2012;5:480-5. [CrossRef]
126. Shi ZT, Bao HY, Feng S. Antitumor activity and structure-activity relationship of seven lanostane-type triterpenes from Fomitopsis pinicola and F. officinalis. Zhongguo Zhong Yao Za Zhi 2017;42:915-22.
127. Liang CJ, Lee CW, Sung HC, Chen YH, Chiang YC, Hsu HY, et al. Ganoderma lucidum polysaccharides reduce lipopolysaccharide-induced interleukin-1 b expression in cultured smooth muscle cells and in thoracic aortas in mice. Evid Based Complement Alternat Med 2014;2014:305149. [CrossRef]
128. Soares R, Meireles M, Rocha A, Pirraco A, Obiol D, Alonso E, et al. Maitake (D fraction) mushroom extract induces apoptosis in breast cancer cells by BAK-1 gene activation. J Med Food 2011;14:563-72. [CrossRef]
129. Alonso EN, Ferronato MJ, Gandini NA, Fermento ME, Obiol DJ, Romero AL, et al. Antitumoral effects of D-fraction from Grifola frondosa (maitake) mushroom in breast cancer. Nutr Cancer 2017;69:29-43. [CrossRef]
130. Mao GH, Ren Y, Li Q, Wu HY, Jin D, Zhao T, et al. Anti-tumor and immunomodulatory activity of selenium (Se)-polysaccharide from Se-enriched Grifola frondosa. Int J Biol Macromol 2016;82:607-13. [CrossRef]
131. Mao GH, Ren Y, Feng WW, Li Q, Wu HY, Zhao T, et al. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr Polym 2015;134:406-12. [CrossRef]
132. Cui F, Zan X, Li Y, Sun W, Yang Y, Ping L. Grifola frondosa glycoprotein GFG-3a arrests S phase, alters proteome, and induces apoptosis in human gastric cancer cells. Nutr Cancer 2016;68:267-79. [CrossRef]
133. Lu H, Yang Y, Gad E, Wenner CA, Chang A, Larson ER, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK Cells. Clin Cancer Res 2011;17:67-76. [CrossRef]
134. Pei JJ, Wang ZB, Ma HL, Yan JK. Structural features and antitumor activity of a novel polysaccharide from alkaline extract of Phellinus linteus mycelia. Carbohydr Polym 2015;115:472-7. [CrossRef]
135. Bi Y, Miao Y, Han Y, Xu J, Wang Q. Biological and physicochemical properties of two polysaccharides from the mycelia of Grifola umbellate. Carbohydr Polym 2013;95:740-5. [CrossRef]
136. Li SC, Yang XM, Ma HL, Yan JK, Guo DZ. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydr Polym 2015;133:24-30. [CrossRef]
137. Luo XJ, Li LL, Deng QP, Yu XF, Yang LF, Luo FJ, et al. Grifolin, a potent antitumour natural product upregulates death-associated protein kinase 1 DAPK1 via p53 in nasopharyngeal carcinoma cells. Eur J Cancer 2011;47:316-25. [CrossRef]
138. Matuszewska A, Stefaniuk D, Jaszek M, Pi?t M, Zaj?c A, Matuszewski ?, et al. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci Rep 2019;9:1975. [CrossRef]
139. Wu Z, Li Y. Grifolin exhibits anti-cancer activity by inhibiting the development and invasion of gastric tumor cells. Oncotarget 2017;8:21454-60. [CrossRef]
140. Luo X, Hong L, Cheng C, Li N, Zhao X, Shi F, et al. DNMT1 mediates metabolic reprogramming induced by Epstein-Barr virus latent membrane protein 1 and reversed by grifolin in nasopharyngeal carcinoma. Cell death Dis 2018;9:619. [CrossRef]
141. Wang Y, Zhang Q, Zhao J, Zhao X, Zhang J, Wang LA. Iso-suillin from the mushroom Suillus flavus induces cell cycle arrest and apoptosis in K562 cell line. Food Chem Toxicol 2014;67:17-25. [CrossRef]
142. Jia ZQ, Chen Y, Yan YX, Zhao JX. Iso-suillin isolated from Suillus luteus, induces G1 phase arrest and apoptosis in human hepatoma SMMC-7721 cells. Asian Pac J Cancer Prev 2014;15:1423-8. [CrossRef]
143. Zhao JX, Zhang QS, Chen Y, Yao SJ, Yan YX, Wang Y, et al. Iso-suillin from Suillus flavus induces apoptosis in human small cell lung cancer H446 cell line. Chin Med J (Engl) 2016;129:1215-23. [CrossRef]
144. Zhao YY, Chao X, Zhang Y, Lin RC, Sun WJ. Cytotoxic steroids from Polyporus umbellatus. Planta Med 2010;76:1755-8. [CrossRef]
145. Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem 2013;139:503-8. [CrossRef]
146. Kang JH, Jang JE, Mishra SK, Lee HJ, Nho CW, Shin D, et al. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J Ethnopharmacol 2015;173:303-12. [CrossRef]
147. Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang TC, et al. Hispolon from Phellinus linteus has antiproliferative effects via MDM2-recruited ERK1/2 activity in breast and bladder cancer cells. Food Chem Toxicol 2009;47:2013-21. [CrossRef]
148. Li YG, Ji DF, Zhong S, Zhu JX, Chen S, Hu GY. Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int J Biol Macromol 2011;48:511-7. [CrossRef]
149. Cai X, Pi Y, Zhou X, Tian L, Qiao S, Lin J. Hepatoma cell growth inhibition by inducing apoptosis with polysaccharide isolated from Turkey tail medicinal mushroom, Trametes versicolor (L.:Fr.) Lloyd (aphyllophoromycetideae). Int J Med Mushrooms 2010;12:257-63. [CrossRef]
150. Zhang Q, Huang N, Wang J, Luo H, He H, Ding M, et al. The H+/K+-ATPase inhibitory activities of Trametenolic acid B from Trametes lactinea (Berk.) Pat, and its effects on gastric cancer cells. Fitoterapia 2013;89:210-7. [CrossRef]
151. Talero E, García-Mauriño S, Ávila-Román J, Rodríguez-Luna A, Alcaide A, Motilva V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar Drugs 2015;13:6152-209. [CrossRef]
152. Andrade KA, Lauritano C, Romano G, Ianora A. Marine microalgae with anti-cancer properties. Mar Drugs 2018;16:165. [CrossRef]
153. Manganyi MC, Ateba CN. Untapped potentials of endophytic fungi:A review of novel bioactive compounds with biological applications. Microorganisms 2020;8:1934. [CrossRef]
154. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 2020;83:770-803. [CrossRef]
155. Landy M, Warren GH, Rosenman SB, Colio LG. Bacillomycin;an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 1948;67:539-41. [CrossRef]
156. Banerjee N, Bose SK. Mode of action of mycobacillin, a new antifungal antibiotic. J Bacteriol 1963;86:387-91. [CrossRef]
157. Kerr JR. Bacterial inhibition of fungal growth and pathogenicity. Microb Ecol Health Dis 1999;11:129-42. [CrossRef]
158. Chernin L, Brandis A, Ismailov Z, Chet I. Pyrrolnitrin production by an Enterobacter agglomerans strain with a broad spectrum of antagonistic activity towards fungal and bacterial phytopathogens. Curr Microbiol 1996;32:208-12. [CrossRef]
159. Li X, Zhang Y, Wei Z, Guan Z, Cai Y, Liao X. Antifungal activity of isolated Bacillus amyloliquefaciens SYBC H47 for the biocontrol of peach gummosis. PLoS One 2016;11:e0162125. [CrossRef]
160. Nyfeler R, Keller-Schierlein W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus:Isolation and structural components. Helv Chim Acta 1974;57:2459-77. [CrossRef]
161. Manivasagan P, Venkatesan J, Sivakumar K, Kim SK. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol Res 2014;169:262-78. [CrossRef]
162. Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, et al. Actinobacteria-derived peptide antibiotics since 2000. Peptides 2018;103:48-59. [CrossRef]
163. Li T, Li L, Du F, Sun L, Shi J, Long M, et al. Activity and mechanism of action of antifungal peptides from microorganisms:A review. Molecules 2021;26:3438. [CrossRef]
164. Wiraswati SM, Rusmana I, Nawangsih AA, Wahyudi AT. Antifungal activities of bacteria producing bioactive compounds isolated from rice phyllosphere against Pyricularia oryzae. J Plant Protect Res 2019;59:86-94.
165. Cools TL, Struyfs C, Cammue BP, Thevissen K. Antifungal plant defensins:Increased insight in their mode of action as a basis for their use to combat fungal infections. Future Microbiol 2017;12:441-54. [CrossRef]
166. Pourakbar L, Moghaddam SS, El Enshasy HA, Sayyed RZ. Antifungal activity of the extract of a macroalgae, Gracilariopsis persica, against four plant pathogenic fungi. Plants (Basel) 2021;10:1781. [CrossRef]
167. Mosbah A, Delavenne E, Souissi Y, Mahjoubi M, Jéhan P, Le Yondre N, et al. Novel antifungal compounds, spermine-like and short cyclic polylactates, produced by Lactobacillus harbinensis K.V9.3.1Np in yogurt. Front Microbiol 2018;9:2252. [CrossRef]
168. Demain AL, Fang A. The natural functions of secondary metabolites. Adv Biochem Eng Biotechnol 2000;69:1-39. [CrossRef]
169. Abbas S, Senthilkumar R, Arjunan S. Isolation and molecular characterization of microorganisms producing novel antibiotics from soil sample. Eur J Exp Biol 2014;4:149-55.
170. Bano S, Naz S, Uzair B, Hussain M, Khan MM, Bibi H, et al. Detection of microorganisms with antibacterial activities from different industrial wastes and GC-MS analysis of crude microbial extract. Braz J Biol 2021;83:e245585. [CrossRef]
171. Vasilchenko A, Vasilchenko AV, Valyshev AV, Rogozhin EA. A novel high-molecular-mass bacteriocin produced by Enterococcus faecium:Biochemical features and mode of action. Probiotics Antimicrob Proteins 2018;10:427-34. [CrossRef]
172. Lv X, Ma H, Sun M, Lin Y, Bai F, Li J, et al. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 2018;89:22-31. [CrossRef]
173. Todorov SD, Dicks LM. Bacteriocin production by Lactobacillus pentosus ST712BZ isolated from boza. Braz J Microbiol 2007;38:166-72. [CrossRef]
174. Tenea GN, Yépez L. Bioactive compounds of lactic acid bacteria. Case study:Evaluation of antimicrobial activity of bacteriocin-producing lactobacilli isolated from native ecological niches of Ecuador. In:Probiotics and Prebiotics in Human Nutrition and Health. London, UK:Intechopen;2016. 149-67. [CrossRef]
175. Sánchez LA, Hedström M, Delgado MA, Delgado OD. Production, purification and characterization of serraticin A, a novel cold-active antimicrobial produced by Serratia proteamaculans 136. J Appl Microbiol 2010;109:936-45. [CrossRef]
176. Pan HQ, Zhang SY, Wang N, Li ZL, Hua HM, Hu JC, et al. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar Drugs 2013;11:3891-901. [CrossRef]
177. Orlo E, Russo C, Nugnes R, Lavorgna M, Isidori M. Natural methoxyphenol compounds:Antimicrobial activity against foodborne pathogens and food spoilage bacteria, and role in antioxidant processes. Foods 2021;10:1807. [CrossRef]
178. Tomaseto AA, Alpiste MC, de Castro Nassar AF, Destéfano SA. Antibacterial activity of phytopathogenic Streptomyces strains against bacteria associated to clinical diseases. Arq Inst Biol 2020;87:1-7. [CrossRef]
179. Cao J, de la Fuente-Nunez C, Ou RW, Torres MD, Pande SG, Sinskey AJ, et al. Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synth Biol 2018;7:896-902. [CrossRef]
180. Li C, Zhu C, Ren B, Yin X, Shim SH, Gao Y, et al. Two optimized antimicrobial peptides with therapeutic potential for clinical antibiotic-resistant Staphylococcus aureus. Eur J Med Chem 2019;183:111686. [CrossRef]
181. Huan Y, Kong Q, Mou H, Yi H. Antimicrobial peptides:Classification, design, application and research progress in multiple fields. Front Microbiol 2020;11:582779. [CrossRef]
182. Ranadive KR, Belsare MH, Deokule SS, Jagtap NV, Jadhav HK, Vaidya JG. Glimpses of antimicrobial activity of fungi from World. J New Biol Rep 2013;2:142-62.
183. Handayani D, Rivai H, Hutabarat M, Rasyid R. Antibacterial activity of endophytic fungi isolated from mangrove plant Sonneratia griffithii Kurz. J Appl Pharm Sci 2017;7:209-12.
184. Rosenthal PJ. Antimalarial drug discovery:Old and new approaches. J Exp Biol 2003;206:3735-44. [CrossRef]
185. Ferreira MC, Cantrell CL, Wedge DE, Gonçalves VN, Jacob MR, Khan S, et al. Antimycobacterial and antimalarial activities of endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from Brazil. Mem Inst Oswaldo Cruz 2017;112:692-7. [CrossRef]
186. Ahmad SJ, Rahim MB, Baharum SN, Baba MS, Zin NM. Discovery of antimalarial drugs from Streptomycetes metabolites using a metabolomic approach. J Trop Med 2017;2017:2189814. [CrossRef]
187. Maskey RP, Helmke E, Kayser O, Fiebig HH, Maier A, Busche A, et al. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J Antibiot (Tikyo) 2004;57:771-9. [CrossRef]
188. Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod 2004;67:257-68. [CrossRef]
189. Castillo UF, Strobel GA, Ford EJ, Hess WM, Porter H, Jensen JB, et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology (Reading) 2002;148:2675-85. [CrossRef]
190. Castillo U, Harper JK, Strobel GA, Sears J, Alesi K, Ford E, et al. Kakadumycins, novel antibiotics from Streptomyces sp NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett 2003;224:183-90. [CrossRef]
191. Castillo UF, Strobel GA, Mullenberg K, Condron MM, Teplow DB, Folgiano V, et al. Munumbicins E-4 and E-5:Novel broad-spectrum antibiotics from Streptomyces NRRL 3052. FEMS Microbiol Lett 2006;255:296-300. [CrossRef]
192. Zin NM, Baba MS, Zainal-Abidin AH, Latip J, Mazlan NW, Edrada-Ebel R. Gancidin W, a potential low-toxicity antimalarial agent isolated from an endophytic Streptomyces SUK10. Drug Des Devel Ther 2017;11:351-63. [CrossRef]
193. McCarthy PJ, Roberts BF, Carbonell A, Roberts J, Wright AE, Chakrabarti D. Marine microbiome as a source of antimalarials. Trop Med Infect Dis 2019;4:103. [CrossRef]
194. Parapini S, Paone S, Erba E, Cavicchini L, Pourshaban M, Celani F, et al. In vitro antimalarial activity of inhibitors of the human GTPase Rac1. Antimicrob Agents Chemother 2022;66:e0149821. [CrossRef]
195. Kharroubi AT, Darwish HM. Diabetes mellitus:The epidemic of the century. World J Diabetes 2015;6:850-67. [CrossRef]
196. Antonceva E, Shamtsyan M. Antidiabetical and hypoglycemic action of mushroom polysaccharides. E3S Web Conf 2020;215:05001. [CrossRef]
197. Das A, Chen CM, Mu SC, Yang SH, Ju YM, Li SC. Medicinal components in edible mushrooms on diabetes mellitus treatment. Pharmaceutics 2022;14:436. [CrossRef]
198. Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 2000;23:1108-12. [CrossRef]
199. Hegazy WA, Rajab AA, Lila AS, Abbas HA. Anti-diabetics and antimicrobials:Harmony of mutual interplay. World J Diabetes 2021;12:1832-55. [CrossRef]
200. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in Type 2 diabetes pathophysiology. EBioMedicine 2020;51:102590. [CrossRef]
201. Ganesan K, Chung SK, Vanamala J, Xu B. Causal relationship between diet-induced gut microbiota changes and diabetes:A novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci 2018;19:3720. [CrossRef]
202. Jayant KK, Vijayakumar BS. In-vitro anti-oxidant and anti-diabetic potential of endophytic fungi associated with Ficus religiosa. Ital J Mycol 2021;50:10-20.
203. Ushasri R, Anusha R. In vitro anti-diabetic activity of ethanolic and acetone extracts of endophytic fungi Syncephalastrum racemosum isolated from the seaweed Gracilaria corticata by alphα-amylase inhibition assay method. Int J Curr Microbiol App Sci 2015;4:254-9.
204. Garrab M, Edziri H, El Mokni R, Mastouri M, Mabrouk H, Douki W. Phenolic composition, antioxidant and anticholinesterase properties of the three mushrooms Agaricus silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus giganteus (Pers.) Karst. in Tunisia. S Afr J Bot 2019;124:359-63. [CrossRef]
205. Friedman M. Mushroom polysaccharides:Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016;5:80. [CrossRef]
206. Hereher F, ElFallal A, Toson E, Abou-Dobara M, Abdelaziz M. Pilot study:Tumor suppressive effect of crude polysaccharide substances extracted from some selected mushroom. Beni Suef Univ J Basic Appl Sci 2018;7:767-75. [CrossRef]
207. Muszy?ska B, Grzywacz-Kisielewska A, Ka?a K, Gdula-Argasi?ska J. Anti-inflammatory properties of edible mushrooms:A review. Food Chem 2018;243:373-81. [CrossRef]
208. Stojkovic D, Smiljkovic M, Ciric A, Glamoclija J, Van Griensven L, Ferreira IC, et al. An insight into antidiabetic properties of six medicinal and edible mushrooms:Inhibition of α-amylase and a-glucosidase linked to Type-2 diabetes. S Afr J Bot 2019;120:100-3. [CrossRef]
209. Kiho T, Tsujimura Y, Sakushima M, Usui S, Ukai S. Polysaccharides in fungi. XXXIII. Hypoglycemic activity of an acidic polysaccharide (AC) from Tremella fuciformis. Yakugaku Zasshi 1994;114:308-15. [CrossRef]
210. Sato M, Tai T, Nunoura Y, Yajima Y, Kawashima S, Tanaka K. Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin. Biol Pharm Bull 2002;25:81-6. [CrossRef]
211. Zhang HN, Lin ZB. Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta Pharmacol Sin 2004;25:191-5.
212. Yang BK, Jung YS, Song CH. Hypoglycemic effects of Ganoderma applanatum and Collybia confluens exo-polymers in streptozotocin-induced diabetic rats. Phytother Res 2007;21:1066-9. [CrossRef]
213. Yuan Z, He P, Cui J, Takeuchi H. Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel. on genetically diabetic KK-Ay mice. Biosci Biotechnol Biochem 1998;62:1898-903. [CrossRef]
214. Kim YW, Kim KH, Choi HJ, Lee DS. Anti-diabetic activity of beta-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol Lett 2005;27:483-7. [CrossRef]
215. Han C, Liu T. A comparison of hypoglycemic activity of three species of basidiomycetes rich in vanadium. Biol Trace Elem Res 2009;127:177-82. [CrossRef]
216. Li SP, Zhang GH, Zeng Q, Huang ZG, Wang YT, Dong TT, et al. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 2006;13:428-33. [CrossRef]
217. Kubo K, Aoki H, Nanba H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biol Pharm Bull 1994;17:1106-10. [CrossRef]
218. Lo HC, Hsu TH, Chen CY. Submerged culture mycelium and broth of Grifola frondosa improve glycemic responses in diabetic rats. Am J Chin Med 2008;36:265-85. [CrossRef]
219. Manohar V, Talpur NA, Echard BW, Lieberman S, Preuss HG. Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes Obes Metab 2002;4:43-8. [CrossRef]
220. Austria AB, Dulay RM, Pambid RC. Mycochemicals, antioxidant and antidiabetic properties of Philippine sawgill mushroom Lentinus swartzii (higher basidiomycetes). Asian J Agric Biol 2021;10:202006365. [CrossRef]
221. Perera PK, Li Y. Mushrooms as a functional food mediator in preventing and ameliorating diabetes. Funct Food Health Dis 2011;1:161-71. [CrossRef]
222. Chatterjee S, Ghosh R, Mandal NC. Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLoS One 2019;14:e0214744. [CrossRef]
223. Gauchan DP, Kandel P, Tuladhar A, Acharya A, Kadel U, Baral A, et al. Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. F1000Res 2020;9:379. [CrossRef]
224. Do Nascimento AM, Soares MG, da Silva Torchelsen FK, de Araujo JA, Lage PS, Duarte MC, et al. Antileishmanial activity of compounds produced by endophytic fungi derived from medicinal plant Vernonia polyanthes and their potential as source of bioactive substances. World J Microbiol Biotechnol 2015;31:1793-800. [CrossRef]
225. Verma A, Gupta P, Rai N, Tiwari RK, Kumar A, Salvi P, et al. Assessment of biological activities of fungal endophytes derived bioactive compounds isolated from Amoora rohituka. J Fungi (Basel) 2022;8:285. [CrossRef]
226. Ramesh C, Pattar MG. Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacognosy Res 2010;2:107-12. [CrossRef]
227. Chowdhury MM, Kubra K, Ahmed SR. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann Clin Microbiol Antimicrob 2015;14:8. [CrossRef]
228. Barros L, Calhelha RC, Vaz JA, Ferreira IC, Baptista P, Estevinho LM. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol 2007;225:151-6. [CrossRef]
229. Erbiai EH, da Silva LP, Saidi R, Lamrani Z, da Silva JC, Maouni A. Chemical composition, bioactive compounds, and antioxidant activity of two wild edible mushrooms Armillaria mellea and Macrolepiota procera from two countries (Morocco and Portugal). Biomolecules 2021;11:575. [CrossRef]
230. Koutrotsios G, Kalogeropoulos N, Kaliora AC, Zervakis GI. Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J Agric Food Chem 2018;66:5971-83. [CrossRef]
231. Zawadzka A, Janczewska A, Kobus-Cisowska J, Dziedzi?ski M, Siwulski M, Czarniecka-Skubina E, et al. The effect of light conditions on the content of selected active ingredients in anatomical parts of the oyster mushroom (Pleurotus ostreatus L.). Plos One 2022;17:e0262279. [CrossRef]
232. Bhoonobtong A, Sawadsitang S, Sodngam S, Mongkolthanaruk W. Characterization of Endophytic Bacteria, Bacillus amyloliquefaciens for Antimicrobial Agents Production. In:International Conference on Biological and Life Sciences;2012. p. 6-11.
233. Beiranvand M, Amin M, Hashemi-Shahraki A, Romani B, Yaghoubi S, Sadeghi P. Antimicrobial activity of endophytic bacterial populations isolated from medical plants of Iran. Iran J Microbiol 2017;9:11-8.
234. Naine SJ, Nasimunislam N, Vaishnavi B, Mohanasrinivasan V, Devi CS. Isolation of soil actinomycetes inhabiting amrithi forest for the potential source of bioactive compounds. Asian J Pharm Clin Res 2012;5:189-92.
235. Elshobary ME, El-Shenody RA, Ashour M, Zabed HM, Qi X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci 2020;35:100567. [CrossRef]
236. Bhuyar P, Rahim MH, Maniam GP, Ramaraj R, Govindan N. Exploration of bioactive compounds and antibacterial activity of marine blue-green microalgae (Oscillatoria sp.) isolated from coastal region of West Malaysia. SN Appl Sci 2020;2:1906. [CrossRef]